Improving Management of Portal Hypertension: The Potential Benefit of Non-Etiological Therapies in Cirrhosis
Abstract
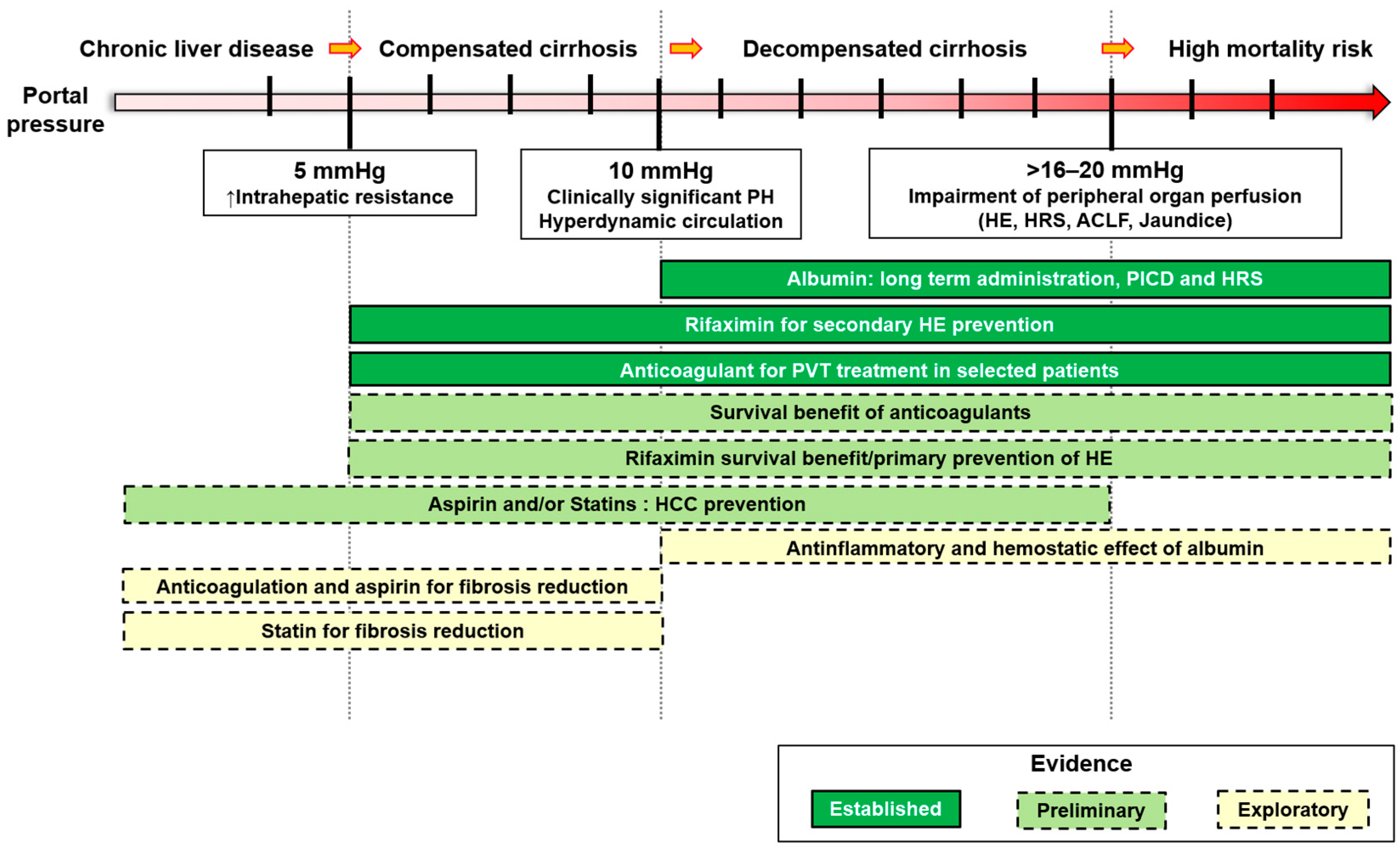
1. Introduction
2. Albumin
3. Rifaximin
4. Statins
5. Aspirin
6. Anticoagulation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosch, J.; Iwakiri, Y. The Portal Hypertension Syndrome: Etiology, Classification, Relevance, and Animal Models. Hepatol. Int. 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Iwakiri, Y.; Groszmann, R.J. The Hyperdynamic Circulation of Chronic Liver Diseases: From the Patient to the Molecule. Hepatology 2006, 43, S121–S131. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, E.; Garcia-Guix, M.; Mirabet, S.; Villanueva, C. The Relationship of Hyperdynamic Circulation and Cardiodynamic States in Cirrhosis. J. Hepatol. 2018, 69, 746–747. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, Y.; Trebicka, J. Portal Hypertension in Cirrhosis: Pathophysiological Mechanisms and Therapy. JHEP Rep. 2021, 3, 100316. [Google Scholar] [CrossRef] [PubMed]
- De Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Abraldes, J.G.; Albillos, A.; Baiges, A.; Bajaj, J.; Bañares, R.; et al. Baveno VII–Renewing Consensus in Portal Hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- La Mura, V.; Nicolini, A.; Tosetti, G.; Primignani, M. Cirrhosis and Portal Hypertension: The Importance of Risk Stratification, the Role of Hepatic Venous Pressure Gradient Measurement. World J. Hepatol. 2015, 7, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Mandorfer, M.; Kozbial, K.; Schwabl, P.; Freissmuth, C.; Schwarzer, R.; Stern, R.; Chromy, D.; Stättermayer, A.F.; Reiberger, T.; Beinhardt, S.; et al. Sustained Virologic Response to Interferon-Free Therapies Ameliorates HCV-Induced Portal Hypertension. J. Hepatol. 2016, 65, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Vorobioff, J.; Groszmann, R.J.; Picabea, E.; Gamen, M.; Villavicencio, R.; Bordato, J.; Morel, I.; Audano, M.; Tanno, H.; Lerner, E.; et al. Prognostic Value of Hepatic Venous Pressure Gradient Measurements in Alcoholic Cirrhosis: A 10-Year Prospective Study. Gastroenterology 1996, 111, 701–709. [Google Scholar] [CrossRef]
- Lampertico, P.; Invernizzi, F.; Viganò, M.; Loglio, A.; Mangia, G.; Facchetti, F.; Primignani, M.; Jovani, M.; Iavarone, M.; Fraquelli, M.; et al. The Long-Term Benefits of Nucleos(t)Ide Analogs in Compensated HBV Cirrhotic Patients with No or Small Esophageal Varices: A 12-Year Prospective Cohort Study. J. Hepatol. 2015, 63, 1118–1125. [Google Scholar] [CrossRef]
- Berzigotti, A.; Albillos, A.; Villanueva, C.; Genescá, J.; Ardevol, A.; Augustín, S.; Calleja, J.L.; Bañares, R.; García-Pagán, J.C.; Mesonero, F.; et al. Effects of an Intensive Lifestyle Intervention Program on Portal Hypertension in Patients with Cirrhosis and Obesity: The SportDiet Study. Hepatology 2017, 65, 1293–1305. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Degasperi, E.; Anolli, M.P.; Fanetti, I.; Borghi, M.; Soffredini, R.; Iavarone, M.; Tosetti, G.; Perbellini, R.; Sangiovanni, A.; et al. Incidence of Liver- and Non-Liver-Related Outcomes in Patients with HCV-Cirrhosis after SVR. J. Hepatol. 2022, 76, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.G.; Mendoza, Y.P.; Bosch, J. Beta-Blockers in Cirrhosis: Evidence-Based Indications and Limitations. JHEP Rep. 2020, 2, 100063. [Google Scholar] [CrossRef]
- Lens, S.; Baiges, A.; Alvarado-Tapias, E.; LLop, E.; Martinez, J.; Fortea, J.I.; Ibáñez-Samaniego, L.; Mariño, Z.; Rodríguez-Tajes, S.; Gallego, A.; et al. Clinical Outcome and Hemodynamic Changes Following HCV Eradication with Oral Antiviral Therapy in Patients with Clinically Significant Portal Hypertension. J. Hepatol. 2020, 73, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Jalan, R.; D’Amico, G.; Trebicka, J.; Moreau, R.; Angeli, P.; Arroyo, V. New Clinical and Pathophysiological Perspectives Defining the Trajectory of Cirrhosis. J. Hepatol. 2021, 75, S14–S26. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Angeli, P.; Moreau, R.; Jalan, R.; Claria, J.; Trebicka, J.; Fernández, J.; Gustot, T.; Caraceni, P.; Bernardi, M. The Systemic Inflammation Hypothesis: Towards a New Paradigm of Acute Decompensation and Multiorgan Failure in Cirrhosis. J. Hepatol. 2020, 74, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Turco, L.; Garcia-Tsao, G.; Magnani, I.; Bianchini, M.; Costetti, M.; Caporali, C.; Colopi, S.; Simonini, E.; De Maria, N.; Banchelli, F.; et al. Cardiopulmonary Hemodynamics and C-Reactive Protein as Prognostic Indicators in Compensated and Decompensated Cirrhosis. J. Hepatol. 2018, 68, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.; Quintarelli, C.; Lattuada, A.; Leo, R.; Alessandroni, M.; Mannucci, P.M.; Violi, F. High Plasma Levels of von Willebrand Factor as a Marker of Endothelial Perturbation in Cirrhosis: Relationship to Endotoxemia. Hepatology 1996, 23, 1377–1383. [Google Scholar] [CrossRef]
- Bellot, P.; García-Pagán, J.C.; Francés, R.; Abraldes, J.G.; Navasa, M.; Pérez-Mateo, M.; Such, J.; Bosch, J. Bacterial DNA Translocation Is Associated with Systemic Circulatory Abnormalities and Intrahepatic Endothelial Dysfunction in Patients with Cirrhosis. Hepatology 2010, 52, 2044–2052. [Google Scholar] [CrossRef]
- Bellot, P.; Francés, R.; Such, J. Pathological Bacterial Translocation in Cirrhosis: Pathophysiology, Diagnosis and Clinical Implications. Liver Int. 2013, 33, 31–39. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef]
- La Mura, V.; Reverter, J.C.; Flores-Arroyo, A.; Raffa, S.; Reverter, E.; Seijo, S.; Abraldes, J.G.; Bosch, J.; García-Pagán, J.C. Von Willebrand Factor Levels Predict Clinical Outcome in Patients with Cirrhosis and Portal Hypertension. Gut 2011, 60, 1133–1138. [Google Scholar] [CrossRef]
- Kalambokis, G.N.; Oikonomou, A.; Christou, L.; Kolaitis, N.I.; Tsianos, E.V.; Christodoulou, D.; Baltayiannis, G. Von Willebrand Factor and Procoagulant Imbalance Predict Outcome in Patients with Cirrhosis and Thrombocytopenia. J. Hepatol. 2016, 65, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Primignani, M.; Lemma, L.; Chantarangkul, V.; Dell’Era, A.; Iannuzzi, F.; Aghemo, A.; Mannucci, P.M. Detection of the Imbalance of Procoagulant versus Anticoagulant Factors in Cirrhosis by a Simple Laboratory Method. Hepatology 2010, 52, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Ferlitsch, M.; Reiberger, T.; Hoke, M.; Salzl, P.; Schwengerer, B.; Ulbrich, G.; Payer, B.A.; Trauner, M.; Peck-Radosavljevic, M.; Ferlitsch, A. Von Willebrand Factor as New Noninvasive Predictor of Portal Hypertension, Decompensation and Mortality in Patients with Liver Cirrhosis. Hepatology 2012, 56, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.; Liguori, E.; La Mura, V. Coagulation, Microenvironment and Liver Fibrosis. Cells 2018, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Peters, T. (Ed.) The Albumin Molecule: Its Structure and Chemical Properties. In All about Albumin; Academic Press: San Diego, CA, USA, 1995; pp. 9–75. ISBN 978-0-12-552110-9. [Google Scholar]
- Bhattacharya, A.A.; Grüne, T.; Curry, S. Crystallographic Analysis Reveals Common Modes of Binding of Medium and Long-Chain Fatty Acids to Human Serum Albumin. J. Mol. Biol. 2000, 303, 721–732. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human Serum Albumin: From Bench to Bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Chaudhury, C.; Mehnaz, S.; Robinson, J.M.; Hayton, W.L.; Pearl, D.K.; Roopenian, D.C.; Anderson, C.L. The Major Histocompatibility Complex-Related Fc Receptor for IgG (FcRn) Binds Albumin and Prolongs Its Lifespan. J. Exp. Med. 2003, 197, 315–322. [Google Scholar] [CrossRef]
- Arroyo, V.; García-Martinez, R.; Salvatella, X. Human Serum Albumin, Systemic Inflammation, and Cirrhosis. J. Hepatol. 2014, 61, 396–407. [Google Scholar] [CrossRef]
- Zhang, W.-J.; Frei, B. Albumin Selectively Inhibits TNF Alpha-Induced Expression of Vascular Cell Adhesion Molecule-1 in Human Aortic Endothelial Cells. Cardiovasc. Res. 2002, 55, 820–829. [Google Scholar] [CrossRef]
- Vila, M.C.; Solà, R.; Molina, L.; Andreu, M.; Coll, S.; Gana, J.; Marquez, J.; Palá, J.; Bory, F.; Pons, S.; et al. Hemodynamic Changes in Patients Developing Effective Hypovolemia after Total Paracentesis. J. Hepatol. 1998, 28, 639–645. [Google Scholar] [CrossRef]
- Ruiz-del-Arbol, L.; Monescillo, A.; Jimenéz, W.; Garcia-Plaza, A.; Arroyo, V.; Rodés, J. Paracentesis-Induced Circulatory Dysfunction: Mechanism and Effect on Hepatic Hemodynamics in Cirrhosis. Gastroenterology 1997, 113, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Caraceni, P.; Navickis, R.J.; Wilkes, M.M. Albumin Infusion in Patients Undergoing Large-Volume Paracentesis: A Meta-Analysis of Randomized Trials. Hepatology 2012, 55, 1172–1181. [Google Scholar] [CrossRef]
- Caraceni, P.; Riggio, O.; Angeli, P.; Alessandria, C.; Neri, S.; Foschi, F.G.; Levantesi, F.; Airoldi, A.; Boccia, S.; Svegliati-Baroni, G.; et al. Long-Term Albumin Administration in Decompensated Cirrhosis (ANSWER): An Open-Label Randomised Trial. Lancet 2018, 391, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, P.; Sherlock, S. The Effect of Repeated Albumin Infusions in Patients with Cirrhosis. Lancet 1962, 2, 1125–1129. [Google Scholar] [CrossRef]
- Di Pascoli, M.; Fasolato, S.; Piano, S.; Bolognesi, M.; Angeli, P. Long-Term Administration of Human Albumin Improves Survival in Patients with Cirrhosis and Refractory Ascites. Liver Int. 2019, 39, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Italian Association for the Study of the Liver (AISF). Portal Hypertension and Ascites: Patient-and Population-Centered Clinical Practice Guidelines by the Italian Association for the Study of the Liver (AISF). Dig. Liver Dis. 2021, 53, 1089–1104. [Google Scholar] [CrossRef]
- Ginès, P.; Solà, E.; Angeli, P.; Wong, F.; Nadim, M.K.; Kamath, P.S. Hepatorenal Syndrome. Nat. Rev. Dis. Primers 2018, 4, 23. [Google Scholar] [CrossRef]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the Management of Patients with Decompensated Cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- EASL Clinical Practice Guidelines on the Management of Ascites, Spontaneous Bacterial Peritonitis, and Hepatorenal Syndrome in Cirrhosis. J. Hepatol. 2010, 53, 397–417. [CrossRef]
- Runyon, B.A. The Evolution of Ascitic Fluid Analysis in the Diagnosis of Spontaneous Bacterial Peritonitis. Am. J. Gastroenterol. 2003, 98, 1675–1677. [Google Scholar] [CrossRef]
- Rimola, A.; García-Tsao, G.; Navasa, M.; Piddock, L.J.; Planas, R.; Bernard, B.; Inadomi, J.M. Diagnosis, Treatment and Prophylaxis of Spontaneous Bacterial Peritonitis: A Consensus Document. International Ascites Club. J. Hepatol. 2000, 32, 142–153. [Google Scholar] [CrossRef]
- Garcia-Martinez, R.; Caraceni, P.; Bernardi, M.; Gines, P.; Arroyo, V.; Jalan, R. Albumin: Pathophysiologic Basis of Its Role in the Treatment of Cirrhosis and Its Complications. Hepatology 2013, 58, 1836–1846. [Google Scholar] [CrossRef]
- Salerno, F.; Navickis, R.J.; Wilkes, M.M. Albumin Infusion Improves Outcomes of Patients with Spontaneous Bacterial Peritonitis: A Meta-Analysis of Randomized Trials. Clin. Gastroenterol. Hepatol. 2013, 11, 123–130.e1. [Google Scholar] [CrossRef]
- Fernández, J.; Angeli, P.; Trebicka, J.; Merli, M.; Gustot, T.; Alessandria, C.; Aagaard, N.K.; de Gottardi, A.; Welzel, T.M.; Gerbes, A.; et al. Efficacy of Albumin Treatment for Patients with Cirrhosis and Infections Unrelated to Spontaneous Bacterial Peritonitis. Clin. Gastroenterol. Hepatol. 2020, 18, 963–973.e14. [Google Scholar] [CrossRef]
- China, L.; Freemantle, N.; Forrest, E.; Kallis, Y.; Ryder, S.D.; Wright, G.; Portal, A.J.; Becares Salles, N.; Gilroy, D.W.; O’Brien, A. A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N. Engl. J. Med. 2021, 384, 808–817. [Google Scholar] [CrossRef]
- Fernández, J.; Clària, J.; Amorós, A.; Aguilar, F.; Castro, M.; Casulleras, M.; Acevedo, J.; Duran-Güell, M.; Nuñez, L.; Costa, M.; et al. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients with Decompensated Cirrhosis. Gastroenterology 2019, 157, 149–162. [Google Scholar] [CrossRef]
- Bernardi, M.; Angeli, P.; Claria, J.; Moreau, R.; Gines, P.; Jalan, R.; Caraceni, P.; Fernandez, J.; Gerbes, A.L.; O’Brien, A.J.; et al. Albumin in Decompensated Cirrhosis: New Concepts and Perspectives. Gut 2020, 69, 1127–1138. [Google Scholar] [CrossRef]
- Solà, E.; Solé, C.; Simón-Talero, M.; Martín-Llahí, M.; Castellote, J.; Garcia-Martínez, R.; Moreira, R.; Torrens, M.; Márquez, F.; Fabrellas, N.; et al. Midodrine and Albumin for Prevention of Complications in Patients with Cirrhosis Awaiting Liver Transplantation. A Randomized Placebo-Controlled Trial. J. Hepatol. 2018, 69, 1250–1259. [Google Scholar] [CrossRef]
- Tufoni, M.; Zaccherini, G.; Caraceni, P. Prolonged Albumin Administration in Patients with Decompensated Cirrhosis: The Amount Makes the Difference. Ann. Transl. Med. 2019, 7, S201. [Google Scholar] [CrossRef]
- Kim, S.B.; Chi, H.S.; Park, J.S.; Hong, C.D.; Yang, W.S. Effect of Increasing Serum Albumin on Plasma D-Dimer, von Willebrand Factor, and Platelet Aggregation in CAPD Patients. Am. J. Kidney Dis. 1999, 33, 312–317. [Google Scholar] [CrossRef]
- Garcia-Martinez, R.; Noiret, L.; Sen, S.; Mookerjee, R.; Jalan, R. Albumin Infusion Improves Renal Blood Flow Autoregulation in Patients with Acute Decompensation of Cirrhosis and Acute Kidney Injury. Liver Int. 2015, 35, 335–343. [Google Scholar] [CrossRef]
- Fernández, J.; Monteagudo, J.; Bargallo, X.; Jiménez, W.; Bosch, J.; Arroyo, V.; Navasa, M. A Randomized Unblinded Pilot Study Comparing Albumin versus Hydroxyethyl Starch in Spontaneous Bacterial Peritonitis. Hepatology 2005, 42, 627–634. [Google Scholar] [CrossRef]
- Northup, P.G.; McMahon, M.M.; Ruhl, A.P.; Altschuler, S.E.; Volk-Bednarz, A.; Caldwell, S.H.; Berg, C.L. Coagulopathy Does Not Fully Protect Hospitalized Cirrhosis Patients from Peripheral Venous Thromboembolism. Am. J. Gastroenterol. 2006, 101, 1524–1528. [Google Scholar] [CrossRef]
- DuPont, H.L. Review Article: The Antimicrobial Effects of Rifaximin on the Gut Microbiota. Aliment. Pharmacol. Ther. 2016, 43 (Suppl. S1), 3–10. [Google Scholar] [CrossRef]
- Scarpignato, C.; Pelosini, I. Rifaximin, a Poorly Absorbed Antibiotic: Pharmacology and Clinical Potential. Chemotherapy 2005, 51 (Suppl. S1), 36–66. [Google Scholar] [CrossRef]
- Baker, D.E. Rifaximin: A Nonabsorbed Oral Antibiotic. Rev. Gastroenterol. Disord. 2005, 5, 19–30. [Google Scholar]
- Robins, G.W.; Wellington, K. Rifaximin: A Review of Its Use in the Management of Traveller’s Diarrhoea. Drugs 2005, 65, 1697–1713. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jimenez, C.; Mookerjee, R.; et al. The PREDICT Study Uncovers Three Clinical Courses of Acutely Decompensated Cirrhosis That Have Distinct Pathophysiology. J. Hepatol. 2020, 73, 842–854. [Google Scholar] [CrossRef]
- Trebicka, J.; Macnaughtan, J.; Schnabl, B.; Shawcross, D.L.; Bajaj, J.S. The Microbiota in Cirrhosis and Its Role in Hepatic Decompensation. J. Hepatol. 2021, 75, S67–S81. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R. Acute-on-Chronic Liver Failure. N. Engl. J. Med. 2020, 382, 2137–2145. [Google Scholar] [CrossRef]
- Tarao, K.; So, K.; Moroi, T.; Ikeuchi, T.; Suyama, T.; Endo, O.; Fukushima, K. Detection of Endotoxin in Plasma and Ascitic Fluid of Patients with Cirrhosis: Its Clinical Significance. Gastroenterology 1977, 73, 539–542. [Google Scholar] [CrossRef]
- Triger, D.R.; Boyer, T.D.; Levin, J. Portal and Systemic Bacteraemia and Endotoxaemia in Liver Disease. Gut 1978, 19, 935–939. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-Chronic Liver Failure Is a Distinct Syndrome That Develops in Patients with Acute Decompensation of Cirrhosis. Gastroenterology 2013, 144, 1426–1437.e9. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, Liver Disease and the Gut Microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions Between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of Fecal Microbial Communities in Patients with Liver Cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered Profile of Human Gut Microbiome Is Associated with Cirrhosis and Its Complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef]
- Sorribas, M.; Jakob, M.O.; Yilmaz, B.; Li, H.; Stutz, D.; Noser, Y.; de Gottardi, A.; Moghadamrad, S.; Hassan, M.; Albillos, A.; et al. FXR Modulates the Gut-Vascular Barrier by Regulating the Entry Sites for Bacterial Translocation in Experimental Cirrhosis. J. Hepatol. 2019, 71, 1126–1140. [Google Scholar] [CrossRef]
- Vlachogiannakos, J.; Saveriadis, A.S.; Viazis, N.; Theodoropoulos, I.; Foudoulis, K.; Manolakopoulos, S.; Raptis, S.; Karamanolis, D.G. Intestinal Decontamination Improves Liver Haemodynamics in Patients with Alcohol-Related Decompensated Cirrhosis. Aliment. Pharmacol. Ther. 2009, 29, 992–999. [Google Scholar] [CrossRef]
- Kimer, N.; Pedersen, J.S.; Tavenier, J.; Christensen, J.E.; Busk, T.M.; Hobolth, L.; Krag, A.; Al-Soud, W.A.; Mortensen, M.S.; Sørensen, S.J.; et al. Rifaximin Has Minor Effects on Bacterial Composition, Inflammation, and Bacterial Translocation in Cirrhosis: A Randomized Trial. J. Gastroenterol. Hepatol. 2018, 33, 307–314. [Google Scholar] [CrossRef]
- Kimer, N.; Pedersen, J.S.; Busk, T.M.; Gluud, L.L.; Hobolth, L.; Krag, A.; Møller, S.; Bendtsen, F. Copenhagen Rifaximin (CoRif) Study Group Rifaximin Has No Effect on Hemodynamics in Decompensated Cirrhosis: A Randomized, Double-Blind, Placebo-Controlled Trial. Hepatology 2017, 65, 592–603. [Google Scholar] [CrossRef]
- Lim, Y.L.; Kim, M.Y.; Jang, Y.O.; Baik, S.K.; Kwon, S.O. Rifaximin and Propranolol Combination Therapy Is More Effective than Propranolol Monotherapy for the Reduction of Portal Pressure: An Open Randomized Controlled Pilot Study. Gut Liver 2017, 11, 702–710. [Google Scholar] [CrossRef]
- Vlachogiannakos, J.; Viazis, N.; Vasianopoulou, P.; Vafiadis, I.; Karamanolis, D.G.; Ladas, S.D. Long-Term Administration of Rifaximin Improves the Prognosis of Patients with Decompensated Alcoholic Cirrhosis. J. Gastroenterol. Hepatol. 2013, 28, 450–455. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, Y.B.; Lee, J.-H.; Nam, J.Y.; Chang, Y.; Cho, H.; Yoo, J.-J.; Cho, Y.Y.; Cho, E.J.; Yu, S.J.; et al. Rifaximin Treatment Is Associated with Reduced Risk of Cirrhotic Complications and Prolonged Overall Survival in Patients Experiencing Hepatic Encephalopathy. Aliment. Pharmacol. Ther. 2017, 46, 845–855. [Google Scholar] [CrossRef]
- Dong, T.; Aronsohn, A.; Gautham Reddy, K.; Te, H.S. Rifaximin Decreases the Incidence and Severity of Acute Kidney Injury and Hepatorenal Syndrome in Cirrhosis. Dig. Dis. Sci. 2016, 61, 3621–3626. [Google Scholar] [CrossRef]
- Salehi, S.; Tranah, T.H.; Lim, S.; Heaton, N.; Heneghan, M.; Aluvihare, V.; Patel, V.C.; Shawcross, D.L. Rifaximin Reduces the Incidence of Spontaneous Bacterial Peritonitis, Variceal Bleeding and All-Cause Admissions in Patients on the Liver Transplant Waiting List. Aliment. Pharmacol. Ther. 2019, 50, 435–441. [Google Scholar] [CrossRef]
- Ibrahim, E.-S.; Alsebaey, A.; Zaghla, H.; Moawad Abdelmageed, S.; Gameel, K.; Abdelsameea, E. Long-Term Rifaximin Therapy as a Primary Prevention of Hepatorenal Syndrome. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1247–1250. [Google Scholar] [CrossRef]
- Kamal, F.; Khan, M.A.; Khan, Z.; Cholankeril, G.; Hammad, T.A.; Lee, W.M.; Ahmed, A.; Waters, B.; Howden, C.W.; Nair, S.; et al. Rifaximin for the Prevention of Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome in Cirrhosis: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1109–1117. [Google Scholar] [CrossRef]
- Hanouneh, M.A.; Hanouneh, I.A.; Hashash, J.G.; Law, R.; Esfeh, J.M.; Lopez, R.; Hazratjee, N.; Smith, T.; Zein, N.N. The Role of Rifaximin in the Primary Prophylaxis of Spontaneous Bacterial Peritonitis in Patients with Liver Cirrhosis. J. Clin. Gastroenterol. 2012, 46, 709–715. [Google Scholar] [CrossRef]
- Mostafa, T.; Badra, G.; Abdallah, M. The Efficacy and the Immunomodulatory Effect of Rifaximin in Prophylaxis of Spontaneous Bacterial Peritonitis in Cirrhotic Egyptian Patients. Turk. J. Gastroenterol. 2015, 26, 163–169. [Google Scholar] [CrossRef]
- Lutz, P.; Parcina, M.; Bekeredjian-Ding, I.; Nischalke, H.D.; Nattermann, J.; Sauerbruch, T.; Hoerauf, A.; Strassburg, C.P.; Spengler, U. Impact of Rifaximin on the Frequency and Characteristics of Spontaneous Bacterial Peritonitis in Patients with Liver Cirrhosis and Ascites. PLoS ONE 2014, 9, e93909. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Rautou, P.-E.; Romero-Gómez, M.; Larsen, F.S.; Shawcross, D.L.; Thabut, D.; Vilstrup, H.; Weissenborn, K. EASL Clinical Practice Guidelines on the Management of Hepatic Encephalopathy. J. Hepatol. 2022, 77, 807–824. [Google Scholar] [CrossRef]
- Bass, N.M.; Mullen, K.D.; Sanyal, A.; Poordad, F.; Neff, G.; Leevy, C.B.; Sigal, S.; Sheikh, M.Y.; Beavers, K.; Frederick, T.; et al. Rifaximin Treatment in Hepatic Encephalopathy. N. Engl. J. Med. 2010, 362, 1071–1081. [Google Scholar] [CrossRef]
- Mullen, K.D.; Sanyal, A.J.; Bass, N.M.; Poordad, F.F.; Sheikh, M.Y.; Frederick, R.T.; Bortey, E.; Forbes, W.P. Rifaximin Is Safe and Well Tolerated for Long-Term Maintenance of Remission from Overt Hepatic Encephalopathy. Clin. Gastroenterol. Hepatol. 2014, 12, 1390–1397. [Google Scholar] [CrossRef]
- Sanyal, A.; Younossi, Z.M.; Bass, N.M.; Mullen, K.D.; Poordad, F.; Brown, R.S.; Vemuru, R.P.; Mazen Jamal, M.; Huang, S.; Merchant, K.; et al. Randomised Clinical Trial: Rifaximin Improves Health-Related Quality of Life in Cirrhotic Patients with Hepatic Encephalopathy-a Double-Blind Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2011, 34, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Bureau, C.; Thabut, D.; Jezequel, C.; Archambeaud, I.; D’Alteroche, L.; Dharancy, S.; Borentain, P.; Oberti, F.; Plessier, A.; De Ledinghen, V.; et al. The Use of Rifaximin in the Prevention of Overt Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt : A Randomized Controlled Trial. Ann. Intern. Med. 2021, 174, 633–640. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Shallcross, L.; O’Brien, A. Antimicrobial Resistance in Liver Disease: Better Diagnostics Are Needed. Lancet Gastroenterol. Hepatol. 2017, 2, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and Fungal Infections in Acute-on-Chronic Liver Failure: Prevalence, Characteristics and Impact on Prognosis. Gut 2018, 67, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Salerno, A.; Pesce, A.; Debbia, E.A.; Schito, G.C. In Vitro Activity of Rifaximin, Metronidazole and Vancomycin against Clostridium Difficile and the Rate of Selection of Spontaneously Resistant Mutants against Representative Anaerobic and Aerobic Bacteria, Including Ammonia-Producing Species. Chemotherapy 2000, 46, 253–266. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Jiang, Z.-D. Influence of Rifaximin Treatment on the Susceptibility of Intestinal Gram-Negative Flora and Enterococci. Clin. Microbiol. Infect. 2004, 10, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Bouza, E.; Alcalá, L.; Marín, M.; Valerio, M.; Reigadas, E.; Muñoz, P.; González-Del Vecchio, M.; de Egea, V. An Outbreak of Clostridium Difficile PCR Ribotype 027 in Spain: Risk Factors for Recurrence and a Novel Treatment Strategy. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1777–1786. [Google Scholar] [CrossRef]
- Huang, J.S.; Jiang, Z.-D.; Garey, K.W.; Lasco, T.; Dupont, H.L. Use of Rifamycin Drugs and Development of Infection by Rifamycin-Resistant Strains of Clostridium Difficile. Antimicrob. Agents Chemother. 2013, 57, 2690–2693. [Google Scholar] [CrossRef]
- Bays, H.; Cohen, D.E.; Chalasani, N.; Harrison, S.A.; The National Lipid Association’s Statin Safety Task Force. An Assessment by the Statin Liver Safety Task Force: 2014 Update. J. Clin. Lipidol. 2014, 8, S47–S57. [Google Scholar] [CrossRef]
- Naci, H.; Brugts, J.J.; Fleurence, R.; Tsoi, B.; Toor, H.; Ades, A. Comparative Benefits of Statins in the Primary and Secondary Prevention of Major Coronary Events and All-Cause Mortality: A Network Meta-Analysis of Placebo-Controlled and Active-Comparator Trials. Eur. J. Prev. Cardiol. 2013, 20, 641–657. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, R.; Barroso, E.; Serrano, L.; Coll, T.; Sánchez, R.M.; Merlos, M.; Palomer, X.; Laguna, J.C.; Vázquez-Carrera, M. Atorvastatin Prevents Carbohydrate Response Element Binding Protein Activation in the Fructose-Fed Rat by Activating Protein Kinase A. Hepatology 2009, 49, 106–115. [Google Scholar] [CrossRef]
- Bakker-Arkema, R.G.; Davidson, M.H.; Goldstein, R.J.; Davignon, J.; Isaacsohn, J.L.; Weiss, S.R.; Keilson, L.M.; Brown, W.V.; Miller, V.T.; Shurzinske, L.J.; et al. Efficacy and Safety of a New HMG-CoA Reductase Inhibitor, Atorvastatin, in Patients with Hypertriglyceridemia. JAMA 1996, 275, 128–133. [Google Scholar] [CrossRef]
- Liao, J.K.; Laufs, U. Pleiotropic Effects of Statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A Century of Cholesterol and Coronaries: From Plaques to Genes to Statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Gruzdeva, O.; Uchasova, E.; Dyleva, Y.; Akbasheva, O.; Karetnikova, V.; Barbarash, O. Early Effects of Treatment Low-Dose Atorvastatin on Markers of Insulin Resistance and Inflammation in Patients with Myocardial Infarction. Front. Pharmacol. 2016, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Schierwagen, R.; Maybüchen, L.; Hittatiya, K.; Klein, S.; Uschner, F.E.; Braga, T.T.; Franklin, B.S.; Nickenig, G.; Strassburg, C.P.; Plat, J.; et al. Statins Improve NASH via Inhibition of RhoA and Ras. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G724–G733. [Google Scholar] [CrossRef]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a Newly Recognized Type of Immunomodulator. Nat. Med. 2000, 6, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Kumar, A.; SenBanerjee, S.; Staniszewski, K.; Parmar, K.; Vaughan, D.E.; Gimbrone, M.A.; Balasubramanian, V.; García-Cardeña, G.; Jain, M.K. Kruppel-like Factor 2 (KLF2) Regulates Endothelial Thrombotic Function. Circ. Res. 2005, 96, e48–e57. [Google Scholar] [CrossRef]
- Marrone, G.; Maeso-Díaz, R.; García-Cardena, G.; Abraldes, J.G.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. KLF2 Exerts Antifibrotic and Vasoprotective Effects in Cirrhotic Rat Livers: Behind the Molecular Mechanisms of Statins. Gut 2014, 64, 1434–1443. [Google Scholar] [CrossRef]
- Trebicka, J.; Amoros, A.; Pitarch, C.; Titos, E.; Alcaraz-Quiles, J.; Schierwagen, R.; Deulofeu, C.; Fernandez-Gomez, J.; Piano, S.; Caraceni, P.; et al. Addressing Profiles of Systemic Inflammation Across the Different Clinical Phenotypes of Acutely Decompensated Cirrhosis. Front. Immunol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Trebicka, J.; Hennenberg, M.; Laleman, W.; Shelest, N.; Biecker, E.; Schepke, M.; Nevens, F.; Sauerbruch, T.; Heller, J. Atorvastatin Lowers Portal Pressure in Cirrhotic Rats by Inhibition of RhoA/Rho-Kinase and Activation of Endothelial Nitric Oxide Synthase. Hepatology 2007, 46, 242–253. [Google Scholar] [CrossRef]
- La Mura, V.; Pasarín, M.; Meireles, C.Z.; Miquel, R.; Rodríguez-Vilarrupla, A.; Hide, D.; Gracia-Sancho, J.; García-Pagán, J.C.; Bosch, J.; Abraldes, J.G. Effects of Simvastatin Administration on Rodents with Lipopolysaccharide-Induced Liver Microvascular Dysfunction. Hepatology 2013, 57, 1172–1181. [Google Scholar] [CrossRef]
- Tripathi, D.M.; Vilaseca, M.; Lafoz, E.; Garcia-Calderó, H.; Haute, G.V.; Fernández-Iglesias, A.; de Oliveira, J.R.; García-Pagán, J.C.; Bosch, J.; Gracia-Sancho, J. Simvastatin Prevents Progression of Acute on Chronic Liver Failure in Rats with Cirrhosis and Portal Hypertension. Gastroenterology 2018, 155, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Rodríguez-Vilarrupla, A.; Graupera, M.; Zafra, C.; García-Calderó, H.; García-Pagán, J.C.; Bosch, J. Simvastatin Treatment Improves Liver Sinusoidal Endothelial Dysfunction in CCl4 Cirrhotic Rats. J. Hepatol. 2007, 46, 1040–1046. [Google Scholar] [CrossRef]
- Deza, Z.; Caimi, G.R.; Noelia, M.; Coli, L.; Ridruejo, E.; Alvarez, L. Atorvastatin Shows Antitumor Effect in Hepatocellular Carcinoma Development by Inhibiting Angiogenesis via TGF-Β1/PERK Signaling Pathway. Mol. Carcinog. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Uschner, F.E.; Ranabhat, G.; Choi, S.S.; Granzow, M.; Klein, S.; Schierwagen, R.; Raskopf, E.; Gautsch, S.; van der Ven, P.F.M.; Fürst, D.O.; et al. Statins Activate the Canonical Hedgehog-Signaling and Aggravate Non-Cirrhotic Portal Hypertension, but Inhibit the Non-Canonical Hedgehog Signaling and Cirrhotic Portal Hypertension. Sci. Rep. 2015, 5, 14573. [Google Scholar] [CrossRef]
- Chang, C.-C.; Wang, S.-S.; Hsieh, H.-G.; Lee, W.-S.; Chuang, C.-L.; Lin, H.-C.; Lee, F.-Y.; Lee, S.-D.; Huang, H.-C. Rosuvastatin Improves Hepatopulmonary Syndrome through Inhibition of Inflammatory Angiogenesis of Lung. Clin. Sci. 2015, 129, 449–460. [Google Scholar] [CrossRef]
- Zafra, C.; Abraldes, J.G.; Turnes, J.; Berzigotti, A.; Fernández, M.; Garca-Pagán, J.C.; Rodés, J.; Bosch, J. Simvastatin Enhances Hepatic Nitric Oxide Production and Decreases the Hepatic Vascular Tone in Patients with Cirrhosis. Gastroenterology 2004, 126, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Albillos, A.; Bañares, R.; Turnes, J.; González, R.; García-Pagán, J.C.; Bosch, J. Simvastatin Lowers Portal Pressure in Patients with Cirrhosis and Portal Hypertension: A Randomized Controlled Trial. Gastroenterology 2009, 136, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Abraldes, J.G.; Villanueva, C.; Aracil, C.; Turnes, J.; Hernandez-Guerra, M.; Genesca, J.; Rodriguez, M.; Castellote, J.; García-Pagán, J.C.; Torres, F.; et al. Addition of Simvastatin to Standard Therapy for the Prevention of Variceal Rebleeding Does Not Reduce Rebleeding but Increases Survival in Patients with Cirrhosis. Gastroenterology 2016, 150, 1160–1170.e3. [Google Scholar] [CrossRef]
- Unger, L.W.; Forstner, B.; Schneglberger, S.; Muckenhuber, M.; Eigenbauer, E.; Bauer, D.; Scheiner, B.; Mandorfer, M.; Trauner, M.; Reiberger, T. Guideline-Conform Statin Use Reduces Overall Mortality in Patients with Compensated Liver Disease. Sci. Rep. 2019, 9, 11674. [Google Scholar] [CrossRef]
- Kaplan, D.E.; Serper, M.; Mehta, R.; Fox, R.; John, B.; Aytaman, A.; Baytarian, M.; Hunt, K.; Albrecht, J.; Njei, B.; et al. Effects of Hypercholesterolemia and Statin Exposure on Survival in a Large National Cohort of Patients with Cirrhosis. Gastroenterology 2019, 156, 1693–1706. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Lee, C.-L.; Yang, S.-S.; Fu, S.-C.; Chen, Y.-Y.; Wang, T.-C.; Hu, J.-T.; Chen, D.-S. Statins Reduce the Risk of Cirrhosis and Its Decompensation in Chronic Hepatitis B Patients: A Nationwide Cohort Study. Am. J. Gastroenterol. 2016, 111, 976–985. [Google Scholar] [CrossRef]
- Chong, L.-W.; Hsu, Y.-C.; Lee, T.-F.; Lin, Y.; Chiu, Y.-T.; Yang, K.-C.; Wu, J.-C.; Huang, Y.-T. Fluvastatin Attenuates Hepatic Steatosis-Induced Fibrogenesis in Rats through Inhibiting Paracrine Effect of Hepatocyte on Hepatic Stellate Cells. BMC Gastroenterol. 2015, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; King, L.Y.; Zheng, H.; Chung, R.T. Statin Use Is Associated with a Reduced Risk of Fibrosis Progression in Chronic Hepatitis C. J. Hepatol. 2014, 62, 18–23. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Chen, W.-C.; Tsan, Y.-T.; Chen, M.-J.; Shih, W.-T.; Tsai, Y.-H.; Chen, P.-C. Statin Use and the Risk of Cirrhosis Development in Patients with Hepatitis C Virus Infection. J. Hepatol. 2015, 63, 1111–1117. [Google Scholar] [CrossRef]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Yang, H.-C.; Li, Y.-C. Statin Use and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Cancers 2020, 12, 671. [Google Scholar] [CrossRef]
- Facciorusso, A.; Abd El Aziz, M.A.; Singh, S.; Pusceddu, S.; Milione, M.; Giacomelli, L.; Sacco, R. Statin Use Decreases the Incidence of Hepatocellular Carcinoma: An Updated Meta-Analysis. Cancers 2020, 12, 874. [Google Scholar] [CrossRef]
- La Mura, V.; Gagliano, N.; Arnaboldi, F.; Sartori, P.; Procacci, P.; Denti, L.; Liguori, E.; Bitto, N.; Ristagno, G.; Latini, R.; et al. Simvastatin Prevents Liver Microthrombosis and Sepsis Induced Coagulopathy in a Rat Model of Endotoxemia. Cells 2022, 11, 1148. [Google Scholar] [CrossRef]
- Bitto, N.; Salerno, F.; Tripodi, A.; La Mura, V. Coagulation and Fibrosis: A Potential Non-Negligible Target of Statins in Chronic Hepatitis. J. Hepatol. 2015, 63, 277–278. [Google Scholar] [CrossRef]
- Violi, F.; Calvieri, C.; Ferro, D.; Pignatelli, P. Statins as Antithrombotic Drugs. Circulation 2013, 127, 251–257. [Google Scholar] [CrossRef]
- Law, M.; Rudnicka, A.R. Statin Safety: A Systematic Review. Am. J. Cardiol. 2006, 97, 52C–60C. [Google Scholar] [CrossRef]
- Björnsson, E.; Jacobsen, E.I.; Kalaitzakis, E. Hepatotoxicity Associated with Statins: Reports of Idiosyncratic Liver Injury Post-Marketing. J. Hepatol. 2012, 56, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Adhyaru, B.B.; Jacobson, T.A. Safety and Efficacy of Statin Therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef]
- Pollo-Flores, P.; Soldan, M.; Santos, U.C.; Kunz, D.G.; Mattos, D.E.; da Silva, A.C.; Marchiori, R.C.; da Motta Rezende, G.F. Three Months of Simvastatin Therapy vs. Placebo for Severe Portal Hypertension in Cirrhosis: A Randomized Controlled Trial. Dig. Liver Dis. 2015, 47, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Choi, J.-Y.; Lee, J.-H.; Ryu, S.; Park, Z.-W.; Lee, J.-G.; Na, H.-S.; Lee, S.-Y.; Oh, W.-Y.; Chung, M.-W.; et al. The Influences of SLCO1B1 and ABCB1 Genotypes on the Pharmacokinetics of Simvastatin, in Relation to CYP3A4 Inhibition. Pharmacogenomics 2017, 18, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Pose, E.; Napoleone, L.; Amin, A.; Campion, D.; Jimenez, C.; Piano, S.; Roux, O.; Uschner, F.E.; de Wit, K.; Zaccherini, G.; et al. Safety of Two Different Doses of Simvastatin plus Rifaximin in Decompensated Cirrhosis (LIVERHOPE-SAFETY): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet Gastroenterol. Hepatol. 2020, 5, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the Evidence for the Efficacy and Safety of Statin Therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Fuster, V.; Sweeny, J.M. Aspirin: A Historical and Contemporary Therapeutic Overview. Circulation 2011, 123, 768–778. [Google Scholar] [CrossRef]
- Smith, J.B.; Araki, H.; Lefer, A.M. Thromboxane A2, Prostacyclin and Aspirin: Effects on Vascular Tone and Platelet Aggregation. Circulation 1980, 62, V19–V25. [Google Scholar]
- Goh, M.J.; Sinn, D.H. Statin and Aspirin for Chemoprevention of Hepatocellular Carcinoma: Time to Use or Wait Further? Clin. Mol. Hepatol. 2022, 28, 380–395. [Google Scholar] [CrossRef]
- Shek, F.W.; Benyon, R.C. How Can Transforming Growth Factor Beta Be Targeted Usefully to Combat Liver Fibrosis? Eur. J. Gastroenterol. Hepatol. 2004, 16, 123–126. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D.A. Role of Hepatic Stellate Cells in Fibrogenesis and the Reversal of Fibrosis. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. S1), S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Xie, J.; Jiang, X.; Xiao, B.; Hu, X.; Xiang, J. Aspirin Attenuates Liver Fibrosis by Suppressing TGF-Β1/Smad Signaling. Mol. Med. Rep. 2022, 25, 181. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-J.; Yang, Z.-H.; Shi, X.-L.; Liu, D.-L. Effects of Aspirin and Enoxaparin in a Rat Model of Liver Fibrosis. World J. Gastroenterol. 2017, 23, 6412–6419. [Google Scholar] [CrossRef]
- Chauhan, A.; Adams, D.H.; Watson, S.P.; Lalor, P.F. Platelets: No Longer Bystanders in Liver Disease. Hepatology 2016, 64, 1774–1784. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ikenaga, N.; Liu, S.B.; Peng, Z.-W.; Chung, J.; Sverdlov, D.Y.; Miyamoto, M.; Kim, Y.O.; Ogawa, S.; Arch, R.H.; et al. Extrahepatic Platelet-Derived Growth Factor-β, Delivered by Platelets, Promotes Activation of Hepatic Stellate Cells and Biliary Fibrosis in Mice. Gastroenterology 2014, 147, 1378–1392. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shahzad, G.; Jawairia, M.; Bostick, R.M.; Mustacchia, P. Association between Aspirin Use and the Prevalence of Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study from the Third National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 2014, 40, 1066–1073. [Google Scholar] [CrossRef]
- Jiang, Z.G.; Feldbrügge, L.; Tapper, E.B.; Popov, Y.; Ghaziani, T.; Afdhal, N.; Robson, S.C.; Mukamal, K.J. Aspirin Use Is Associated with Lower Indices of Liver Fibrosis among Adults in the United States. Aliment. Pharmacol. Ther. 2016, 43, 734–743. [Google Scholar] [CrossRef]
- Simon, T.G.; Henson, J.; Osganian, S.; Masia, R.; Chan, A.T.; Chung, R.T.; Corey, K.E. Daily Aspirin Use Associated with Reduced Risk for Fibrosis Progression In Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 2776–2784. [Google Scholar] [CrossRef] [PubMed]
- Kopp, E.; Ghosh, S. Inhibition of NF-ΚB by Sodium Salicylate and Aspirin. Science 1994, 265, 956–959. [Google Scholar] [CrossRef]
- Liu, Y.-X.; Feng, J.-Y.; Sun, M.-M.; Liu, B.-W.; Yang, G.; Bu, Y.-N.; Zhao, M.; Wang, T.-J.; Zhang, W.-Y.; Yuan, H.-F.; et al. Aspirin Inhibits the Proliferation of Hepatoma Cells through Controlling GLUT1-Mediated Glucose Metabolism. Acta Pharmacol. Sin. 2019, 40, 122–132. [Google Scholar] [CrossRef]
- Shi, T.; Fujita, K.; Gong, J.; Nakahara, M.; Iwama, H.; Liu, S.; Yoneyama, H.; Morishita, A.; Nomura, T.; Tani, J.; et al. Aspirin Inhibits Hepatocellular Carcinoma Cell Proliferation in Vitro and in Vivo via Inducing Cell Cycle Arrest and Apoptosis. Oncol. Rep. 2020, 44, 457–468. [Google Scholar] [CrossRef]
- Xie, Z.-Y.; Liu, M.-S.; Zhang, C.; Cai, P.-C.; Xiao, Z.-H.; Wang, F.-F. Aspirin Enhances the Sensitivity of Hepatocellular Carcinoma Side Population Cells to Doxorubicin via MiR-491/ABCG2. Biosci. Rep. 2018, 38, BSR20180854. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dai, W.; Mo, W.; Li, J.; Feng, J.; Wu, L.; Liu, T.; Yu, Q.; Xu, S.; Wang, W.; et al. By Inhibiting PFKFB3, Aspirin Overcomes Sorafenib Resistance in Hepatocellular Carcinoma. Int. J. Cancer 2017, 141, 2571–2584. [Google Scholar] [CrossRef]
- Lucotti, S.; Cerutti, C.; Soyer, M.; Gil-Bernabé, A.M.; Gomes, A.L.; Allen, P.D.; Smart, S.; Markelc, B.; Watson, K.; Armstrong, P.C.; et al. Aspirin Blocks Formation of Metastatic Intravascular Niches by Inhibiting Platelet-Derived COX-1/Thromboxane A2. J. Clin. Investig. 2019, 129, 1845–1862. [Google Scholar] [CrossRef]
- Graupera, M.; García-Pagán, J.-C.; Abraldes, J.G.; Peralta, C.; Bragulat, M.; Corominola, H.; Bosch, J.; Rodés, J. Cyclooxygenase-Derived Products Modulate the Increased Intrahepatic Resistance of Cirrhotic Rat Livers. Hepatology 2003, 37, 172–181. [Google Scholar] [CrossRef]
- Graupera, M.; García-Pagán, J.-C.; Parés, M.; Abraldes, J.G.; Roselló, J.; Bosch, J.; Rodés, J. Cyclooxygenase-1 Inhibition Corrects Endothelial Dysfunction in Cirrhotic Rat Livers. J. Hepatol. 2003, 39, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.; Ahn, S.H.; Yoon, J.-H.; Kim, B.K. Clinical Indication of Aspirin Associated with Reduced Risk of Liver Cancer in Chronic Hepatitis B: A Nationwide Cohort Study. Am. J. Gastroenterol. 2022, 117, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, Y.B.; Moon, H.; Chung, J.-W.; Nam, J.Y.; Cho, E.J.; Lee, J.-H.; Yu, S.J.; Kim, Y.J.; Lee, J.; et al. Aspirin Use and Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B with or without Cirrhosis. Hepatology 2022, 76, 492–501. [Google Scholar] [CrossRef]
- Choi, W.-M.; Kim, H.J.; Jo, A.J.; Choi, S.H.; Han, S.; Ko, M.J.; Lim, Y.-S. Association of Aspirin and Statin Use with the Risk of Liver Cancer in Chronic Hepatitis B: A Nationwide Population-Based Study. Liver Int. 2021, 41, 2777–2785. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Hsu, R.-J.; Wang, T.-H.; Wu, C.-T.; Huang, S.-Y.; Hsu, C.-Y.; Su, Y.-C.; Hsu, W.-L.; Liu, D.-W. Aspirin Decreases Hepatocellular Carcinoma Risk in Hepatitis C Virus Carriers: A Nationwide Cohort Study. BMC Gastroenterol. 2020, 20, 6. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Hsu, Y.-C.; Tseng, H.-C.; Lin, J.-T.; Wu, M.-S.; Wu, C.-Y. Association of Daily Aspirin Therapy with Hepatocellular Carcinoma Risk in Patients with Chronic Hepatitis C Virus Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2784–2792.e7. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.C.; Chang, J.; Kim, K.; Park, S.M. Aspirin Use and Risk of Hepatocellular Carcinoma in a National Cohort Study of Korean Adults. Sci. Rep. 2018, 8, 4968. [Google Scholar] [CrossRef]
- Lee, M.; Chung, G.E.; Lee, J.-H.; Oh, S.; Nam, J.Y.; Chang, Y.; Cho, H.; Ahn, H.; Cho, Y.Y.; Yoo, J.-J.; et al. Antiplatelet Therapy and the Risk of Hepatocellular Carcinoma in Chronic Hepatitis B Patients on Antiviral Treatment. Hepatology 2017, 66, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Duberg, A.-S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, T.; Xu, X.; Jin, J. Association of Aspirin and Nonaspirin NSAIDs Therapy with the Incidence Risk of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis on Cohort Studies. Eur. J. Cancer Prev. 2022, 31, 35–43. [Google Scholar] [CrossRef]
- Tan, R.Z.H.; Lockart, I.; Abdel Shaheed, C.; Danta, M. Systematic Review with Meta-Analysis: The Effects of Non-Steroidal Anti-Inflammatory Drugs and Anti-Platelet Therapy on the Incidence and Recurrence of Hepatocellular Carcinoma. Aliment. Pharmacol. Ther. 2021, 54, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Memel, Z.N.; Arvind, A.; Moninuola, O.; Philpotts, L.; Chung, R.T.; Corey, K.E.; Simon, T.G. Aspirin Use Is Associated with a Reduced Incidence of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Hepatol. Commun. 2021, 5, 133–143. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Ryan, P.M.; Dang, M.; Clark, C.; Kontogiannis, V.; Rahmani, J.; Varkaneh, H.K.; Salehisahlabadi, A.; Day, A.S.; et al. Association of Aspirin Therapy with Risk of Hepatocellular Carcinoma: A Systematic Review and Dose-Response Analysis of Cohort Studies with 2.5 Million Participants. Pharmacol. Res. 2020, 151, 104585. [Google Scholar] [CrossRef]
- Lai, Q.; De Matthaeis, N.; Finotti, M.; Galati, G.; Marrone, G.; Melandro, F.; Morisco, F.; Nicolini, D.; Pravisani, R.; Giannini, E.G.; et al. The Role of Antiplatelet Therapies on Incidence and Mortality of Hepatocellular Carcinoma. Eur. J. Clin. Investig. 2023, 53, e13870. [Google Scholar] [CrossRef]
- Guidotti, L.G.; La Vecchia, C.; Colombo, M. Low-Dose Aspirin Reduces the Risk of HBV-Associated HCC Even When Administered Short-Term: Too Good to Be True? Hepatology 2022, 76, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chalasani, N.P.; Schwantes-An, L.; Björnsson, E.S. Review Article: The Safety of Anticoagulants and Antiplatelet Agents in Patients with Cirrhosis. Aliment. Pharmacol. Ther. 2023, 57, 52–71. [Google Scholar] [CrossRef] [PubMed]
- La Mura, V.; Bitto, N.; Tripodi, A. Rational Hemostatic Management in Cirrhosis: From Old Paradigms to New Clinical Challenges. Expert Rev. Hematol. 2022, 15, 1031–1044. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lee, K.-T.; Lee, C.T.-C.; Lai, W.-T.; Huang, Y.-B. Effectiveness and Safety of Antiplatelet Therapy in Stroke Recurrence Prevention in Patients with Liver Cirrhosis: A 2-Year Follow-up Study. Pharmacoepidemiol. Drug Saf. 2012, 21, 1334–1343. [Google Scholar] [CrossRef]
- Russo, M.W.; Pierson, J.; Narang, T.; Montegudo, A.; Eskind, L.; Gulati, S. Coronary Artery Stents and Antiplatelet Therapy in Patients with Cirrhosis. J. Clin. Gastroenterol. 2012, 46, 339–344. [Google Scholar] [CrossRef]
- Krill, T.; Brown, G.; Weideman, R.A.; Cipher, D.J.; Spechler, S.J.; Brilakis, E.; Feagins, L.A. Patients with Cirrhosis Who Have Coronary Artery Disease Treated with Cardiac Stents Have High Rates of Gastrointestinal Bleeding, but No Increased Mortality. Aliment. Pharmacol. Ther. 2017, 46, 183–192. [Google Scholar] [CrossRef]
- Patel, S.S.; Guzman, L.A.; Lin, F.-P.; Pence, T.; Reichman, T.; John, B.; Celi, F.S.; Liptrap, E.; Bhati, C.; Siddiqui, M.S. Utilization of Aspirin and Statin in Management of Coronary Artery Disease in Patients with Cirrhosis Undergoing Liver Transplant Evaluation. Liver Transpl. 2018, 24, 872–880. [Google Scholar] [CrossRef]
- Wu, V.C.-C.; Chen, S.-W.; Chou, A.-H.; Ting, P.-C.; Chang, C.-H.; Wu, M.; Hsieh, M.-J.; Wang, C.-Y.; Chang, S.-H.; Lin, M.-S.; et al. Dual Antiplatelet Therapy in Patients with Cirrhosis and Acute Myocardial Infarction-A 13-Year Nationwide Cohort Study. PLoS ONE 2019, 14, e0223380. [Google Scholar] [CrossRef] [PubMed]
- Seifert, L.L.; Schindler, P.; Sturm, L.; Gu, W.; Seifert, Q.E.; Weller, J.F.; Jansen, C.; Praktiknjo, M.; Meyer, C.; Schoster, M.; et al. Aspirin Improves Transplant-Free Survival after TIPS Implantation in Patients with Refractory Ascites: A Retrospective Multicentre Cohort Study. Hepatol. Int. 2022, 16, 658–668. [Google Scholar] [CrossRef]
- Tripodi, A.; Mannucci, P.M. The Coagulopathy of Chronic Liver Disease. N. Engl. J. Med. 2011, 365, 147–156. [Google Scholar] [CrossRef]
- Lisman, T.; Porte, R.J. Rebalanced Hemostasis in Patients with Liver Disease: Evidence and Clinical Consequences. Blood 2010, 116, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T.; Bongers, T.N.; Adelmeijer, J.; Janssen, H.L.A.; de Maat, M.P.M.; de Groot, P.G.; Leebeek, F.W.G. Elevated Levels of von Willebrand Factor in Cirrhosis Support Platelet Adhesion despite Reduced Functional Capacity. Hepatology 2006, 44, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Primignani, M.; Chantarangkul, V.; Clerici, M.; Dell’Era, A.; Fabris, F.; Salerno, F.; Mannucci, P.M. Thrombin Generation in Patients with Cirrhosis: The Role of Platelets. Hepatology 2006, 44, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T.; Hernandez-Gea, V.; Magnusson, M.; Roberts, L.; Stanworth, S.; Thachil, J.; Tripodi, A. The Concept of Rebalanced Hemostasis in Patients with Liver Disease: Communication from the ISTH SSC Working Group on Hemostatic Management of Patients with Liver Disease. J. Thromb. Haemost. 2021, 19, 1116–1122. [Google Scholar] [CrossRef]
- Senzolo, M.; Garcia-Tsao, G.; García-Pagán, J.C. Current Knowledge and Management of Portal Vein Thrombosis in Cirrhosis. J. Hepatol. 2021, 75, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Northup, P.G.; Garcia-Pagan, J.C.; Garcia-Tsao, G.; Intagliata, N.M.; Superina, R.A.; Roberts, L.N.; Lisman, T.; Valla, D.C. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients with Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 73, 366–413. [Google Scholar] [CrossRef]
- Bhangui, P.; Lim, C.; Levesque, E.; Salloum, C.; Lahat, E.; Feray, C.; Azoulay, D. Novel Classification of Non-Malignant Portal Vein Thrombosis: A Guide to Surgical Decision-Making during Liver Transplantation. J. Hepatol. 2019, 71, 1038–1050. [Google Scholar] [CrossRef]
- Loffredo, L.; Pastori, D.; Farcomeni, A.; Violi, F. Effects of Anticoagulants in Patients with Cirrhosis and Portal Vein Thrombosis: A Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 480–487. [Google Scholar] [CrossRef]
- Bianchini, M.; Cavani, G.; Bonaccorso, A.; Turco, L.; Vizzutti, F.; Sartini, A.; Gitto, S.; Merighi, A.; Banchelli, F.; Villa, E.; et al. Low Molecular Weight Heparin Does Not Increase Bleeding and Mortality Post-Endoscopic Variceal Band Ligation in Cirrhotic Patients. Liver Int. 2018, 38, 1253–1262. [Google Scholar] [CrossRef]
- La Mura, V.; Braham, S.; Tosetti, G.; Branchi, F.; Bitto, N.; Moia, M.; Fracanzani, A.L.; Colombo, M.; Tripodi, A.; Primignani, M. Harmful and Beneficial Effects of Anticoagulants in Patients with Cirrhosis and Portal Vein Thrombosis. Clin. Gastroenterol. Hepatol. 2018, 16, 1146–1152.e4. [Google Scholar] [CrossRef]
- Pettinari, I.; Vukotic, R.; Stefanescu, H.; Pecorelli, A.; Morelli, M.; Grigoras, C.; Sparchez, Z.; Andreone, P.; Piscaglia, F. the BO-LIVES (BOlogna LIVEr vascular Studies) Clinical Impact and Safety of Anticoagulants for Portal Vein Thrombosis in Cirrhosis. Am. J. Gastroenterol. 2019, 114, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Naymagon, L.; Tremblay, D.; Zubizarreta, N.; Moshier, E.; Mascarenhas, J.; Schiano, T. Safety, Efficacy, and Long-Term Outcomes of Anticoagulation in Cirrhotic Portal Vein Thrombosis. Dig. Dis. Sci. 2021, 66, 3619–3629. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, X.; Xu, X.; De Stefano, V.; Plessier, A.; Noronha Ferreira, C.; Qi, X. Anticoagulation Favors Thrombus Recanalization and Survival in Patients with Liver Cirrhosis and Portal Vein Thrombosis: Results of a Meta-Analysis. Adv. Ther. 2021, 38, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Senzolo, M.; Sartori, T.M.; Rossetto, V.; Burra, P.; Cillo, U.; Boccagni, P.; Gasparini, D.; Miotto, D.; Simioni, P.; Tsochatzis, E.; et al. Prospective Evaluation of Anticoagulation and Transjugular Intrahepatic Portosistemic Shunt for the Management of Portal Vein Thrombosis in Cirrhosis. Liver Int. 2012, 32, 919–927. [Google Scholar] [CrossRef]
- Lv, Y.; Bai, W.; Li, K.; Wang, Z.; Guo, W.; Luo, B.; Wang, J.; Wang, Q.; Wang, E.; Xia, D.; et al. Anticoagulation and Transjugular Intrahepatic Portosystemic Shunt for the Management of Portal Vein Thrombosis in Cirrhosis: A Prospective Observational Study. Am. J. Gastroenterol. 2021, 116, 1447–1464. [Google Scholar] [CrossRef]
- Tripodi, A.; Anstee, Q.M.; Sogaard, K.K.; Primignani, M.; Valla, D.C. Hypercoagulability in Cirrhosis: Causes and Consequences. J. Thromb. Haemost. 2011, 9, 1713–1723. [Google Scholar] [CrossRef]
- Wanless, I.R. The Role of Vascular Injury and Congestion in the Pathogenesis of Cirrhosis: The Congestive Escalator and the Parenchymal Extinction Sequence. Curr. Hepatol. Rep. 2020, 19, 40–53. [Google Scholar] [CrossRef]
- Wanless, I.R.; Wong, F.; Blendis, L.M.; Greig, P.; Heathcote, E.J.; Levy, G. Hepatic and Portal Vein Thrombosis in Cirrhosis: Possible Role in Development of Parenchymal Extinction and Portal Hypertension. Hepatology 1995, 21, 1238–1247. [Google Scholar]
- La Mura, V.; Tripodi, A.; Tosetti, G.; Cavallaro, F.; Chantarangkul, V.; Colombo, M.; Primignani, M. Resistance to Thrombomodulin Is Associated with de Novo Portal Vein Thrombosis and Low Survival in Patients with Cirrhosis. Liver Int. 2016, 36, 1322–1330. [Google Scholar] [CrossRef]
- Praktiknjo, M.; Trebicka, J.; Carnevale, R.; Pastori, D.; Queck, A.; Ettorre, E.; Violi, F. Von Willebrand and Factor VIII Portosystemic Circulation Gradient in Cirrhosis: Implications for Portal Vein Thrombosis. Clin. Transl. Gastroenterol. 2020, 11, e00123. [Google Scholar] [CrossRef]
- Scheiner, B.; Balcar, L.; Nussbaumer, R.J.; Weinzierl, J.; Paternostro, R.; Simbrunner, B.; Hartl, L.; Jachs, M.; Bauer, D.; Stättermayer, A.F.; et al. Factor VIII/Protein C Ratio Independently Predicts Liver-Related Events but Does Not Indicate a Hypercoagulable State in ACLD. J. Hepatol. 2022, 76, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Kassel, K.M.; Sullivan, B.P.; Cui, W.; Copple, B.L.; Luyendyk, J.P. Therapeutic Administration of the Direct Thrombin Inhibitor Argatroban Reduces Hepatic Inflammation in Mice with Established Fatty Liver Disease. Am. J. Pathol. 2012, 181, 1287–1295. [Google Scholar] [CrossRef]
- Duplantier, J.G.; Dubuisson, L.; Senant, N.; Freyburger, G.; Laurendeau, I.; Herbert, J.-M.; Desmoulière, A.; Rosenbaum, J. A Role for Thrombin in Liver Fibrosis. Gut 2004, 53, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Abe, W.; Ikejima, K.; Lang, T.; Okumura, K.; Enomoto, N.; Kitamura, T.; Takei, Y.; Sato, N. Low Molecular Weight Heparin Prevents Hepatic Fibrogenesis Caused by Carbon Tetrachloride in the Rat. J. Hepatol. 2007, 46, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Goldin, R.D.; Wright, M.; Martinelli, A.; Cox, R.; Thursz, M.R. Coagulation Status Modulates Murine Hepatic Fibrogenesis: Implications for the Development of Novel Therapies. J. Thromb. Haemost. 2008, 6, 1336–1343. [Google Scholar] [CrossRef]
- Simonetto, D.A.; Yang, H.; Yin, M.; de Assuncao, T.M.; Kwon, J.H.; Hilscher, M.; Pan, S.; Yang, L.; Bi, Y.; Beyder, A.; et al. Chronic Passive Venous Congestion Drives Hepatic Fibrogenesis via Sinusoidal Thrombosis and Mechanical Forces. Hepatology 2015, 61, 648–659. [Google Scholar] [CrossRef]
- Cerini, F.; Vilaseca, M.; Lafoz, E.; García-Irigoyen, O.; García-Calderó, H.; Tripathi, D.M.; Avila, M.; Reverter, J.C.; Bosch, J.; Gracia-Sancho, J.; et al. Enoxaparin Reduces Hepatic Vascular Resistance and Portal Pressure in Cirrhotic Rats. J. Hepatol. 2016, 64, 834–842. [Google Scholar] [CrossRef]
- Vilaseca, M.; García-Calderó, H.; Lafoz, E.; García-Irigoyen, O.; Avila, M.A.; Reverter, J.C.; Bosch, J.; Hernández-Gea, V.; Gracia-Sancho, J.; García-Pagán, J.C. The Anticoagulant Rivaroxaban Lowers Portal Hypertension in Cirrhotic Rats Mainly by Deactivating Hepatic Stellate Cells. Hepatology 2017, 65, 2031–2044. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E.; Distrutti, E.; Severino, B.; Fiorentina, R.; Baldoni, M.; Caliendo, G.; Santagada, V.; Morelli, A.; Cirino, G. PAR1 Antagonism Protects against Experimental Liver Fibrosis. Role of Proteinase Receptors in Stellate Cell Activation. Hepatology 2004, 39, 365–375. [Google Scholar] [CrossRef]
- Rullier, A.; Gillibert-Duplantier, J.; Costet, P.; Cubel, G.; Haurie, V.; Petibois, C.; Taras, D.; Dugot-Senant, N.; Deleris, G.; Bioulac-Sage, P.; et al. Protease-Activated Receptor 1 Knockout Reduces Experimentally Induced Liver Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G226–G235. [Google Scholar] [CrossRef]
- Knight, V.; Tchongue, J.; Lourensz, D.; Tipping, P.; Sievert, W. Protease-Activated Receptor 2 Promotes Experimental Liver Fibrosis in Mice and Activates Human Hepatic Stellate Cells. Hepatology 2012, 55, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; Lourensz, D.; Tchongue, J.; Correia, J.; Tipping, P.; Sievert, W. Cytoplasmic Domain of Tissue Factor Promotes Liver Fibrosis in Mice. World J. Gastroenterol. 2017, 23, 5692–5699. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Cammà, C.; Marietta, M.; Luongo, M.; Critelli, R.; Colopi, S.; Tata, C.; Zecchini, R.; Gitto, S.; Petta, S.; et al. Enoxaparin Prevents Portal Vein Thrombosis and Liver Decompensation in Patients with Advanced Cirrhosis. Gastroenterology 2012, 143, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Bianchini, M.; Blasi, A.; Denys, A.; Giannini, E.G.; de Gottardi, A.; Lisman, T.; de Raucourt, E.; Ripoll, C.; Rautou, P.-E. EASL Clinical Practice Guidelines on Prevention and Management of Bleeding and Thrombosis in Patients with Cirrhosis. J. Hepatol. 2022, 76, 1151–1184. [Google Scholar] [CrossRef]
- De Gottardi, A.; Trebicka, J.; Klinger, C.; Plessier, A.; Seijo, S.; Terziroli, B.; Magenta, L.; Semela, D.; Buscarini, E.; Langlet, P.; et al. Antithrombotic Treatment with Direct-Acting Oral Anticoagulants in Patients with Splanchnic Vein Thrombosis and Cirrhosis. Liver Int. 2017, 37, 694–699. [Google Scholar] [CrossRef]
- Semmler, G.; Pomej, K.; Bauer, D.J.M.; Balcar, L.; Simbrunner, B.; Binter, T.; Hartl, L.; Becker, J.; Pinter, M.; Quehenberger, P.; et al. Safety of Direct Oral Anticoagulants in Patients with Advanced Liver Disease. Liver Int. 2021, 41, 2159–2170. [Google Scholar] [CrossRef]
- Menichelli, D.; Ronca, V.; Di Rocco, A.; Pignatelli, P.; Marco Podda, G. CAR Direct Oral Anticoagulants and Advanced Liver Disease: A Systematic Review and Meta-Analysis. Eur. J. Clin. Investig. 2021, 51, e13397. [Google Scholar] [CrossRef]
- Violi, F.; Vestri, A.; Menichelli, D.; Di Rocco, A.; Pastori, D.; Pignatelli, P. Direct Oral Anticoagulants in Patients with Atrial Fibrillation and Advanced Liver Disease: An Exploratory Meta-Analysis. Hepatol. Commun. 2020, 4, 1034–1040. [Google Scholar] [CrossRef]
- Oldham, M.; Palkimas, S.; Hedrick, A. Safety and Efficacy of Direct Oral Anticoagulants in Patients with Moderate to Severe Cirrhosis. Ann. Pharmacother. 2022, 56, 782–790. [Google Scholar] [CrossRef]
- Serper, M.; Weinberg, E.M.; Cohen, J.B.; Reese, P.P.; Taddei, T.H.; Kaplan, D.E. Mortality and Hepatic Decompensation in Patients with Cirrhosis and Atrial Fibrillation Treated with Anticoagulation. Hepatology 2021, 73, 219–232. [Google Scholar] [CrossRef]
| Drug | Main Established and Potential Non-Etiological Effects | Research Agenda |
|---|---|---|
| Albumin |
| |
| Rifaximin |
| |
| Statins |
|
|
| Aspirin |
|
|
| Anti-coagulants |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bitto, N.; Ghigliazza, G.; Lavorato, S.; Caputo, C.; La Mura, V. Improving Management of Portal Hypertension: The Potential Benefit of Non-Etiological Therapies in Cirrhosis. J. Clin. Med. 2023, 12, 934. https://doi.org/10.3390/jcm12030934
Bitto N, Ghigliazza G, Lavorato S, Caputo C, La Mura V. Improving Management of Portal Hypertension: The Potential Benefit of Non-Etiological Therapies in Cirrhosis. Journal of Clinical Medicine. 2023; 12(3):934. https://doi.org/10.3390/jcm12030934
Chicago/Turabian StyleBitto, Niccolò, Gabriele Ghigliazza, Stanislao Lavorato, Camilla Caputo, and Vincenzo La Mura. 2023. "Improving Management of Portal Hypertension: The Potential Benefit of Non-Etiological Therapies in Cirrhosis" Journal of Clinical Medicine 12, no. 3: 934. https://doi.org/10.3390/jcm12030934
APA StyleBitto, N., Ghigliazza, G., Lavorato, S., Caputo, C., & La Mura, V. (2023). Improving Management of Portal Hypertension: The Potential Benefit of Non-Etiological Therapies in Cirrhosis. Journal of Clinical Medicine, 12(3), 934. https://doi.org/10.3390/jcm12030934






