Gas Chromatography–Mass Spectrometry (GC–MS) Metabolites Analysis in Endometriosis Patients: A Prospective Observational Translational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Sample Preparation
2.3. GC–MS Analysis
2.4. Multivariate Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angioni, S. New insights on endometriosis. Minerva Obstet. Gynecol. 2017, 69, 438–439. [Google Scholar] [CrossRef]
- Melis, G.B.; Neri, M.; Corda, V.; Malune, M.E.; Piras, B.; Pirarba, S.; Guerriero, S.; Orrù, M.; D’Alterio, M.N.; Angioni, S.; et al. Overview of elagolix for the treatment of endometriosis. Expert Opin. Drug Metab. Toxicol. 2016, 12, 581–588. [Google Scholar] [CrossRef]
- Angioni, S.; Cofelice, V.; Pontis, A.; Tinelli, R.; Socolov, R. New trends of progestins treatment of endometriosis. Gynecol. Endocrinol. 2014, 30, 769–773. [Google Scholar] [CrossRef]
- Zajec, V.; Mikuš, M.; Vitale, S.G.; D’Alterio, M.N.; Gregov, M.; Šarić, M.J.; Carugno, J.; Angioni, S.; Ćorić, M. Current status and challenges of drug development for hormonal treatment of endometriosis: A systematic review of randomized control trials. Gynecol. Endocrinol. 2022, 38, 713–720. [Google Scholar] [CrossRef]
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef]
- Melis, I.; Agus, M.; Pluchino, N.; Sardo, A.D.S.; Litta, P.; Melis, G.B.; Angioni, S. Alexithymia in Women with Deep Endometriosis? A Pilot Study. J. Endometr. Pelvic Pain Disord. 2014, 6, 26–33. [Google Scholar] [CrossRef]
- Melis, I.; Penna, M.P.; Murru, M.; Pontis, A.; Agus, M.; Angioni, S. In Multidimensional assessment of pain in women with endo-metriosis: Preliminary results of the experience in Cagliari. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Benevento, Italy, 15–18 May 2016; pp. 1–6. [Google Scholar]
- Stochino-Loi, E.; Pontis, A.; Cofelice, V.; Pirarba, S.; Fais, M.F.; Daniilidis, A.; Melis, I.; Paoletti, A.M.; Angioni, S. Effect of ultramicronized-palmitoylethanolamide and co-micronized palmitoylethanolamide/polydatin on chronic pelvic pain and quality of life in endometriosis patients: An open-label pilot study. Int. J. Women’s Health 2019, ume 11, 443–449. [Google Scholar] [CrossRef]
- D’Alterio, M.N.; Saponara, S.; Agus, M.; Laganà, A.S.; Noventa, M.; Loi, E.S.; Feki, A.; Angioni, S. Medical and surgical interventions to improve the quality of life for endometriosis patients: A systematic review. Gynecol. Surg. 2021, 18, 1–14. [Google Scholar] [CrossRef]
- Angioni, S.; Cela, V.; Sedda, F.; Loi, E.S.; Cofelice, V.; Pontis, A.; Melis, G.B. Focusing on surgery results in infertile patients with deep endometriosis. Gynecol. Endocrinol. 2015, 31, 595–598. [Google Scholar] [CrossRef]
- Laganà, A.S.; Vitale, S.G.; Salmeri, F.M.; Triolo, O.; Frangež, H.B.; Vrtačnik-Bokal, E.; Stojanovska, L.; Apostolopoulos, V.; Granese, R.; Sofo, V. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med. Hypotheses 2017, 103, 10–20. [Google Scholar] [CrossRef]
- Angioni, S.; D’Alterio, M.N.; Coiana, A.; Anni, F.; Gessa, S.; Deiana, D. Genetic Characterization of Endometriosis Patients: Review of the Literature and a Prospective Cohort Study on a Mediterranean Population. Int. J. Mol. Sci. 2020, 21, 1765. [Google Scholar] [CrossRef] [PubMed]
- Deiana, D.; Gessa, S.; Anardu, M.; Daniilidis, A.; Nappi, L.; D’Alterio, M.N.; Pontis, A.; Angioni, S. Genetics of endometriosis: A comprehensive review. Gynecol. Endocrinol. 2019, 35, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yan, Y.; Liu, Z.; Wang, Y. Inflammation and endometriosis. Front. Biosci. 2016, 21, 941–948. [Google Scholar]
- Velho, R.V.; Taube, E.; Sehouli, J.; Mechsner, S. Neurogenic Inflammation in the Context of Endometriosis-What Do We Know? Int. J. Mol. Sci. 2021, 22, 13102. [Google Scholar] [CrossRef] [PubMed]
- Viganó, D.; Zara, F.; Pinto, S.; Loddo, E.; Casula, L.; Soru, M.B.; D’Ancona, G.; D’Alterio, M.N.; Giuliani, C.; Angioni, S.; et al. How is small bowel permeability in endometriosis patients? a case control pilot study. Gynecol. Endocrinol. 2020, 36, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Dorien, F.O.; Flores, I.; Waelkens, E.; D’Hooghe, T. Noninvasive diagnosis of endometriosis: Review of current peripheral blood and endometrial biomarkers. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 72–83. [Google Scholar] [CrossRef]
- Fuldeore, M.J.; Soliman, A.M. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol. Obstet. Investig. 2016, 82, 453–461. [Google Scholar] [CrossRef] [PubMed]
- D’Alterio, M.N.; Giuliani, C.; Scicchitano, F.; Lagana, A.S.; Oltolina, N.M.; Sorrentino, F.; Nappi, L.; Orru, G.; Angioni, S. Possible role of microbiome in the pathogenesis of endometriosis. Minerva Obstet. Gynecol. 2021, 73, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Angioni, S.; Saponara, S.; Succu, A.G.; Sigilli, M.; Scicchitano, F.; D’Alterio, M.N. Metabolomic Characteristics in Endome-triosis Patients. In Endometriosis Pathogenesis, Clinical Impact and Management; Springer: New York, NY, USA, 2021; pp. 9–17. [Google Scholar]
- Fulghesu, A.M.; Piras, C.; Dessì, A.; Succu, C.; Atzori, L.; Pintus, R.; Gentile, C.; Angioni, S.; Fanos, V. Urinary Metabolites Reveal Hyperinsulinemia and Insulin Resistance in Polycystic Ovarian Syndrome (PCOS). Metabolites 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Angioni, S.; D’Alterio, M.N.; Pirarba, S.; Noto, A.; Santoru, M.L.; Tronci, L.; Fanos, V.; Atzori, L.; Congiu, F. Metabolic Profile of Patients with Severe Endometriosis: A Prospective Experimental Study. Reprod. Sci. 2020, 28, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Boja, E.S.; Kinsinger, C.R.; Rodriguez, H.; Srinivas, P. Integration of omics sciences to advance biology and medicine. Clin. Proteom. 2014, 11, 45. [Google Scholar] [CrossRef]
- Syggelou, A.; Iacovidou, N.; Atzori, L.; Xanthos, T.; Fanos, V. Metabolomics in the Developing Human Being. Pediatr. Clin. N. Am. 2012, 59, 1039–1058. [Google Scholar] [CrossRef]
- Arakaki, A.K.; Skolnick, J.; McDonald, J.F. Marker metabolites can be therapeutic targets as well. Nature 2008, 456, 443. [Google Scholar] [CrossRef]
- Murgia, F.; Muroni, A.; Puligheddu, M.; Polizzi, L.; Barberini, L.; Orofino, G.; Solla, P.; Poddighe, S.; Del Carratore, F.; Griffin, J.L.; et al. Metabolomics As a Tool for the Characterization of Drug-Resistant Epilepsy. Front. Neurol. 2017, 8, 459. [Google Scholar] [CrossRef]
- Papadimitropoulos, M.-E.P.; Vasilopoulou, C.G.; Maga-Nteve, C.; Klapa, M.I. Untargeted GC-MS Metabolomics. Methods Mol. Biol. 2018, 1738, 133–147. [Google Scholar] [CrossRef]
- Tokarz, J.; Adamski, J.; Rižner, T.L. Metabolomics for Diagnosis and Prognosis of Uterine Diseases? A Systematic Review. J. Pers. Med. 2020, 10, 294. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef]
- Emwas, A.H. The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol. Biol. 2015, 1277, 161–193. [Google Scholar]
- Kim, S.J.; Song, H.E.; Lee, H.Y.; Yoo, H.J. Mass Spectrometry-based Metabolomics in Translational Research. Adv. Exp. Med. Biol. 2021, 1310, 509–531. [Google Scholar] [CrossRef]
- Letsiou, S.; Peterse, D.P.; Fassbender, A.; Hendriks, M.M.; Broek, N.J.V.D.; Berger, R.; O, D.F.; Vanhie, A.; Vodolazkaia, A.; Van Langendonckt, A.; et al. Endometriosis is associated with aberrant metabolite profiles in plasma. Fertil. Steril. 2017, 107, 699–706.e6. [Google Scholar] [CrossRef]
- Loy, S.L.; Zhou, J.; Cui, L.; Tan, T.Y.; Ee, T.X.; Chern, B.S.M.; Chan, J.K.Y.; Lee, Y.H. Discovery and validation of peritoneal endometriosis biomarkers in peritoneal fluid and serum. Reprod. Biomed. Online 2021, 43, 727–737. [Google Scholar] [CrossRef]
- Vouk, K.; Ribič-Pucelj, M.; Adamski, J.; Rižner, T.L. Altered levels of acylcarnitines, phosphatidylcholines, and sphingomyelins in peritoneal fluid from ovarian endometriosis patients. J. Steroid Biochem. Mol. Biol. 2016, 159, 60–69. [Google Scholar] [CrossRef]
- May, K.E.; Conduit-Hulbert, S.A.; Villar, J.; Kirtley, S.; Kennedy, S.H.; Becker, C.M. Peripheral biomarkers of endometriosis: A systematic review. Hum. Reprod. Updat. 2010, 16, 651–674. [Google Scholar] [CrossRef]
- Ghazi, N.; Arjmand, M.; Akbari, Z.; Mellati, A.O.; Saheb-Kashaf, H.; Zamani, Z. (1)H NMR- based metabolomics approaches as non- invasive tools for diagnosis of endometriosis. Int. J. Reprod. BioMed. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Dutta, M.; Singh, B.; Joshi, M.; Das, D.; Subramani, E.; Maan, M.; Jana, S.K.; Sharma, U.; Das, S.; Dasgupta, S.; et al. Metabolomics reveals perturbations in endometrium and serum of minimal and mild en-dometriosis. Sci. Rep. 2018, 8, 6466. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cui, L.; Fang, J.; Chern, B.S.M.; Tan, H.H.; Chan, J.K.Y. Limited value of pro-inflammatory oxylipins and cytokines as circulating biomarkers in endometriosis—a targeted ‘omics study. Sci. Rep. 2016, 6, 26117. [Google Scholar] [CrossRef]
- Vicente-Munoz, S.; Morcillo, I.; Puchades-Carrasco, L.; Paya, V.; Pellicer, A.; Pineda-Lucena, A. Nuclear magnetic resonance metabolomic profiling of urine provides a noninvasive alternative to the identification of biomarkers associated with endo-metriosis. Fertil. Steril. 2015, 104, 1202–1209. [Google Scholar] [CrossRef]
- Cordeiro, F.B.; Cataldi, T.R.; Perkel, K.J.; do Vale Teixeira da Costa, L.; Rochetti, R.C.; Stevanato, J.; Eberlin, M.N.; Zylbersztejn, D.S.; Cedenho, A.P.; Lo Turco, E.G. Lipidomics analysis of follicular fluid by ESI-MS reveals potential biomarkers for ovarian endometriosis. J. Assist. Reprod. Genet. 2015, 32, 1817–1825. [Google Scholar] [CrossRef]
- Honour, J.W. Gas chromatography-mass spectrometry. Methods Mol. Biol. 2006, 324, 53–74. [Google Scholar]
- Fiehn, O. Metabolomics by Gas Chromatography–Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.4.1–30.4.32. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Haas, D.; Shebl, O.; Shamiyeh, A.; Oppelt, P. The rASRM score and the Enzian classification for endometriosis: Their strengths and weaknesses. Acta Obstet. et Gynecol. Scand. 2012, 92, 3–7. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Liggi, S.; Hinz, C.; Hall, Z.; Santoru, M.L.; Poddighe, S.; Fjeldsted, J.; Atzori, L.; Griffin, J.L. KniMet: A pipeline for the pro-cessing of chromatography-mass spectrometry metabolomics data. Metabolomics 2018, 14, 52. [Google Scholar] [CrossRef]
- Trygg, J.; Holmes, .A.E.; Lundstedt, .T. Chemometrics in Metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef]
- Vitale, S.G.; Capriglione, S.; Peterlunger, I.; La Rosa, V.L.; Vitagliano, A.; Noventa, M.; Valenti, G.; Sapia, F.; Angioli, R.; Lopez, S.; et al. The Role of Oxidative Stress and Membrane Transport Systems during Endometriosis: A Fresh Look at a Busy Corner. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Angioni, S.; Nappi, L.; Sorrentino, F.; Peiretti, M.; Daniilidis, A.; Pontis, A.; Tinelli, R.; D’Alterio, M.N. Laparoscopic treatment of deep endometriosis with a diode laser: Our experience. Arch. Gynecol. Obstet. 2021, 304, 1221–1231. [Google Scholar] [CrossRef]
- Angioni, S.; Pontis, A.; Cela, V.; Sedda, F.; Genazzani, A.D.; Nappi, L. Surgical technique of endometrioma excision impacts on the ovarian reserve. Single-port access laparoscopy versus multiport access laparoscopy: A case control study. Gynecol. Endocrinol. 2015, 31, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Stochino-Loi, E.; Darwish, B.; Mircea, O.; Touleimat, S.; Millochau, J.C.; Abo, C.; Angioni, S.; Roman, H. Does preoperative antimullerian hormone level influence postoperative pregnancy rate in women undergoing surgery for severe endometriosis? Fertil. Steril. 2017, 107, 707–713.e3. [Google Scholar] [CrossRef] [PubMed]
- Socolov, R.; Butureanu, S.; Angioni, S.; Sindilar, A.; Boiculese, L.; Cozma, L.; Socolov, D. The value of serological markers in the diagnosis and prognosis of endometriosis: A prospective case–control study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 154, 215–217. [Google Scholar] [CrossRef]
- Mohamed, M.L.; El Behery, M.M.; Mansour, S.A. Comparative study between VEGF-A and CA-125 in diagnosis and fol-low-up of advanced endometriosis after conservative laparoscopic surgery. Arch. Gynecol. Obstet. 2013, 287, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Matalliotakis, I.M.; Goumenou, A.G.; Mulayim, N.; Karkavitsas, N.; Koumantakis, E.E. High concentrations of the CA-125, CA 19-9 and CA 15-3 in the peritoneal fluid between patients with and without endometriosis. Arch. Gynecol. Obstet. 2004, 271, 40–45. [Google Scholar] [CrossRef]
- Vodolazkaia, A.; El-Aalamat, Y.; Popovic, D.; Mihalyi, A.; Bossuyt, X.; Kyama, C.M.; Fassbender, A.; Bokor, A.; Schols, D.; Huskens, D.; et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum. Reprod. 2012, 27, 2698–2711. [Google Scholar] [CrossRef]
- Mol, B.W.; Bayram, N.; Lijmer, J.G.; Wiegerinck, M.A.; Bongers, M.Y.; van der Veen, F.; Bossuyt, P.M. The performance of CA-125 measurement in the detection of endometriosis: A meta-analysis. Fertil. Steril. 1998, 70, 1101–1108. [Google Scholar] [CrossRef]
- Patel, N.R.; McPhail, M.J.; Shariff, M.I.; Keun, H.C.; Taylor-Robinson, S.D. Biofluid metabonomics using (1)H NMR spec-troscopy: The road to biomarker discovery in gastroenterology and hepatology. Expert Rev. Gastroenterol. Hepatol. 2012, 6, 239–251. [Google Scholar] [CrossRef]
- Weiss, R.H.; Kim, K. Metabolomics in the study of kidney diseases. Nat. Rev. Nephrol. 2011, 8, 22–33. [Google Scholar] [CrossRef]
- Rhee, E.P.; E Gerszten, R. Metabolomics and Cardiovascular Biomarker Discovery. Clin. Chem. 2012, 58, 139–147. [Google Scholar] [CrossRef]
- Muthubharathi, B.C.; Gowripriya, T.; Balamurugan, K. Metabolomics: Small molecules that matter more. Mol. Om. 2021, 17, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Stoop, M.P.; Coulier, L.; Rosenling, T.; Shi, S.; Smolinska, A.M.; Buydens, L.; Ampt, K.; Stingl, C.; Dane, A.; Muilwijk, B.; et al. Quantitative proteomics and metabolomics analysis of normal human cerebrospinal fluid samples. Mol. Cell. Proteom. 2010, 9, 2063–2075. [Google Scholar] [CrossRef]
- Vigano, P.; Candiani, M.; Monno, A.; Giacomini, E.; Vercellini, P.; Somigliana, E. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 2017, 33, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.J.; Fendt, S.-M.; Becker, D.F. The Proline Cycle As a Potential Cancer Therapy Target. Biochemistry 2018, 57, 3433–3444. [Google Scholar] [CrossRef] [PubMed]
- Jussila, T.; Kauppila, S.; Bode, M.; Tapanainen, J.; Risteli, J.; Risteli, L.; Kauppila, A.; Stenbäck, F. Synthesis and maturation of type I and type III collagens in endometrial adenocarcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, 66–74. [Google Scholar] [CrossRef]
- Palka, J.; Oscilowska, I.; Szoka, L. Collagen metabolism as a regulator of proline dehydrogenase/proline oxidase-dependent apoptosis/autophagy. Am. Acids 2021, 53, 1917–1925. [Google Scholar] [CrossRef]
- Santonastaso, M.; Pucciarelli, A.; Costantini, S.; Caprio, F.; Sorice, A.; Capone, F.; Natella, A.; Iardino, P.; Colacurci, N.; Chiosi, E. Correction: Metabolomic profiling and biochemical evaluation of the follicular fluid of endometriosis patients. Mol. Biosyst. 2017, 13, 1246. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yang, J.X.; Allen, J.C.; Tan, C.S.; Chern, B.S.M.; Tan, T.Y.; Tan, H.H.; Mattar, C.N.Z.; Chan, J.K.Y. Elevated peritoneal fluid ceramides in human endometriosis-associated infertility and their effects on mouse oocyte maturation. Fertil. Steril. 2018, 110, 767–777.e5. [Google Scholar] [CrossRef]
- Thomas, M.; Hughes, R. A relationship between ascorbic acid and threonic acid in guinea-pigs. Food Chem. Toxicol. 1983, 21, 449–452. [Google Scholar] [CrossRef]
- Ansariniya, H.; Yavari, A.; Javaheri, A.; Zare, F. Oxidative stress-related effects on various aspects of endometriosis. Am. J. Reprod. Immunol. 2022, 88, e13593. [Google Scholar] [CrossRef]
- Maignien, C.; Santulli, P.; Kateb, F.; Caradeuc, C.; Marcellin, L.; Pocate-Cheriet, K.; Bourdon, M.; Chouzenoux, S.; Batteux, F.; Bertho, G.; et al. Endometriosis phenotypes are associated with specific serum metabolic profiles determined by pro-ton-nuclear magnetic resonance. Reprod. Biomed. Online 2020, 41, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia (acetoacetate) upregulates NADPH oxidase 4 and elevates oxidative stress, ICAM-1, and monocyte adhesivity in endothelial cells. Cell. Physiol. Biochem. 2015, 35, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Röhling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Burmester, G.-R.; Brand, M.D. Bioenergetics of immune functions: Fundamental and therapeutic aspects. Immunol. Today 2000, 21, 194–199. [Google Scholar] [CrossRef]
- Straub, R.H.; Cutolo, M.; Buttgereit, F.; Pongratz, G. Energy regulation and neuroendocrine-immune control in chronic in-flammatory diseases. J. Intern. Med. 2010, 267, 543–560. [Google Scholar] [CrossRef]
- Kaufmann, T.B.; Drillich, M.; Tenhagen, B.A.; Heuwieser, W. Correlations between periparturient serum concentrations of non-esterified fatty acids, beta-hydroxybutyric acid, bilirubin, and urea and the occurrence of clinical and subclinical post-partum bovine endometritis. BMC Vet. Res. 2010, 6, 47. [Google Scholar] [CrossRef]
- Hilvo, M.; de Santiago, I.; Gopalacharyulu, P.; Schmitt, W.D.; Budczies, J.; Kuhberg, M.; Dietel, M.; Aittokallio, T.; Markowetz, F.; Denkert, C.; et al. Accumulated Metabolites of Hydroxybutyric Acid Serve as Diagnostic and Prognostic Biomarkers of Ovarian High-Grade Serous Carcinomas. Cancer Res 2016, 76, 796–804. [Google Scholar] [CrossRef]
- Ishikawa, M.; Nakayama, K.; Nakamura, K.; Ono, R.; Sanuki, K.; Yamashita, H.; Ishibashi, T.; Minamoto, T.; Iida, K.; Razia, S.; et al. Affinity-purified DNA-based mutation profiles of endometriosis-related ovarian neoplasms in Japanese patients. Oncotarget 2018, 9, 14754–14763. [Google Scholar] [CrossRef]
- Jana, S.K.; Dutta, M.; Joshi, M.; Srivastava, S.; Chakravarty, B.; Chaudhury, K. 1H NMR Based Targeted Metabolite Profiling for Understanding the Complex Relationship Connecting Oxidative Stress with Endometriosis. BioMed. Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Felig, P.; Owen, O.E.; Wahren, J.; Cahill, G.F. Amino acid metabolism during prolonged starvation. J. Clin. Investig. 1969, 48, 584–594. [Google Scholar] [CrossRef]
- Sherwin, R.S.; Hendler, R.G.; Felig, P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes 1976, 25, 776–784. [Google Scholar] [CrossRef]
- Soto-Mota, A.; Norwitz, N.G.; Evans, R.D.; Clarke, K. Exogenous d-beta-hydroxybutyrate lowers blood glucose in part by decreasing the availability of L-alanine for gluconeogenesis. Endocrinol. Diabetes Metab. 2022, 5, e00300. [Google Scholar] [CrossRef]
- Dutta, M.; Joshi, M.; Srivastava, S.; Lodh, I.; Chakravarty, B.; Chaudhury, K. A metabonomics approach as a means for identification of potential biomarkers for early diagnosis of endometriosis. Mol. Biosyst. 2012, 8, 3281–3287. [Google Scholar] [CrossRef]
- Sivanand, S.; Heiden, M.G.V. Emerging Roles for Branched-Chain Amino Acid Metabolism in Cancer. Cancer Cell 2020, 37, 147–156. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Wilkinson, A.C. Branched-chain amino acid metabolism in cancer. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 64–70. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Powell, J.D.; Hutson, S.M. Leucine Metabolism in T Cell Activation: mTOR Signaling and Beyond. Adv. Nutr. Int. Rev. J. 2016, 7, 798S–805S. [Google Scholar] [CrossRef]
- Bonvini, A.; Coqueiro, A.Y.; Tirapegui, J.; Calder, P.C.; Rogero, M.M. Immunomodulatory role of branched-chain amino acids. Nutr. Rev. 2018, 76, 840–856. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Vallvé-Juanico, J.; Houshdaran, S.; Giudice, L.C. The endometrial immune environment of women with endometriosis. Hum. Reprod. Updat. 2019, 25, 565–592. [Google Scholar] [CrossRef]
- Strasser, B.; Sperner-Unterweger, B.; Fuchs, D.; Gostner, J.M. Mechanisms of Inflammation-Associated Depression: Immune Influences on Tryptophan and Phenylalanine Metabolisms. Curr. Top. Behav. Neurosci. 2016, 31, 95–115. [Google Scholar] [CrossRef]
- Luporini, R.L.; Pott-Junior, H.; Leal, M.C.B.D.M.; Castro, A.; Ferreira, A.G.; Cominetti, M.R.; Anibal, F.D.F. Phenylalanine and COVID-19: Tracking disease severity markers. Int. Immunopharmacol. 2021, 101, 108313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-H.; Zhang, A.-H.; Wang, L.; Tan, Y.-L.; Guan, Y.; Han, Y.; Sun, H.; Wang, X.-J. Novel chinmedomics strategy for discovering effective constituents from ShenQiWan acting on ShenYangXu syndrome. Chin. J. Nat. Med. 2016, 14, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Trabanelli, S.; Ocadlikova, D.; Evangelisti, C.; Parisi, S.; Curti, A. Induction of Regulatory T Cells by Dendritic Cells through Indoleamine 2,3- dioxygenase: A Potent Mechanism of Acquired Peripheral Tolerance. Curr. Med. Chem. 2011, 18, 2234–2239. [Google Scholar] [CrossRef]
- Song, H.; Park, H.; Kim, Y.-S.; Kim, K.D.; Lee, H.-K.; Cho, D.-H.; Yang, J.-W.; Hur, D.Y. l-Kynurenine-induced apoptosis in human NK cells is mediated by reactive oxygen species. Int. Immunopharmacol. 2011, 11, 932–938. [Google Scholar] [CrossRef]
- Zaher, S.S.; Germain, C.; Fu, H.; Larkin, D.F.; George, A.J. 3-hydroxykynurenine suppresses CD4+ T-cell proliferation, in-duces T-regulatory-cell development, and prolongs corneal allograft survival. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2640–2648. [Google Scholar] [CrossRef]
- Osuga, Y.; Koga, K.; Hirota, Y.; Hirata, T.; Yoshino, O.; Taketani, Y. Lymphocytes in Endometriosis. Am. J. Reprod. Immunol. 2010, 65, 1–10. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune ac-tivation. Front. Biosci. 2015, 20, 1116–1143. [Google Scholar]
- Urata, Y.; Koga, K.; Hirota, Y.; Akiyama, I.; Izumi, G.; Takamura, M.; Nagai, M.; Harada, M.; Hirata, T.; Yoshino, O.; et al. IL-1β Increases Expression of Tryptophan 2,3-dioxygenase and Stimulates Tryptophan Catabolism in Endometrioma Stromal Cells. Am. J. Reprod. Immunol. 2014, 72, 496–503. [Google Scholar] [CrossRef]
- Gunther, J.; Fallarino, F.; Fuchs, D.; Wirthgen, E. Editorial: Immunomodulatory Roles of Tryptophan Metabolites in Inflam-mation and Cancer. Front. Immunol. 2020, 11, 1497. [Google Scholar] [CrossRef]
- Moffett, J.R.; Arun, P.; Puthillathu, N.; Vengilote, R.; Ives, J.A.; Badawy, A.A.-B.; Namboodiri, A.M. Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD+ Synthesis During Inflammation and Infection. Front. Immunol. 2020, 11, 31. [Google Scholar] [CrossRef]
- Biefer, H.R.C.; Vasudevan, A.; Elkhal, A. Aspects of Tryptophan and Nicotinamide Adenine Dinucleotide in Immunity: A New Twist in an Old Tale. Int. J. Tryptophan Res. 2017, 10, 1178646917713491. [Google Scholar] [CrossRef]
- Grant, R.S. Indoleamine 2,3-Dioxygenase Activity Increases NAD+ Production in IFN-gamma-Stimulated Human Primary Mononuclear Cells. Int. J. Tryptophan Res. 2018, 11, 1178646917751636. [Google Scholar] [CrossRef]
- Wu, X.-H.; Zhao, C.; Zhang, A.-H.; Zhang, J.-Q.; Wang, X.; Sun, X.-L.; Sun, Z.; Wang, X.-J. High-throughput metabolomics used to identify potential therapeutic targets of Guizhi Fuling Wan against endometriosis of cold coagulation and blood stasis. RSC Adv. 2018, 8, 19238–19250. [Google Scholar] [CrossRef]

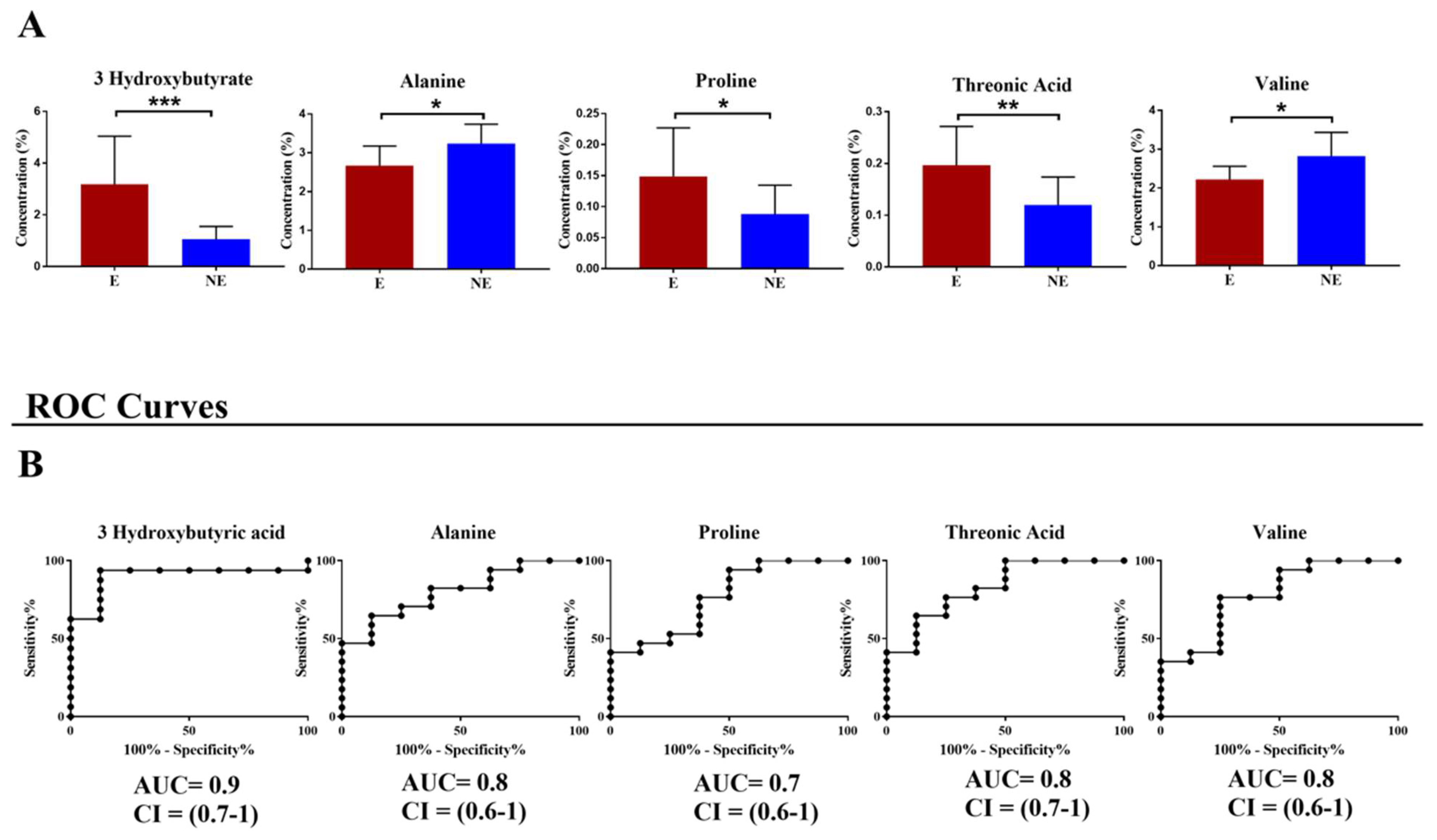
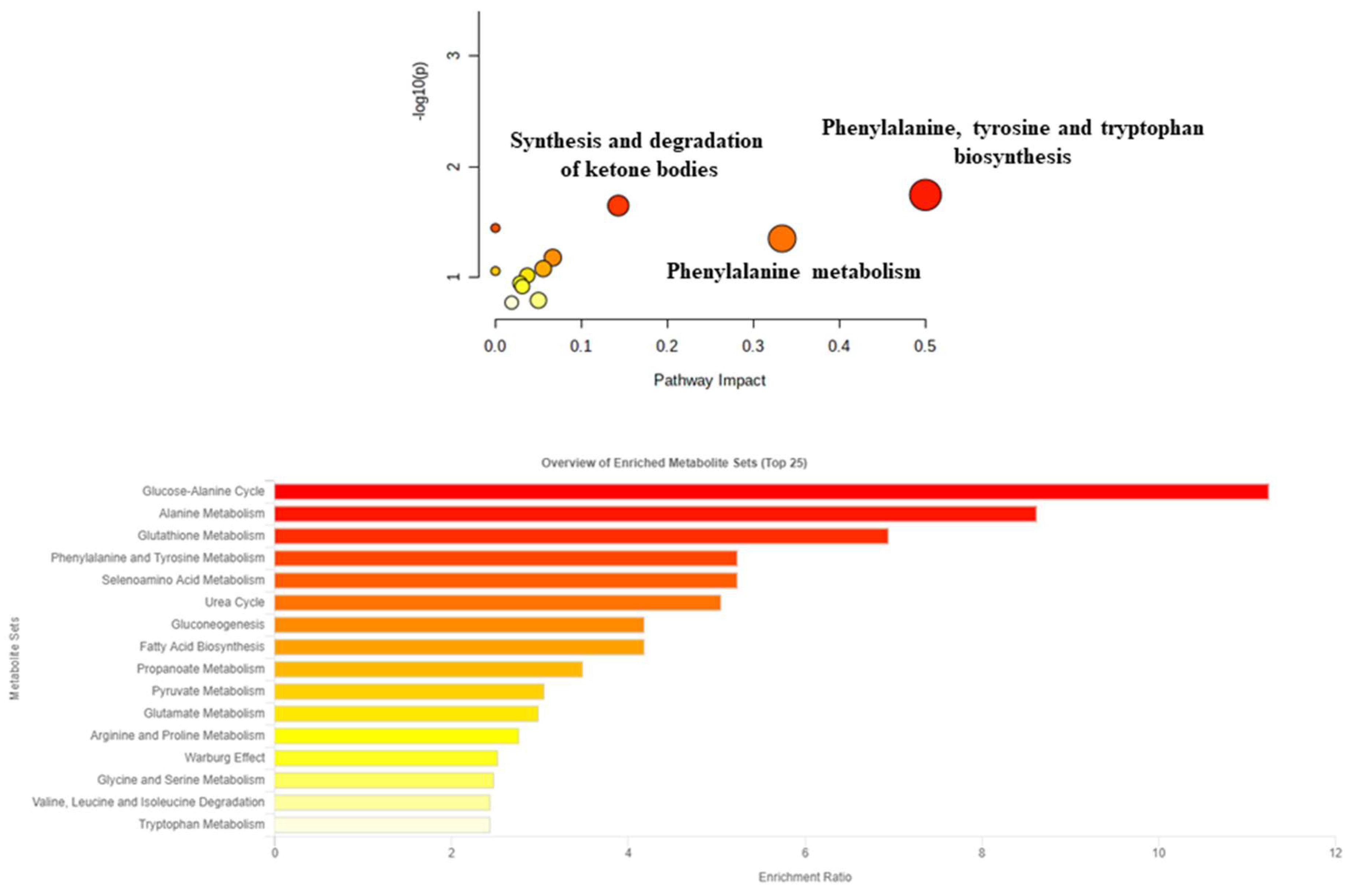
| Clinical Variables | Group Endometriosis (n = 22) |
|---|---|
| Dysmenorrhea (VAS median [range]) | 8 (7–10) |
| Chronic pelvic pain (VAS median, [range]) | 7 (5–9) |
| Dyspareunia (VAS median, [range]) | 8 (7–10) |
| Dischezia (VAS median, [range]) | 3 (0–4) |
| Dysuria (VAS median, [range]) | 1 (0–5) |
| Surgical variables | |
| Single ovarian endometrioma (n; %) | 18; 81.8% |
| Measurements in cm (median, [range]) | 7 (5–9) |
| Bilateral ovarian endometrioma (n; %) | 6; 27.3% |
| Measurements in cm (median, [range]) | 4 (4–6) |
| Deep infiltrating endometriosis (DIE) (n; %) | 22; 100% |
| Variables | Endometriosis (n = 22) | No Endometriosis (n = 10) | p-Value |
|---|---|---|---|
| Age (median, range) | 34 (22–43) | 41 (33–50) | <0.05 |
| BMI (median, range) | 20.4 (18.5–29) | 23.4 (19.5–31.2) | NS |
| Ethnicity | |||
| Caucasian | 22 | 10 | |
| Age at menarche, mean | 11.7 (1.4) | 11.8 (1.5) | NS |
| Marital status % | |||
| Married | 64 | 50 | NS |
| Divorced | 18 | 20 | NS |
| Single | 18 | 30 | NS |
| Parity% | 18 | 20 | NS |
| One | 13.5 | 20 | NS |
| two | 4.5 | - | NS |
| Cigarette smoking% | |||
| Non smoker | 77.3 | 80 | NS |
| Current smoker daily | 22.7 | 20 | |
| Cigarettes (mean ± SD) | 10.6 ± 6.2 | 9.9 ± 6.1 | NS |
| Schooling % | |||
| Elementary and middle school | 13.6 | 20 | NS |
| High school | 60 | 60 | NS |
| Higher education | 26.4 | 20 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angioni, S.; Congiu, F.; Vitale, S.G.; D’Alterio, M.N.; Noto, A.; Monni, G.; Santoru, M.L.; Fanos, V.; Murgia, F.; Atzori, L. Gas Chromatography–Mass Spectrometry (GC–MS) Metabolites Analysis in Endometriosis Patients: A Prospective Observational Translational Study. J. Clin. Med. 2023, 12, 922. https://doi.org/10.3390/jcm12030922
Angioni S, Congiu F, Vitale SG, D’Alterio MN, Noto A, Monni G, Santoru ML, Fanos V, Murgia F, Atzori L. Gas Chromatography–Mass Spectrometry (GC–MS) Metabolites Analysis in Endometriosis Patients: A Prospective Observational Translational Study. Journal of Clinical Medicine. 2023; 12(3):922. https://doi.org/10.3390/jcm12030922
Chicago/Turabian StyleAngioni, Stefano, Francesca Congiu, Salvatore Giovanni Vitale, Maurizio Nicola D’Alterio, Antonio Noto, Giovanni Monni, Maria Laura Santoru, Vassilios Fanos, Federica Murgia, and Luigi Atzori. 2023. "Gas Chromatography–Mass Spectrometry (GC–MS) Metabolites Analysis in Endometriosis Patients: A Prospective Observational Translational Study" Journal of Clinical Medicine 12, no. 3: 922. https://doi.org/10.3390/jcm12030922
APA StyleAngioni, S., Congiu, F., Vitale, S. G., D’Alterio, M. N., Noto, A., Monni, G., Santoru, M. L., Fanos, V., Murgia, F., & Atzori, L. (2023). Gas Chromatography–Mass Spectrometry (GC–MS) Metabolites Analysis in Endometriosis Patients: A Prospective Observational Translational Study. Journal of Clinical Medicine, 12(3), 922. https://doi.org/10.3390/jcm12030922










