Antitumor Immunity Exerted by Natural Killer and Natural Killer T Cells in the Liver
Abstract
1. Introduction
2. NK Cells
2.1. Overview
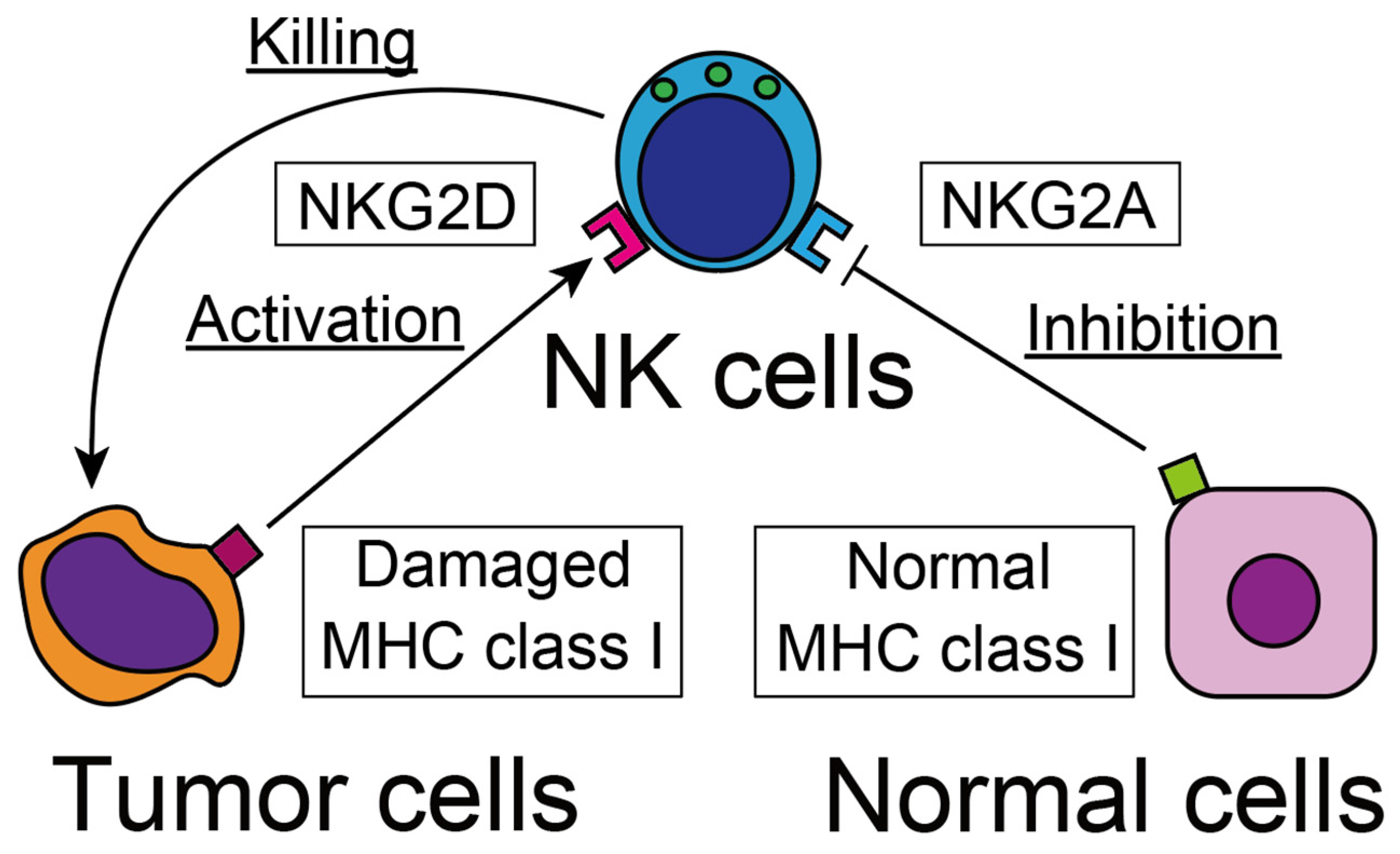
2.2. Recognition of Tumor Cells
2.3. Cytotoxicity against Tumor Cells
2.4. Possible Therapeutic Strategy against Human Cancer
3. NKT Cells
3.1. Overview
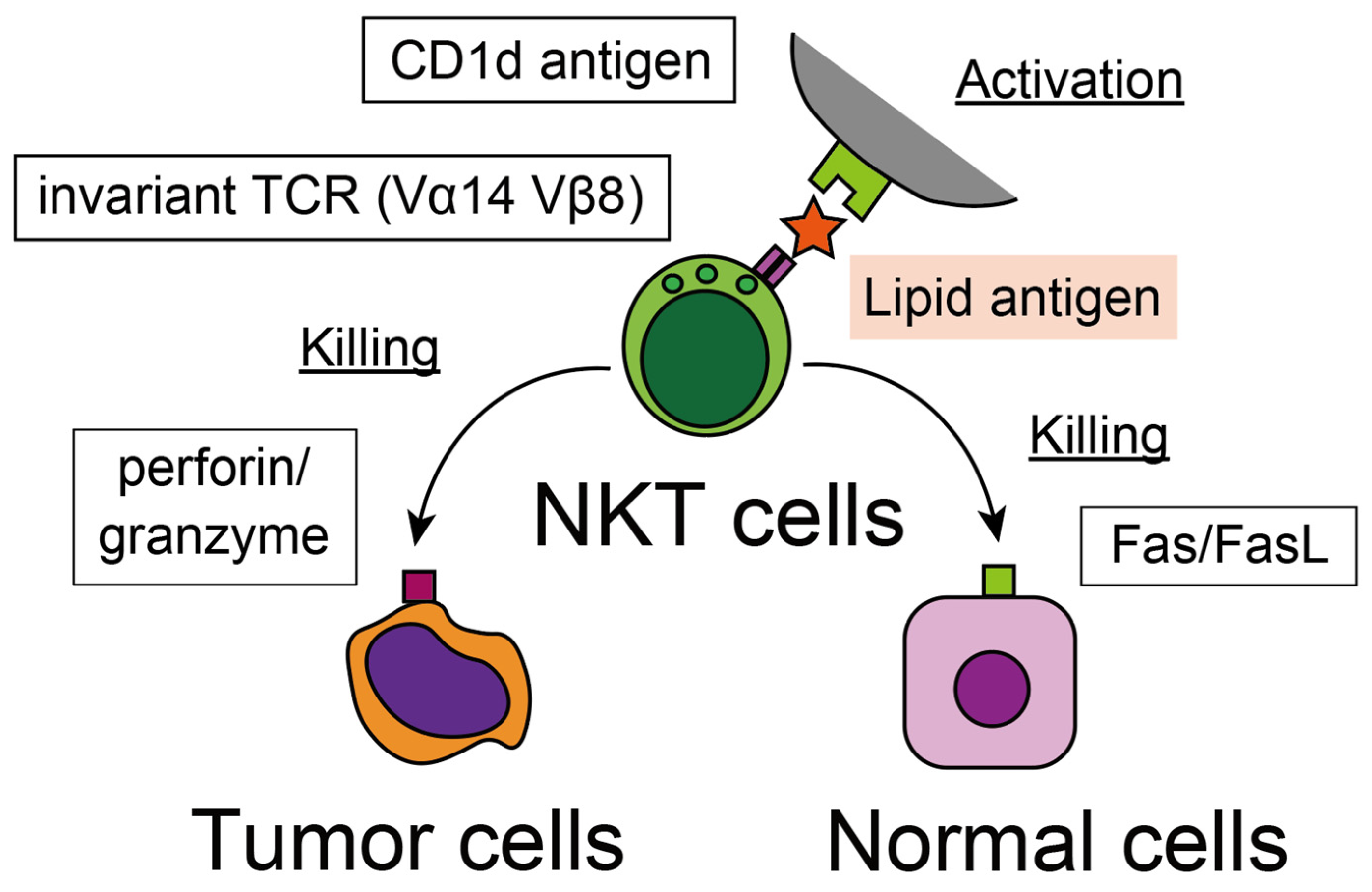
3.2. Recognition of Tumor Cells
3.3. Cytotoxicity against Tumor Cells
3.4. Possible Therapeutic Strategy against Human Cancer
4. Concluding Remarks
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Herberman, R.B.; Nunn, M.E.; Holden, H.T.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 1975, 16, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, C.; Chasson, L.; Luci, C.; Tomasello, E.; Geissmann, F.; Vivier, E.; Walzer, T. The trafficking of natural killer cells. Immunol. Rev. 2007, 220, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Kawarabayashi, N.; Seki, S.; Hatsuse, K.; Ohkawa, T.; Koike, Y.; Aihara, T.; Habu, Y.; Nakagawa, R.; Ami, K.; Hiraide, H.; et al. Decrease of CD56(+)T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology 2000, 32, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.; Ahmad, F.; Hong, H.S.; Eberhard, J.; Lu, I.N.; Ballmaier, M.; Schmidt, R.E.; Jacobs, R.; Meyer-Olson, D. FcgammaRIII (CD16)-mediated ADCC by NK cells is regulated by monocytes and FcgammaRII (CD32). Eur. J. Immunol. 2014, 44, 3368–3379. [Google Scholar] [CrossRef] [PubMed]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.; Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef]
- Gowen, B.G.; Chim, B.; Marceau, C.D.; Greene, T.T.; Burr, P.; Gonzalez, J.R.; Hesser, C.R.; Dietzen, P.A.; Russell, T.; Iannello, A.; et al. A forward genetic screen reveals novel independent regulators of ULBP1, an activating ligand for natural killer cells. eLife 2015, 4, e08474. [Google Scholar] [CrossRef]
- Nice, T.J.; Coscoy, L.; Raulet, D.H. Posttranslational regulation of the NKG2D ligand Mult1 in response to cell stress. J. Exp. Med. 2009, 206, 287–298. [Google Scholar] [CrossRef]
- Gasser, S.; Orsulic, S.; Brown, E.J.; Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005, 436, 1186–1190. [Google Scholar] [CrossRef]
- Liao, N.S.; Bix, M.; Zijlstra, M.; Jaenisch, R.; Raulet, D. MHC class I deficiency: Susceptibility to natural killer (NK) cells and impaired NK activity. Science 1991, 253, 199–202. [Google Scholar] [CrossRef]
- Ljunggren, H.G.; Karre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef]
- Lanier, L.L. NK cell recognition. Annu. Rev. Immunol. 2005, 23, 225–274. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, T.; Seki, S.; Dobashi, H.; Koike, Y.; Habu, Y.; Ami, K.; Hiraide, H.; Sekine, I. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology 2001, 103, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Ahmad, M.; Bennett, I.M.; Azzoni, L.; Alnemri, E.S.; Perussia, B. Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 1998, 188, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jaw, J.J.; Stutzman, N.C.; Zou, Z.; Sun, P.D. Natural killer cell-produced IFN-gamma and TNF-alpha induce target cell cytolysis through up-regulation of ICAM-1. J. Leukoc. Biol. 2012, 91, 299–309. [Google Scholar] [CrossRef]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Chang, A.E.; Avis, F.P.; Leitman, S.; Linehan, W.M.; Robertson, C.N.; Lee, R.E.; Rubin, J.T.; et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med. 1987, 316, 889–897. [Google Scholar] [CrossRef]
- Levin, A.M.; Bates, D.L.; Ring, A.M.; Krieg, C.; Lin, J.T.; Su, L.; Moraga, I.; Raeber, M.E.; Bowman, G.R.; Novick, P.; et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature 2012, 484, 529–533. [Google Scholar] [CrossRef]
- Ardolino, M.; Azimi, C.S.; Iannello, A.; Trevino, T.N.; Horan, L.; Zhang, L.; Deng, W.; Ring, A.M.; Fischer, S.; Garcia, K.C.; et al. Cytokine therapy reverses NK cell anergy in MHC-deficient tumors. J. Clin. Investig. 2014, 124, 4781–4794. [Google Scholar] [CrossRef] [PubMed]
- Andre, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Blery, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e13. [Google Scholar] [CrossRef]
- Doorduijn, E.M.; Sluijter, M.; Salvatori, D.C.; Silvestri, S.; Maas, S.; Arens, R.; Ossendorp, F.; van der Burg, S.H.; van Hall, T. CD4(+) T Cell and NK Cell Interplay Key to Regression of MHC Class I(low) Tumors upon TLR7/8 Agonist Therapy. Cancer Immunol. Res. 2017, 5, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; Gerster, J.F.; Owens, M.L.; Slade, H.B.; Tomai, M.A. Imiquimod applied topically: A novel immune response modifier and new class of drug. Int. J. Immunopharmacol. 1999, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; MacDonald, H.R.; Kronenberg, M.; Smyth, M.J.; Van Kaer, L. NKT cells: What’s in a name? Nat. Rev. Immunol. 2004, 4, 231–237. [Google Scholar] [CrossRef]
- Sykes, M.; Hoyles, K.A.; Romick, M.L.; Sachs, D.H. In vitro and in vivo analysis of bone marrow-derived CD3+, CD4-, CD8-, NK1.1+ cell lines. Cell. Immunol. 1990, 129, 478–493. [Google Scholar] [CrossRef]
- Sykes, M. Unusual T cell populations in adult murine bone marrow. Prevalence of CD3+CD4-CD8- and alpha beta TCR+NK1.1+ cells. J. Immunol. 1990, 145, 3209–3215. [Google Scholar] [CrossRef]
- Ohteki, T.; Seki, S.; Abo, T.; Kumagai, K. Liver is a possible site for the proliferation of abnormal CD3+4-8- double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J. Exp. Med. 1990, 172, 7–12. [Google Scholar] [CrossRef]
- Seki, S.; Abo, T.; Ohteki, T.; Sugiura, K.; Kumagai, K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J. Immunol. 1991, 147, 1214–1221. [Google Scholar] [CrossRef]
- Levitsky, H.I.; Golumbek, P.T.; Pardoll, D.M. The fate of CD4-8- T cell receptor-alpha beta+ thymocytes. J. Immunol. 1991, 146, 1113–1117. [Google Scholar] [CrossRef]
- Arase, H.; Arase, N.; Nakagawa, K.; Good, R.A.; Onoe, K. NK1.1+ CD4+ CD8- thymocytes with specific lymphokine secretion. Eur. J. Immunol. 1993, 23, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Lantz, O.; Bendelac, A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J. Exp. Med. 1994, 180, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Makino, Y.; Koseki, H.; Adachi, Y.; Akasaka, T.; Tsuchida, K.; Taniguchi, M. Extrathymic differentiation of a T cell bearing invariant V alpha 14J alpha 281 TCR. Int. Rev. Immunol. 1994, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kono, D.H.; Balderas, R.S.; Theofilopoulos, A.N. V beta repertoire of murine hepatic T cells. Implication for selection of double negative alpha beta + T cells. J. Immunol. 1994, 153, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Miyaji, C.; Kawachi, Y.; Iiai, T.; Ohtsuka, K.; Iwanage, T.; Takahashi-Iwanaga, H.; Abo, T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J. Immunol. 1995, 155, 2972–2983. [Google Scholar] [CrossRef]
- Makino, Y.; Kanno, R.; Ito, T.; Higashino, K.; Taniguchi, M. Predominant expression of invariant V alpha 14+ TCR alpha chain in NK1.1+ T cell populations. Int. Immunol. 1995, 7, 1157–1161. [Google Scholar] [CrossRef]
- Bendelac, A.; Lantz, O.; Quimby, M.E.; Yewdell, J.W.; Bennink, J.R.; Brutkiewicz, R.R. CD1 recognition by mouse NK1+ T lymphocytes. Science 1995, 268, 863–865. [Google Scholar] [CrossRef]
- Beckman, E.M.; Porcelli, S.A.; Morita, C.T.; Behar, S.M.; Furlong, S.T.; Brenner, M.B. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature 1994, 372, 691–694. [Google Scholar] [CrossRef]
- Hix, L.M.; Shi, Y.H.; Brutkiewicz, R.R.; Stein, P.L.; Wang, C.R.; Zhang, M. CD1d-expressing breast cancer cells modulate NKT cell-mediated antitumor immunity in a murine model of breast cancer metastasis. PLoS ONE 2011, 6, e20702. [Google Scholar] [CrossRef]
- Dockry, E.; O’Leary, S.; Gleeson, L.E.; Lyons, J.; Keane, J.; Gray, S.G.; Doherty, D.G. Epigenetic induction of CD1d expression primes lung cancer cells for killing by invariant natural killer T cells. Oncoimmunology 2018, 7, e1428156. [Google Scholar] [CrossRef]
- Fujii, S.; Shimizu, K.; Kronenberg, M.; Steinman, R.M. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat. Immunol. 2002, 3, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Bezbradica, J.S.; Stanic, A.K.; Matsuki, N.; Bour-Jordan, H.; Bluestone, J.A.; Thomas, J.W.; Unutmaz, D.; Van Kaer, L.; Joyce, S. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J. Immunol. 2005, 174, 4696–4705. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Motoki, K.; Ueno, H.; Nakagawa, R.; Sato, H.; Kondo, E.; et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 1997, 278, 1626–1629. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Cui, J.; Koezuka, Y.; Toura, I.; Kaneko, Y.; Sato, H.; Kondo, E.; Harada, M.; Koseki, H.; Nakayama, T.; et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. USA 1998, 95, 5690–5693. [Google Scholar] [CrossRef]
- Kawano, T.; Nakayama, T.; Kamada, N.; Kaneko, Y.; Harada, M.; Ogura, N.; Akutsu, Y.; Motohashi, S.; Iizasa, T.; Endo, H.; et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999, 59, 5102–5105. [Google Scholar] [PubMed]
- Kagi, D.; Ledermann, B.; Burki, K.; Zinkernagel, R.M.; Hengartner, H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 1996, 14, 207–232. [Google Scholar] [CrossRef]
- Metelitsa, L.S.; Naidenko, O.V.; Kant, A.; Wu, H.W.; Loza, M.J.; Perussia, B.; Kronenberg, M.; Seeger, R.C. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J. Immunol. 2001, 167, 3114–3122. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, R.; Nagafune, I.; Tazunoki, Y.; Ehara, H.; Tomura, H.; Iijima, R.; Motoki, K.; Kamishohara, M.; Seki, S. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J. Immunol. 2001, 166, 6578–6584. [Google Scholar] [CrossRef]
- Inui, T.; Nakagawa, R.; Ohkura, S.; Habu, Y.; Koike, Y.; Motoki, K.; Kuranaga, N.; Fukasawa, M.; Shinomiya, N.; Seki, S. Age-associated augmentation of the synthetic ligand- mediated function of mouse NK1.1 ag(+) T cells: Their cytokine production and hepatotoxicity in vivo and in vitro. J. Immunol. 2002, 169, 6127–6132. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Nakashima, H.; Habu, Y.; Nakagawa, R.; Fukasawa, M.; Kinoshita, M.; Shinomiya, N.; Seki, S. Neutralization of tumor necrosis factor abrogates hepatic failure induced by alpha-galactosylceramide without attenuating its antitumor effect in aged mice. J. Hepatol. 2005, 43, 670–678. [Google Scholar] [CrossRef]
- Nakagawa, R.; Inui, T.; Nagafune, I.; Tazunoki, Y.; Motoki, K.; Yamauchi, A.; Hirashima, M.; Habu, Y.; Nakashima, H.; Seki, S. Essential role of bystander cytotoxic CD122+CD8+ T cells for the antitumor immunity induced in the liver of mice by alpha-galactosylceramide. J. Immunol. 2004, 172, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Molling, J.W.; Kolgen, W.; van der Vliet, H.J.; Boomsma, M.F.; Kruizenga, H.; Smorenburg, C.H.; Molenkamp, B.G.; Langendijk, J.A.; Leemans, C.R.; von Blomberg, B.M.; et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int. J. Cancer 2005, 116, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.M.; Cheng, O.; Shaulov, A.; Koezuka, Y.; Bubley, G.J.; Wilson, S.B.; Balk, S.P.; Exley, M.A. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J. Immunol. 2001, 167, 4046–4050. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, K.; Seino, K.; Ishikawa, Y.; Nozue, M.; Todoroki, T.; Fukao, K. Impaired proliferative response of V alpha 24 NKT cells from cancer patients against alpha-galactosylceramide. J. Immunol. 2002, 168, 6494–6499. [Google Scholar] [CrossRef]
- Yoneda, K.; Morii, T.; Nieda, M.; Tsukaguchi, N.; Amano, I.; Tanaka, H.; Yagi, H.; Narita, N.; Kimura, H. The peripheral blood Valpha24+ NKT cell numbers decrease in patients with haematopoietic malignancy. Leuk. Res. 2005, 29, 147–152. [Google Scholar] [CrossRef]
- Fuji, N.; Ueda, Y.; Fujiwara, H.; Toh, T.; Yoshimura, T.; Yamagishi, H. Antitumor effect of alpha-galactosylceramide (KRN7000) on spontaneous hepatic metastases requires endogenous interleukin 12 in the liver. Clin. Cancer Res. 2000, 6, 3380–3387. [Google Scholar]
- Giaccone, G.; Punt, C.J.; Ando, Y.; Ruijter, R.; Nishi, N.; Peters, M.; von Blomberg, B.M.; Scheper, R.J.; van der Vliet, H.J.; van den Eertwegh, A.J.; et al. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin. Cancer Res. 2002, 8, 3702–3709. [Google Scholar]
- Richter, J.; Neparidze, N.; Zhang, L.; Nair, S.; Monesmith, T.; Sundaram, R.; Miesowicz, F.; Dhodapkar, K.M.; Dhodapkar, M.V. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood 2013, 121, 423–430. [Google Scholar] [CrossRef]
- Uchida, T.; Horiguchi, S.; Tanaka, Y.; Yamamoto, H.; Kunii, N.; Motohashi, S.; Taniguchi, M.; Nakayama, T.; Okamoto, Y. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol. Immunother. 2008, 57, 337–345. [Google Scholar] [CrossRef]
- Greenbaum, U.; Mahadeo, K.M.; Kebriaei, P.; Shpall, E.J.; Saini, N.Y. Chimeric Antigen Receptor T-Cells in B-Acute Lymphoblastic Leukemia: State of the Art and Future Directions. Front. Oncol. 2020, 10, 1594. [Google Scholar] [CrossRef]
- Tian, G.; Courtney, A.N.; Jena, B.; Heczey, A.; Liu, D.; Marinova, E.; Guo, L.; Xu, X.; Torikai, H.; Mo, Q.; et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J. Clin. Investig. 2016, 126, 2341–2355. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, A.; Caputo, V.S.; Holubova, M.; Baxan, N.; Dubois, O.; Chaudhry, M.S.; Xiao, X.; Goudevenou, K.; Pitcher, D.S.; Petevi, K.; et al. Enhanced Anti-lymphoma Activity of CAR19-iNKT Cells Underpinned by Dual CD19 and CD1d Targeting. Cancer Cell 2018, 34, 596–610.e11. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, W.; Heczey, A.; Liu, D.; Guo, L.; Wood, M.; Jin, J.; Courtney, A.N.; Liu, B.; Di Pierro, E.J.; et al. NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced In Vivo Persistence and Antitumor Activity against Neuroblastoma. Clin. Cancer Res. 2019, 25, 7126–7138. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Lukacs, J.D.; Johnston, B. The Current Landscape of NKT Cell Immunotherapy and the Hills Ahead. Cancers 2021, 13, 5174. [Google Scholar] [CrossRef]
| Identifier | Title | Conditions | Locations |
| NCT05487651 | Allogeneic NK T-Cells Expressing CD19 Specific CAR in B-Cell Malignancies (ANCHOR2) | NHL, Relapsed, Adult B-cell Lymphoma B-cell Leukemia | University of California, San Francisco, San Francisco, CA, USA |
| NCT03774654 | CD19.CAR Allogeneic NKT for Patients with Relapsed or Refractory B-Cell Malignancies (ANCHOR) | Refractory B-Cell Non-Hodgkin Lymphoma | Houston Methodist Hospital, Houston, TX, USA |
| NCT03294954 | GD2 Specific CAR and Interleukin-15 Expressing Autologous NKT Cells to Treat Children with Neuroblastoma (GINAKIT2) | Neuroblastoma | Texas Children’s Hospital, Houston, TX, USA |
| NCT03093688 | Clinical Safety and Efficacy Study of Infusion of iNKT Cells and CD8 + T Cells in Patients with Advanced Solid Tumor | Non-small Cell Lung Cancer Small Cell Lung Cancer Pancreas Cancer | Shanghai Public Health Clinical Center, Shanghai, China |
| NCT02562963 | Clinical Efficacy and Safety of NKT Cell Infusion in Patients with Advanced Solid Tumor | Non-small Cell Lung Cancer Gastric Cancer | Hua Xin Hospital First Hospital of Tsinghua University, Beijing, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakashima, H.; Kinoshita, M. Antitumor Immunity Exerted by Natural Killer and Natural Killer T Cells in the Liver. J. Clin. Med. 2023, 12, 866. https://doi.org/10.3390/jcm12030866
Nakashima H, Kinoshita M. Antitumor Immunity Exerted by Natural Killer and Natural Killer T Cells in the Liver. Journal of Clinical Medicine. 2023; 12(3):866. https://doi.org/10.3390/jcm12030866
Chicago/Turabian StyleNakashima, Hiroyuki, and Manabu Kinoshita. 2023. "Antitumor Immunity Exerted by Natural Killer and Natural Killer T Cells in the Liver" Journal of Clinical Medicine 12, no. 3: 866. https://doi.org/10.3390/jcm12030866
APA StyleNakashima, H., & Kinoshita, M. (2023). Antitumor Immunity Exerted by Natural Killer and Natural Killer T Cells in the Liver. Journal of Clinical Medicine, 12(3), 866. https://doi.org/10.3390/jcm12030866






