Retinopathy of Prematurity in the 21st Century and the Complex Impact of Supplemental Oxygen
Abstract
1. Introduction
2. Animal Models of ROP
Oxygen-Induced Retinopathy
3. Clinical Manifestations of ROP
3.1. Traditional ROP in Extremely Preterm Infants with Strict Oxygen Regulation
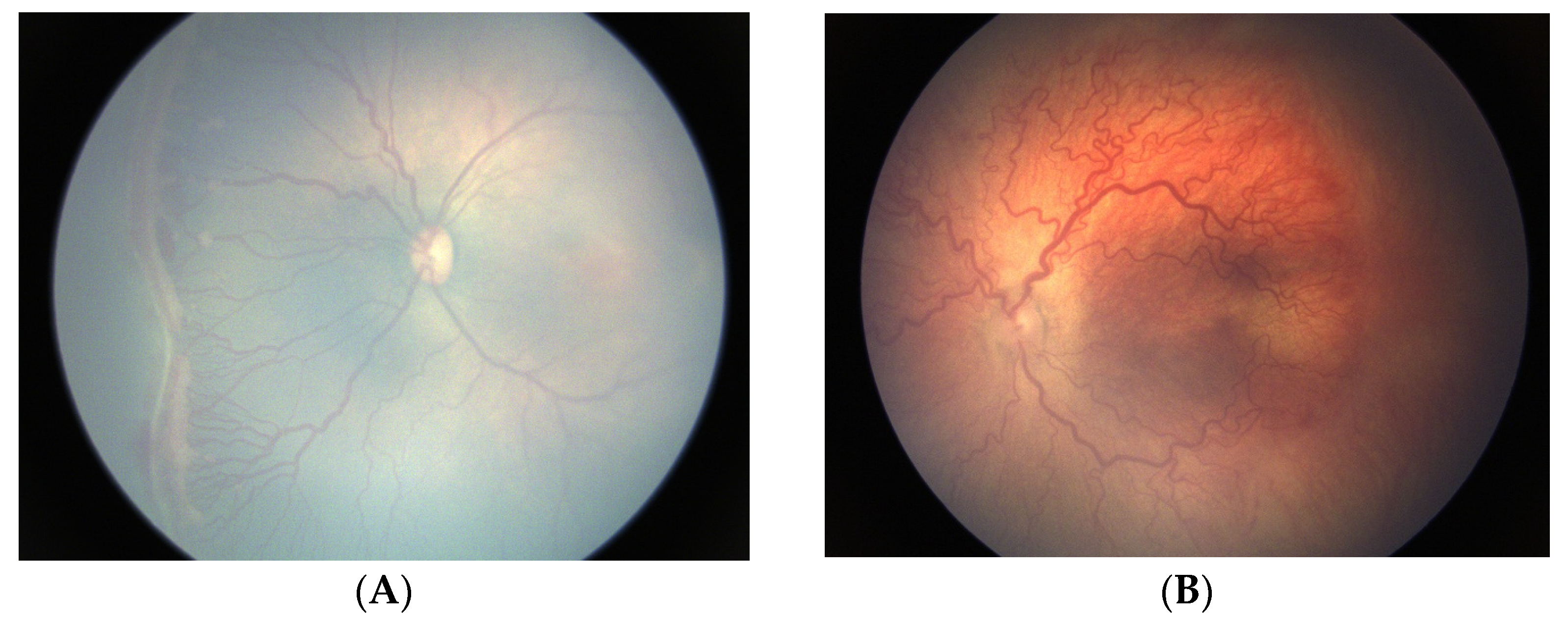
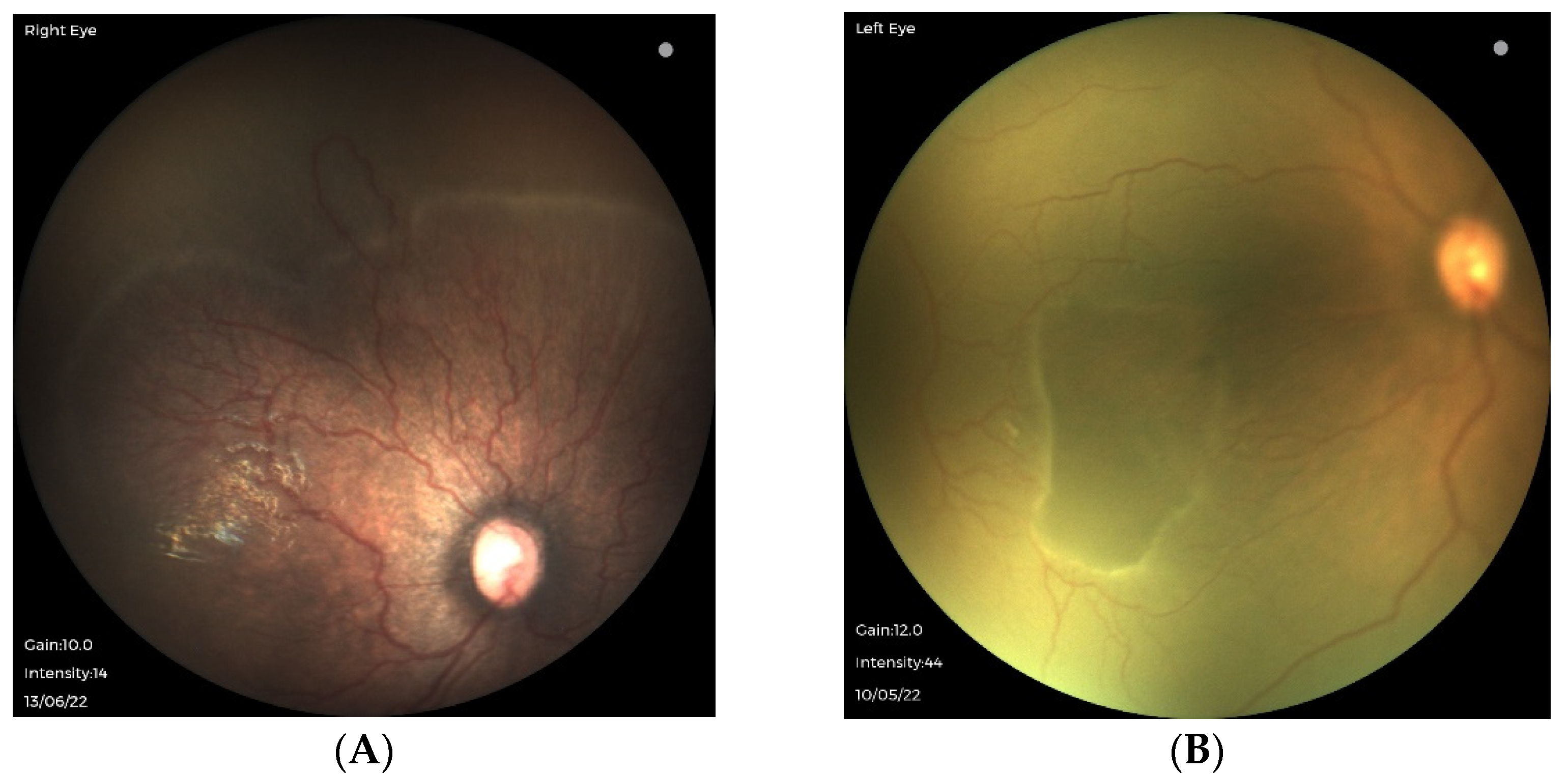
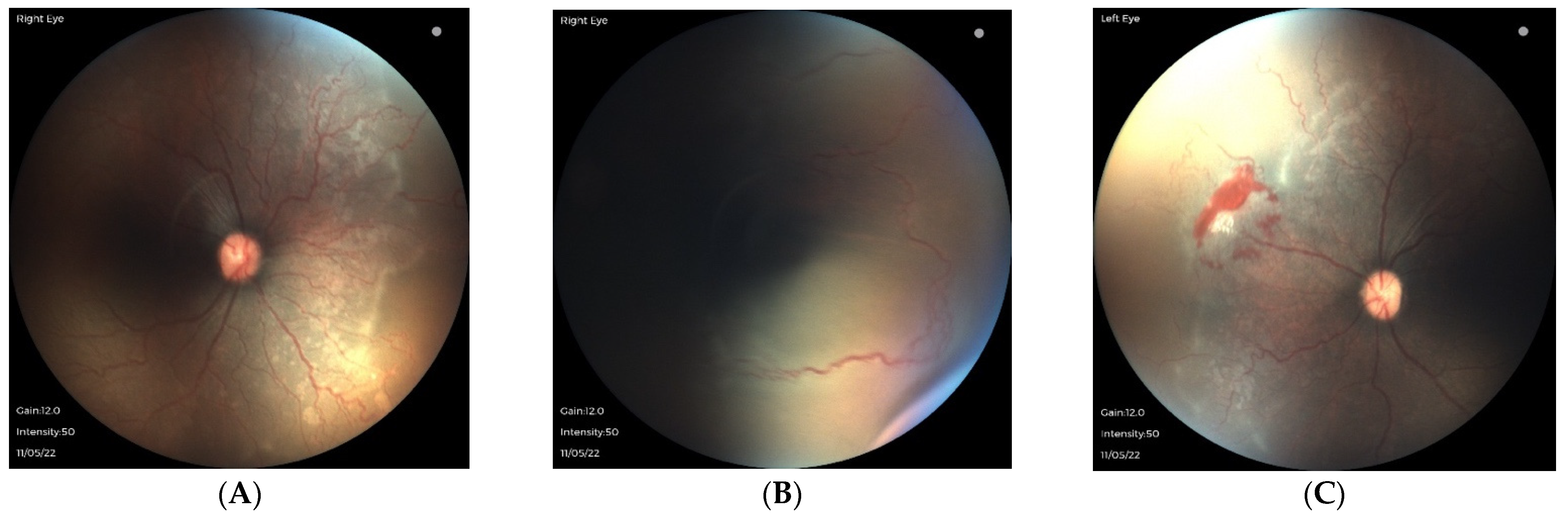
3.2. Oxygen-Associated ROP Seen in Larger Preterm Infants
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Terry, T.L. Fibroblastic overgrowth of persistent tunica vasculosa lentis in infants born prematurely: II. Report of clinical aspects. Trans. Am. Ophthalmol. Soc. 1942, 40, 262–284. [Google Scholar] [PubMed]
- Gilbert, C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum. Dev. 2008, 84, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia; a clinical approach. Med. J. Aust. 1951, 2, 48–50. [Google Scholar] [CrossRef] [PubMed]
- Patz, A.H.L.; De La Cruz, E. Studies on the effect of high oxygen administration in retrolental fibroplasia. I. Nursery observations. Am. J. Ophthalmol. 1952, 35, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, V.E. Retrolental fibroplasia: Cooperative study of retrolental fibroplasia and the use of oxygen. AMA Arch. Ophthalmol. 1956, 56, 481–543. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, M.E.; Lane, R.H. Effects of oxygen on the development and severity of retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2013, 17, 229–234. [Google Scholar] [CrossRef]
- Hellstrom, A.; Smith, L.; Dammann, O. Retinopathy of prematurity. Lancet 2013, 382, 1445–1457. [Google Scholar] [CrossRef]
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch. Ophthalmol. 1984, 102, 1130–1134. [Google Scholar] [CrossRef]
- Shah, P.K.; Prabhu, V.; Karandikar, S.; Ranjan, R.; Narendran, V.; Kalpana, N. Retinopathy of prematurity: Past, present, and future. World J. Clin. Pediatr. 2016, 5, 35–46. [Google Scholar] [CrossRef]
- Sabri, K.; Ells, A.L.; Lee, E.Y.; Dutta, S.; Vinekar, A. Retinopathy of Prematurity: A Global Perspective and Recent Developments. Pediatrics 2022, 150, e2021053924. [Google Scholar] [CrossRef]
- Blencowe, H.; Lawn, J.E.; Vazquez, T.; Fielder, A.; Gilbert, C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at reginal and global levels for 2010. Pediatr. Res. 2013, 74, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Malik, A.; Nahar, N.; Das, W.K.; Visser, L.; Sitati, S.; Ademola-Popoola, D.S. Epidemiology of ROP update—Africa is the new frontier. Semin. Perinatol. 2019, 43, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Romero, L.C.; Lundgren, P.; Gutierrez-Padilla, J.A.; Gomez-Ruiz, L.M.; Corona, M.Q.; Orozco-Monroy, J.V.; Barragan-Sánchez, A.; Razo-Cervantes, J.C.; Löfqvist, C.; Hård, A.L.; et al. Oxygen monitoring reduces the risk for retinopathy of prematurity in a Mexican population. Neonatology 2016, 110, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, L.; Villanueva, A.M.; Quinn, G.E. A practical method for reducing blindness due to retinopathy of prematurity in a developing country. J. Perinat. Med. 2012, 40, 577–582. [Google Scholar] [CrossRef]
- Hariharan, L.; Gilbert, C.E.; Quinn, G.E.; Barg, F.K.; Lomuto, C.; Quiroga, A.; McLeod-Omawale, J.; Zin, A.; Ortiz, Z.; Alda, E.; et al. Reducing blindness from retinopathy of prematurity (ROP) in Argentina through collaboration, advocacy and policy implementation. Health Policy Plan. 2018, 1, 654–665. [Google Scholar] [CrossRef]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, reginal, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health. 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Lloyd, T.; Isenberg, S.J.; Lambert, S. Current management of retinopathy of prematurity in sub-Saharan Africa. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, 151.e1–151.e6. [Google Scholar] [CrossRef]
- Herrod, S.K.; Stevenson, A.; Vaucher, Y.E.; Lambert, S.R.; Isenberg, S.J.; Yap, V.L.; Ezeaka, V.C.; Carlo, W.A. Oxygen management among infants in neonatal units in sub-Saharan Africa: A cross-sectional survey. J. Perinatol. 2021, 41, 2631–2638. [Google Scholar] [CrossRef]
- Herrod, S.; Adio, A.; Isenberg, S.; Lambert, S. Blindness secondary to retinopathy of prematurity in sub-Saharan Africa. Ophthalmic Epidemiol. 2022, 29, 156–163. [Google Scholar] [CrossRef]
- Shah, P.K.; Narendran, V.; Kalpana, N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch. Dis. Child. Fetal. Neonatal. Ed. 2012, 97, F371–F375. [Google Scholar] [CrossRef]
- Hartnett, M.E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 2015, 122, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.; Ward, B.; Serpell, G. Role of oxygen in the genesis of retrolental fibroplasia; a preliminary report. Br. J. Ophthalmol. 1953, 37, 513–520. [Google Scholar] [CrossRef] [PubMed]
- McLeod, D.S.; Brownstein, R.; Lutty, G.A. Vaso-obliteration in the canine model of oxygen-induced retinopathy. IOVS 1996, 37, 300–311. [Google Scholar]
- Nicolaides, K.H.; Economides, D.L.; Soothill, P.W. Blood gases, pH, and lactate in appropriate- and small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 1989, 161, 996–1001. [Google Scholar] [CrossRef]
- Chen, J.; Smith, L.E. Retinopathy of prematurity. Angiogenesis 2007, 10, 133–140. [Google Scholar] [CrossRef]
- Hartnett, M.E. Advances in understanding retinopathy of prematurity. Surv. Ophthalmol. 2017, 62, 257–276. [Google Scholar] [CrossRef]
- Bolton, D.P.; Cross, K.W. Further observations on cost of preventing retrolental fibroplasia. Lancet 1974, 1, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Askie, L.M.; Darlow, B.A.; Finer, N.; Schmidt, B.; Stenson, B.; Tarnow-Mordi, W.; Davis, P.G.; Carlo, W.A.; Brocklehurst, P.; Davies, L.C.; et al. Neonatal Oxygenation Prospective Meta-analysis (NeOProM) Collaboration. Association between oxygen saturation targetting and death or disability in extremely preterm infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA 2018, 319, 2190–2201. [Google Scholar] [CrossRef]
- Saugstad, O.D.; Aune, D. Optimal Oxygenation of Extremely Low Birth Weight Infants: A Meta-Analysis and Systematic Review of Oxygen Saturation Targets. Neonatology 2014, 105, 55–63. [Google Scholar] [CrossRef]
- Gilbert, C. Retinopathy of prematurity: Epidemiology. J. Community Eye Health 1997, 10, 22–24. [Google Scholar]
- Good, W.V.; Hardy, R.J.; Dobson, V.; Palmer, E.A.; Phelps, D.L.; Quintos, M.; Tung, B. The incidence and course of retinopathy of prematurity: Findings from the early treatment for retinopathy of prematurity study. Pediatrics 2005, 116, 15–23. [Google Scholar] [PubMed]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: Preliminary results. Pediatrics 1988, 81, 697–706. [Google Scholar] [CrossRef]
- Hunter, D.G.; Repka, M.X. Diode laser photocoagulation for threshold retinopathy of prematurity: A randomized study. Ophthalmology 1993, 100, 238–244. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.L.; Wherley, A.J.; Fellows, R.R.; Bremer, D.L.; Rogers, G.L.; Letson, A.D. A comparison of cryotherapy versus diode laser retinopexy in 100 consecutive infants treated for threshold retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 1998, 2, 360–364. [Google Scholar] [CrossRef]
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: Results of the early treatment for retinopathy of prematurity randomized trial. Arch. Ophthalmol. 2003, 121, 1684–1696. [Google Scholar] [CrossRef]
- Mintz-Hittner, H.; Kennedy, K.A.; Chuang, A.Z. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N. Engl. J. Med. 2011, 364, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.P.; Garcia Gonzalez, J.M.; Snyder, L.; Schechet, S.; Greenwald, M.J.; Shapiro, M.J.; Rodriguez, S.H. Bevacizumab or laser for aggressive posterior retinopathy of prematurity. Taiwan J. Ophthalmol. 2018, 8, 243–248. [Google Scholar] [CrossRef]
- Geloneck, M.M.; Chuang, A.Z.; Clark, W.L.; Hunt, M.G.; Norman, A.A.; Packwood, E.A.; Tawansy, K.A.; Mintz-Hittner, H.A.; BEAT-ROP cooperative group. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: A randomized clinical trial. JAMA Ophthalmol. 2014, 132, 1327–1333. [Google Scholar] [CrossRef]
- Rodriguez, S.H.; Schechet, S.; Shapiro, M.J.; Blair, M.P. Late visual outcomes in infants treated with primary bevacizumab for type 1 retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2020, 24, 149.e1–149.e5. [Google Scholar] [CrossRef]
- Anand, N.; Blair, M.P.; Greenwald, M.J.; Rodriguez, S.H. Refractive outcomes comparing primary laser to primary bevacizumab with delayed laser for type 1 ROP. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2019, 23, 88.e1–88.e6. [Google Scholar] [CrossRef]
- Fierson, W.M. American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2018, 142, e20183061. [Google Scholar] [PubMed]
- Hajrasouliha, A.R.; Garcia Gonzalez, J.M.; Shapiro, M.J.; Yoon, H.; Blair, M.P. Reactivation of retinopathy of prematurity three years after treatment with bevacizumab. Ophthalmic. Surg. Lasers Imaging Retina 2017, 48, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Blair, M.P.; Shapiro, M.J.; Lichenstein, S.J.; Galasso, F.M.; Kapur, R. Reactivation of retinopathy of prematurity after bevacizumab injection. JAMA Ophthalmol. 2012, 130, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Ittiara, S.; Blair, M.P.; Shapiro, M.J.; Lichtenstein, S.J. Exudative retinopathy and detachment: A late reactivation of retinopathy of prematurity after intravitreal bevacizumab. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2013, 17, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.L.; Garcia Gonzalez, J.M.; Shapiro, M.J.; Blair, M.P. Very late reactivation of retinopathy of prematurity after monotherapy with intravitreal bevacizumab. Ophthalmic. Surg. Lasers Imaging Retina 2016, 47, 280–283. [Google Scholar] [CrossRef]
- Taylor, K.; Ghergherehchi, L.; Rao, P.; Harper, C.A., 3rd; Chang, E. Very late-onset reactivation of retinopathy of prematurity post anti-VEGF bevacizumab treatment for type 1 ROP: A case report. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2021, 25, 180–184. [Google Scholar] [CrossRef]
- Yonekawa, Y.; Wu, W.C.; Nitulescu, C.E.; Chan, R.V.P.; Thanos, A.; Thomas, B.J.; Todorich, B.; Drenser, K.A.; Trese, M.T.; Capone, A., Jr. Progressive retinal detachment in infants with retinopathy of prematurity treated with intravitreal bevacizumab or ranibizumab. Retina 2018, 38, 1079–1083. [Google Scholar] [CrossRef]
- Garcia Gonzalez, J.M.; Snyder, L.; Blair, M.; Rohr, A.; Shapiro, M.; Greenwald, M. Prophylactic peripheral laser and fluorescein angiography after bevacizumab for retinopathy of prematurity. Retina 2018, 38, 764–772. [Google Scholar] [CrossRef]
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch. Ophthalmol. 2005, 123, 991–999. [Google Scholar] [CrossRef]
- Fielder, A.; Blencowe, H.; O’Connor, A.; Gilbert, C. Impact of retinopathy of prematurity on ocular structures and visual functions. Arch. Dis. Child. Fetal. Neonatal. Ed. 2015, 100, F79–F84. [Google Scholar] [CrossRef]
- Gilbert, C.; Fielder, A.; Gordillo, L.; Quinn, G.; Semiglia, R.; Visintin, P.; Zin, A.; International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate and high levels of development: Implications for screening programs. Pediatrics 2005, 115, e518–e525. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.F.; Ostmo, S.R.; Chan, R.P.; Berrocal, A.; Binenbaum, G.; Blair, M.; Campbell, J.P.; Capone, A., Jr.; et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Castellanos, M.A.; Velez-Montoya, R.; Price, K.; Henaine-Berra, A.; García-Aguirre, G.; Morales-Canton, V.; Cernichiaro-Espinosa, L.A. Vascular changes on fluorescein angiography of premature infants with low risk of retinopathy of prematurity after high oxygen exposure. Int. J. Retin. Vitr. 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Blair, M.P.; Rodriguez, S.H.; Schechet, S.A.; Shapiro, M.J. Aggressive posteiror retinopathy of prematurity. In A Quick Guide to Pediatric Retina; Spring Nature: Singapore, 2021. [Google Scholar]
- Szewczyk, T.S. Retrolental fribroplasia: Etiology and prophylaxis. Am. J. Ophthalmol. 1952, 35, 301–310. [Google Scholar] [CrossRef] [PubMed]
- STOP-ROP Multicenter Study Group. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: Primary outcomes. Pediatrics 2000, 105, 295–310. [Google Scholar] [CrossRef]
- Sears, J.E.; Pietz, J.; Sonnie, C.; Dolcini, D.; Hoppe, G. A change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology 2009, 116, 513–518. [Google Scholar] [CrossRef]
- Colaizy, T.T.; Longmuir, S.; Gertsch, K.; Abramoff, M.D.; Klein, J.K. Use of a supplemental oxygen protocol to suppress progression of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 2017, 58, 887–891. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, S.H.; Ells, A.L.; Blair, M.P.; Shah, P.K.; Harper, C.A., 3rd; Martinez-Castellanos, M.A.; Prakalapakorn, S.G.; Denis, E.; Lusobya, R.C.; Greenwald, M.J.; et al. Retinopathy of Prematurity in the 21st Century and the Complex Impact of Supplemental Oxygen. J. Clin. Med. 2023, 12, 1228. https://doi.org/10.3390/jcm12031228
Rodriguez SH, Ells AL, Blair MP, Shah PK, Harper CA 3rd, Martinez-Castellanos MA, Prakalapakorn SG, Denis E, Lusobya RC, Greenwald MJ, et al. Retinopathy of Prematurity in the 21st Century and the Complex Impact of Supplemental Oxygen. Journal of Clinical Medicine. 2023; 12(3):1228. https://doi.org/10.3390/jcm12031228
Chicago/Turabian StyleRodriguez, Sarah H., Anna L. Ells, Michael P. Blair, Parag K. Shah, C. Armitage Harper, 3rd, Maria Ana Martinez-Castellanos, S. Grace Prakalapakorn, Erima Denis, Rebecca C. Lusobya, Mark J. Greenwald, and et al. 2023. "Retinopathy of Prematurity in the 21st Century and the Complex Impact of Supplemental Oxygen" Journal of Clinical Medicine 12, no. 3: 1228. https://doi.org/10.3390/jcm12031228
APA StyleRodriguez, S. H., Ells, A. L., Blair, M. P., Shah, P. K., Harper, C. A., 3rd, Martinez-Castellanos, M. A., Prakalapakorn, S. G., Denis, E., Lusobya, R. C., Greenwald, M. J., Isenberg, S. J., Lambert, S. R., Vaucher, Y. E., Carroll, A., & Namakula, L. (2023). Retinopathy of Prematurity in the 21st Century and the Complex Impact of Supplemental Oxygen. Journal of Clinical Medicine, 12(3), 1228. https://doi.org/10.3390/jcm12031228




