The Relative Contributions of Occupational and Community Risk Factors for COVID-19 among Hospital Workers: The HOP-COVID Cohort Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Participants
2.2. Data Collection and Study Variables
2.3. Outcome
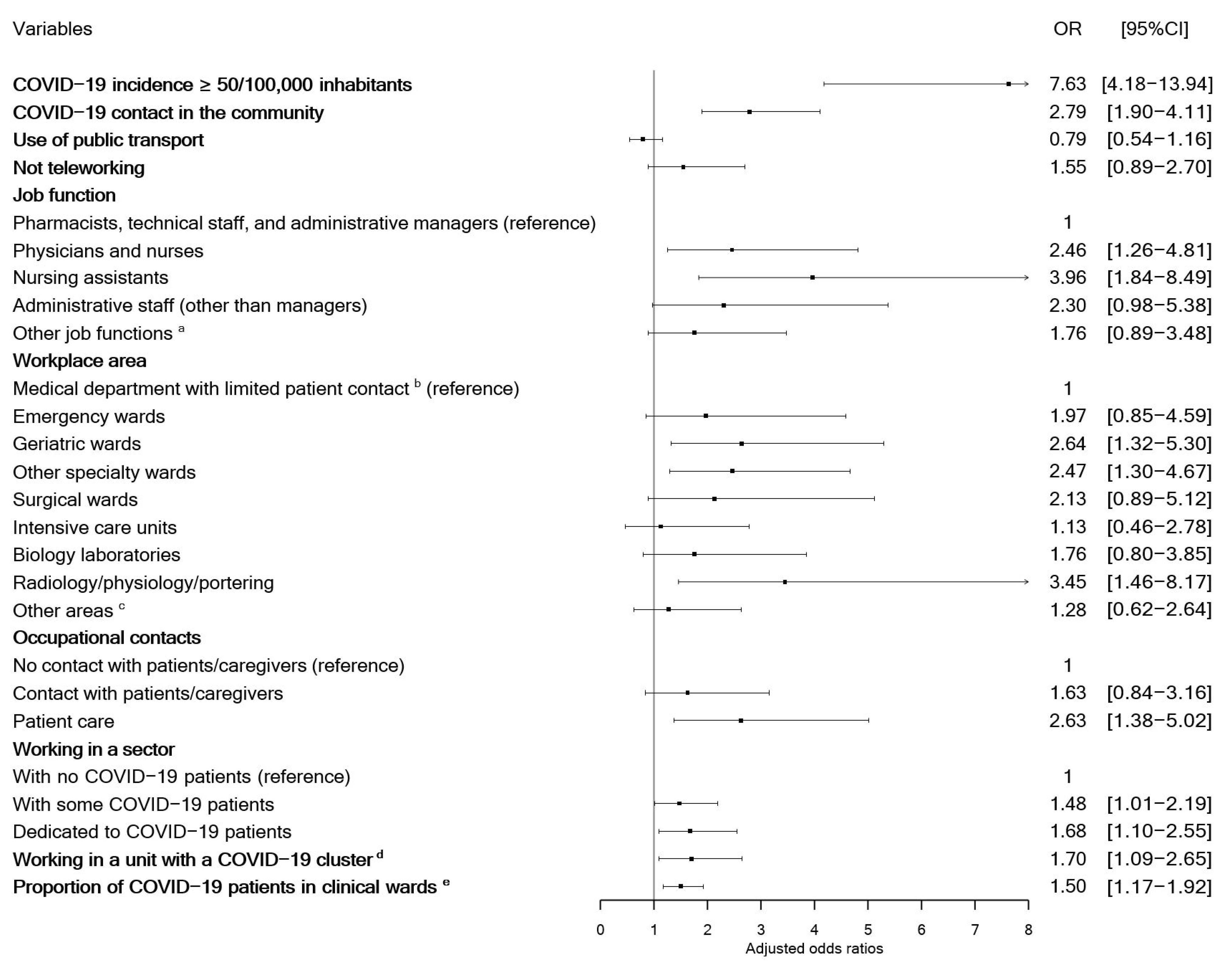
2.4. Statistical Analysis
3. Results
3.1. Participants
3.2. Outcome
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferland, L.; Carvalho, C.; Gomes Dias, J.; Lamb, F.; Adlhoch, C.; Suetens, C.; Beauté, J.; Kinross, P.; Plachouras, D.; Hannila-Handelberg, T.; et al. Risk of hospitalization and death for healthcare workers with COVID-19 in nine European countries, January 2020-January 2021. J. Hosp. Infect. 2022, 119, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef] [PubMed]
- Sikkema, R.S.; Pas, S.D.; Nieuwenhuijse, D.F.; O’Toole, Á.; Verweij, J.; van der Linden, A.; Chestakova, I.; Schapendonk, C.; Pronk, M.; Lexmond, P.; et al. COVID-19 in health-care workers in three hospitals in the south of the Netherlands: A cross-sectional study. Lancet. Infect. Dis. 2020, 20, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.M.; Moreno, G.K.; Buys, A.; Somsen, E.D.; Bobholz, M.; Accola, M.A.; Anderson, L.; Rehrauer, W.M.; Baker, D.A.; Safdar, N.; et al. Viral Sequencing to Investigate Sources of SARS-CoV-2 Infection in US Healthcare Personnel. Clin. Infect. Dis. Publ. Infect. Dis. Soc. Am. 2021, 73, e1329–e1336. [Google Scholar] [CrossRef]
- Sikkens, J.J.; Buis, D.T.P.; Peters, E.J.G.; Dekker, M.; Schinkel, M.; Reijnders, T.D.Y.; Schuurman, A.R.; de Brabander, J.; Lavell, A.H.A.; Maas, J.J.; et al. Serologic Surveillance and Phylogenetic Analysis of SARS-CoV-2 Infection Among Hospital Health Care Workers. JAMA Netw. Open 2021, 4, e2118554. [Google Scholar] [CrossRef]
- Jacob, J.T.; Baker, J.M.; Fridkin, S.K.; Lopman, B.A.; Steinberg, J.P.; Christenson, R.H.; King, B.; Leekha, S.; O’Hara, L.M.; Rock, P.; et al. Risk Factors Associated With SARS-CoV-2 Seropositivity Among US Health Care Personnel. JAMA Netw. Open 2021, 4, e211283. [Google Scholar] [CrossRef]
- Elfström, K.M.; Blomqvist, J.; Nilsson, P.; Hober, S.; Pin, E.; Månberg, A.; Pimenoff, V.N.; Arroyo Mühr, L.S.; Lundgren, K.C.; Dillner, J. Differences in risk for SARS-CoV-2 infection among healthcare workers. Prev. Med. Rep. 2021, 24, 101518. [Google Scholar] [CrossRef]
- Iversen, K.; Bundgaard, H.; Hasselbalch, R.B.; Kristensen, J.H.; Nielsen, P.B.; Pries-Heje, M.; Knudsen, A.D.; Christensen, C.E.; Fogh, K.; Norsk, J.B.; et al. Risk of COVID-19 in health-care workers in Denmark: An observational cohort study. Lancet. Infect. Dis. 2020, 20, 1401–1408. [Google Scholar] [CrossRef]
- Ochoa-Leite, C.; Bento, J.; Rocha, D.R.; Vasques, I.; Cunha, R.; Oliveira, Á.; Rocha, L. Occupational management of healthcare workers exposed to COVID-19. Occup. Med. 2021, 71, 359–365. [Google Scholar] [CrossRef]
- Zabarsky, T.F.; Bhullar, D.; Silva, S.Y.; Mana, T.S.C.; Ertle, M.T.; Navas, M.E.; Donskey, C.J. What are the sources of exposure in healthcare personnel with coronavirus disease 2019 infection? Am. J. Infect. Control. 2021, 49, 392–395. [Google Scholar] [CrossRef]
- Baker, J.M.; Nelson, K.N.; Overton, E.; Lopman, B.A.; Lash, T.L.; Photakis, M.; Jacob, J.T.; Roback, J.D.; Fridkin, S.K.; Steinberg, J.P. Quantification of Occupational and Community Risk Factors for SARS-CoV-2 Seropositivity Among Health Care Workers in a Large U.S. Health Care System. Ann. Intern. Med. 2021, 174, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Dana, T.; Buckley, D.I.; Selph, S.; Fu, R.; Totten, A.M. Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers: A Living Rapid Review. Ann. Intern. Med. 2020, 173, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, C.R.; Persi, R.; Güsewell, S.; Egger, T.; Leal-Neto, O.B.; Sumer, J.; Flury, D.; Brucher, A.; Lemmenmeier, E.; Möller, J.C.; et al. Non-occupational and occupational factors associated with specific SARS-CoV-2 antibodies among hospital workers—A multicentre cross-sectional study. Clin. Microbiol. Infect. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021, 27, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, M.M.; El-Saed, A.; Arabi, Y.M.; Zunitan, M.A.; Farahat, F.M.; Bonnie, H.B.; Matalqa, M.; Othman, F.; Almohrij, S. Risk of COVID-19 in healthcare workers working in intensive care setting. Am. J. Infect. Control. 2022, 50, 988–993. [Google Scholar] [CrossRef]
- Hausfater, P.; Boutolleau, D.; Lacombe, K.; Beurton, A.; Dumont, M.; Constantin, J.M.; Ghosn, J.; Combes, A.; Cury, N.; Guedj, R.; et al. Cumulative incidence of SARS-CoV-2 infection and associated risk factors among frontline health care workers in Paris: The SEROCOV cohort study. Sci. Rep. 2022, 12, 7211. [Google Scholar] [CrossRef] [PubMed]
- Wiggen, T.D.; Bohn, B.; Ulrich, A.K.; Stovitz, S.D.; Strickland, A.J.; Naumchik, B.M.; Walsh, S.; Smith, S.; Baumgartner, B.; Kline, S.; et al. SARS-CoV-2 seroprevalence among healthcare workers. PLoS One 2022, 17, e0266410. [Google Scholar] [CrossRef] [PubMed]
- Harith, A.A.; Ab Gani, M.H.; Griffiths, R.; Abdul Hadi, A.; Abu Bakar, N.A.; Myers, J.; Mahjom, M.; Robat, R.M.; Zubir, M.Z. Incidence, Prevalence, and Sources of COVID-19 Infection among Healthcare Workers in Hospitals in Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 12485. [Google Scholar] [CrossRef]
- Mandic-Rajcevic, S.; Masci, F.; Crespi, E.; Franchetti, S.; Longo, A.; Bollina, I.; Velocci, S.; Amorosi, A.; Baldelli, R.; Boselli, L.; et al. Source and symptoms of COVID-19 among hospital workers in Milan. Occup. Med. 2020, 70, 672–679. [Google Scholar] [CrossRef]
- Mihai, A.M.; Barben, J.; Dipanda, M.; Vovelle, J.; Nuss, V.; Baudin-Senegas, C.; Putot, A.; Manckoundia, P. Analysis of COVID-19 in Professionals Working in Geriatric Environment: Multicenter Prospective Study. Int. J. Environ. Res. Public Health 2021, 18, 9735. [Google Scholar] [CrossRef]
- Feaster, M.; Goh, Y.Y. High Proportion of Asymptomatic SARS-CoV-2 Infections in 9 Long-Term Care Facilities, Pasadena, California, USA, April 2020. Emerg. Infect. Dis. 2020, 26, 2416–2419. [Google Scholar] [CrossRef]
- Höring, S.; Fussen, R.; Neusser, J.; Kleines, M.; Laurentius, T.; Bollheimer, L.C.; Keller, D.; Lemmen, S. Management of a Hospital-Wide COVID-19 Outbreak Affecting Patients and Healthcare Workers. SN Compr. Clin. Med. 2020, 2, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Ouslander, J.G.; Grabowski, D.C. COVID-19 in Nursing Homes: Calming the Perfect Storm. J. Am. Geriatr. Soc. 2020, 68, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Mas Romero, M.; Avendaño Céspedes, A.; Tabernero Sahuquillo, M.T.; Cortés Zamora, E.B.; Gómez Ballesteros, C.; Sánchez-Flor Alfaro, V.; López Bru, R.; López Utiel, M.; Celaya Cifuentes, S.; Peña Longobardo, L.M.; et al. COVID-19 outbreak in long-term care facilities from Spain. Many lessons to learn. PLoS ONE 2020, 15, e0241030. [Google Scholar] [CrossRef]
- Mody, L.; Akinboyo, I.C.; Babcock, H.M.; Bischoff, W.E.; Cheng, V.C.; Chiotos, K.; Claeys, K.C.; Coffey, K.C.; Diekema, D.J.; Donskey, C.J.; et al. Coronavirus disease 2019 (COVID-19) research agenda for healthcare epidemiology. Infect. Control. Hosp. Epidemiol. 2022, 43, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Galanis, P.; Vraka, I.; Fragkou, D.; Bilali, A.; Kaitelidou, D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: A systematic review and meta-analysis. J. Hosp. Infect. 2021, 108, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, M.M.; El-Saed, A.; Al Zunitan, M.; Almulhem, R.; Almohrij, S. Risk of COVID-19 morbidity and mortality among healthcare workers working in a Large Tertiary Care Hospital. Int. J. Infect. Dis. IJID Publ. Int. Soc. Infect. Dis. 2021, 109, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Dana, T.; Buckley, D.I.; Selph, S.; Fu, R.; Totten, A.M. Update Alert 10: Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers. Ann. Intern. Med. 2022, 175, W8–W9. [Google Scholar] [CrossRef] [PubMed]
- Brousseau, N.; Morin, L.; Ouakki, M.; Savard, P.; Quach, C.; Longtin, Y.; Cheng, M.P.; Carignan, A.; Dufresne, S.F.; Leduc, J.M.; et al. SARS-CoV-2 seroprevalence in health care workers from 10 hospitals in Quebec, Canada: A cross-sectional study. CMAJ Can. Med. Assoc. J. = J. De L’association Med. Can. 2021, 193, E1868–E1877. [Google Scholar] [CrossRef]
- Barrett, E.S.; Horton, D.B.; Roy, J.; Gennaro, M.L.; Brooks, A.; Tischfield, J.; Greenberg, P.; Andrews, T.; Jagpal, S.; Reilly, N.; et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers in New Jersey, at the onset of the U.S. COVID-19 pandemic. BMC Infect. Dis. 2020, 20, 853. [Google Scholar] [CrossRef]
- Sims, M.D.; Maine, G.N.; Childers, K.L.; Podolsky, R.H.; Voss, D.R.; Berkiw-Scenna, N.; Oh, J.; Heinrich, K.E.; Keil, H.; Kennedy, R.H.; et al. Coronavirus Disease 2019 (COVID-19) Seropositivity and Asymptomatic Rates in Healthcare Workers Are Associated with Job Function and Masking. Clin. Infect. Dis. Publ. Infect. Dis. Soc. Am. 2021, 73, S154–S162. [Google Scholar] [CrossRef]
- Poletti, P.; Tirani, M.; Cereda, D.; Guzzetta, G.; Trentini, F.; Marziano, V.; Toso, C.; Piatti, A.; Piccarreta, R.; Melegaro, A.; et al. Seroprevalence of and Risk Factors Associated With SARS-CoV-2 Infection in Health Care Workers During the Early COVID-19 Pandemic in Italy. JAMA Netw. Open 2021, 4, e2115699. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.S.; Horton, D.B.; Roy, J.; Xia, W.; Greenberg, P.; Andrews, T.; Gennaro, M.L.; Parmar, V.; Russell, W.D.; Reilly, N.; et al. Risk Factors for Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Hospital Workers: Results From a Screening Study in New Jersey, United States in Spring 2020. Open Forum Infect. Dis. 2020, 7, ofaa534. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.F.; Giavina-Bianchi, P.; Buss, L.; Mesquita Peres, C.H.; Rafael, M.M.; dos Santos, L.G.N.; Bedin, A.A.; Francisco, M.C.P.B.; Satakie, F.M.; Jesus Menezes, M.A.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Seroprevalence and Risk Factors Among Oligo/Asymptomatic Healthcare Workers: Estimating the Impact of Community Transmission. Clin. Infect. Dis. Publ. Infect. Dis. Soc. Am. 2020, 73, e1214–e1218. [Google Scholar] [CrossRef] [PubMed]
- Machnicki, S.; Patel, D.; Singh, A.; Talwar, A.; Mina, B.; Oks, M.; Makkar, P.; Naidich, D.; Mehta, A.; Hill, N.S.; et al. The Usefulness of Chest CT Imaging in Patients with Suspected or Diagnosed COVID-19: A Review of Literature. Chest 2021, 160, 652–670. [Google Scholar] [CrossRef]

| Total | Diagnosis of COVID-19 | |||
|---|---|---|---|---|
| Definite | Probable | p-Values a | ||
| N = 213 | N = 175 | N = 38 | ||
| Fever and respiratory symptoms | 93 (43.9) | 76 (43.7) | 17 (44.7) | 0.91 |
| Anosmia and/or ageusia | 89 (42.0) | 76 (43.7) | 13 (34.2) | 0.28 |
| Other symptoms b | 159 (74.6) | 127 (72.6) | 32 (84.2) | 0.15 |
| SARS-CoV-2 RT-PCR test | ||||
| Not performed | 49 (23.0) | 30 (17.1) | 19 (50.0) | - |
| Not helpful c | 26 (12.2) | 7 (4.0) | 19 (50.0) | |
| Negative d | 3 (1.4) | 3 (1.7) | 0 (-) | |
| Positive | 135 (63.4) | 135 (77.1) | 0 (-) | |
| SARS-CoV-2 serology test | ||||
| Not performed | 84 (39.4) | 52 (29.7) | 32 (84.2) | - |
| Not helpful e | 58 (27.2) | 52 (29.7) | 6 (15.8) | |
| Negative | 6 (2.8) | 6 (3.4) | 0 (-) | |
| Positive | 65 (30.5) | 65 (37.1) | 0 (-) | |
| Patient classification | ||||
| Positive RT-PCR test | 110 (51.6) | 110 (62.9) | 0 (-) | - |
| Positive RT-PCR and serology tests | 25 (11.7) | 25 (14.3) | 0 (-) | |
| Positive serology test | 40 (18.8) | 40 (22.9) | 0 (-) | |
| Clinical diagnosis/sick leave | 38 (17.8 | 0 (-) | 38 (100) | |
| COVID-19 acquisition | 0.59 | |||
| Probably hospital-acquired | 121 (56.8) | 99 (56.6) | 22 (57.9) | |
| Probably community-acquired | 51 (23.9) | 44 (25.1) | 7 (18.4) | |
| Indefinite f | 41 (19.3) | 32 (18.3) | 9 (23.7) | |
| Diagnosis of COVID-19 | |||||
|---|---|---|---|---|---|
| Total | No | Definite/Probable | p | ||
| Characteristics | (n = 1369) a | (n = 1156) b | (n = 213) b | Value c | OR [95% CI] |
| Recruitment period | 0.003 | ||||
| 18 May 2020–5 November 2020 | 809 (59.1) | 700 (86.5) | 109 (13.5) | 1.4 [0.7–2.8] | |
| 6 November 2020–28 February 2021 | 471 (34.4) | 376 (79.8) | 95 (20.2) | 2.3 [1.1–4.6] | |
| 1 March 2021–16 July 2021 | 89 (6.5) | 80 (89.9) | 9 (10.1) | Ref. | |
| Hospital (n = 1368, 1155/213) | <0.001 | ||||
| Henri Mondor | 1000 (73.1) | 877 (87.7) | 123 (12.2) | 0.8 [0.3–1.7] | |
| Albert Chenevier | 105 (7.7) | 93 (88.6) | 12 (11.4) | Ref. | |
| Emile Roux (geriatric hospital) | 123 (9.0) | 83 (67.5) | 40 (32.5) | 3.4 [2.3–5.2] | |
| Joffre-Dupuytren (geriatric hospital) | 68 (5.0) | 43 (63.2) | 25 (36.8) | 4.2 [2.5–7.0] | |
| Georges Clemenceau (geriatric hospital) | 72 (5.3) | 59 (81.9) | 13 (18.1) | 1.4 [0.5–3.6] | |
| Regional daily COVID-19 incidence per 100,000 inhabitants | <0.001 | ||||
| <50 | 392 (28.6) | 379 (96.7) | 13 (3.3) | Ref. | |
| 50 to 149 | 157 (11.5) | 130 (82.8) | 27 (17.2) | 6.1 [3.0–12.1] | |
| 150 to 249 | 140 (10.2) | 120 (85.7) | 20 (14.3) | 4.9 [2.4–10.1] | |
| ≥250 | 246 (18.0) | 194 (78.9) | 52 (21.1) | 7.8 [4.1–14.6] | |
| Before data were available (May 2020) | 434 (31.7) | 333 (76.7) | 101 (23.3) | 8.8 [4.9–16.1] | |
| Age, median [IQR], years (n = 1368, 1155/213) | 43 [32–53] | 43 [32–53] | 43 [33–53] | 0.68 | - |
| Sex | 0.46 | ||||
| Male | 375 (27.4) | 321 (85.6) | 54 (14.4) | ||
| Female | 994 (72.6) | 835 (84.0) | 159 (16.0) | - | |
| Blood group (n = 1221, 1036/185) | 0.31 | ||||
| O | 517 (42.3) | 445 (86.1) | 72 (13.9) | - | |
| A, B or AB | 704 (57.7) | 591 (83.9) | 113 (16.0) | ||
| Living with children attending primary school (n = 1361, 1149/212) | 0.22 | ||||
| No | 971 (71.4) | 827 (85.2) | 144 (14.8) | - | |
| Yes | 389 (28.6) | 321 (82.6) | 68 (17.5) | ||
| Living with children attending junior high or high school (n = 1361, 1149/212) | 0.53 | ||||
| No | 964 (70.9) | 810 (84.0) | 154 (16.0) | . | - |
| Yes | 396 (29.1) | 338 (85.4) | 58 (14.6) | ||
| Use of public transport (n = 1368, 1155/213) | 0.15 | ||||
| No | 1010 (73.9) | 844 (83.6) | 166 (16.4) | Ref. | |
| Yes | 357 (26.1) | 310 (86.8) | 47 (13.2) | 0.8 [0.5–1.1] | |
| COVID-19 contact in the community (n = 1352,1139/213) | <0.001 | ||||
| No | 1167 (86.3) | 1012 (86.7) | 155 (13.28) | Ref. | |
| Yes | 185 (13.7) | 127 (68.65) | 58 (31.35) | 3.0 [2.1–4.3] | |
| Diagnosis of COVID-19 | |||||
|---|---|---|---|---|---|
| Total | No | Definite/Probable | p | ||
| Characteristics | (n = 1369) a | (n=1156) b | (n = 213) b | Value c | OR [95% CI] |
| Activity (n = 1359, 1146/213) | 0.06 | ||||
| Teleworking | 49 (3.6) | 45 (91.8) | 4 (8.2) | Ref. | |
| Alternate teleworking and in-hospital work | 127 (9.4) | 114 (89.8) | 13 (10.2) | ||
| In-hospital work | 1183 (87.0) | 987 (83.4) | 196 (16.6) | 1.9 [1.1–3.1] | |
| Job function (n = 1367, 1154/213) | 0.003 | ||||
| Physicians | 374 (27.4) | 311 (83.2) | 63 (16.8) | 2.2 [1.2–4.1] | |
| Nurses | 157 (11.5) | 131 (83.4) | 26 (16.8) | ||
| Nursing assistants | 115 (8.4) | 83 (72.2) | 32 (27.8) | 4.1 [2.0–8.5] | |
| Nurse managers | 102 (7.5) | 90 (88.2) | 12 (11.8) | 1.6 [0.9–3.1] | |
| Allied health professionals d | 101 (7.4) | 88 (87.1) | 13 (12.9) | ||
| Medical and other students | 94 (6.9) | 83 (88.3) | 11 (11.7) | ||
| Laboratory technicians | 57 (4.2) | 49 (86.0) | 8 (14.0) | ||
| Administrative staff in clinical units | 73 (5.3) | 62 (84.9) | 11 (15.1) | ||
| Researchers and research support staff | 63 (4.6) | 54 (85.7) | 9 (14.3) | ||
| Pharmacists | 30 (2.2) | 28 (93.3) | 2 (6.7) | Ref. | |
| Technical and administrative unit managers | 61 (4.5) | 56 (91.8) | 5 (8.2) | ||
| Technical staff (other than managers) | 50 (3.7) | 45 (90.0) | 5 (10.0) | ||
| Administrative staff (other than managers) | 90 (6.6) | 74 (82.2) | 16 (17.8) | 2.3 [1.0–5.2] | |
| Workplace area (n = 1319, 1106/213) | 0.02 | ||||
| Emergency departments | 92 (7.0) | 80 (87.0) | 12 (13.0) | 1.3 [0.6–2.9] | |
| Geriatric wards | 210 (15.9) | 150 (71.4) | 60 (28.6) | 3.5 [1.9–6.4] | |
| Other specialty wards | 303 (23.0) | 250 (82.5) | 53 (17.5) | 1.9 [1.1–3.4] | |
| Surgical wards | 71 (5.4) | 60 (84.5) | 11 (15.5) | 1.6 [0.7–3.7] | |
| Intensive care units | 133 (10.1) | 124 (93.2) | 9 (6.8) | 0.6 [0.3–1.5] | |
| Medical biology laboratories | 125 (9.5) | 109 (87.2) | 16 (12.8) | 1.3 [0.6–2.7] | |
| Radiology/physiology/functional assessment facilities | 50 (3.8) | 40 (80.0) | 10 (20.0) | 2.6 [1.1–5.7] | |
| Stretcher services | 8 (0.6) | 5 (62.5) | 3 (37.5) | ||
| Medical departments with limited patient contact e | 157 (11.9) | 141 (89.8) | 16 (10.2) | Ref. | |
| Other services f | 170 (12.9) | 147 (86.5) | 23 (13.5) | 1.4 [0.7–2.7] | |
| Type of geriatric ward (n = 197, 148/49) | 0.90 | ||||
| Acute care ward | 65 (33.0) | 50 (76.9) | 15 (23.1) | - | |
| Rehabilitation ward | 103 (52.3) | 76 (73.8) | 27 (26.2) | ||
| Long-term care ward | 29 (14.7) | 22 (75.9) | 7 (24.1) | ||
| Type of laboratory (n = 124, 109/15) | 0.13 | ||||
| Microbiology | 42 (33.9) | 35 (83.3) | 7 (16.7) | 2.4 [0.8–7.4] | |
| Pathology | 18 (14.5) | 15 (83.3) | 3 (16.7) | ||
| Other | 64 (51.6) | 59 (92.2) | 5 (7.8) | Ref | |
| Contact with patients or caregivers (n = 1358, 1145/213) | 0.006 | ||||
| Patient care | 622 (45.8) | 502 (80.7) | 120 (19.3) | 2.1 [1.2–3.9] | |
| Patient contact without care provision | 255 (18.8) | 218 (85.5) | 37 (14.5) | 1.3 [0.7–2.5] | |
| Contact with caregivers | 355 (26.1) | 310 (87.3) | 43 (12.1) | ||
| No or limited contact with caregivers | 128 (9.4) | 115 (89.8) | 13 (10.2) | Ref. | |
| COVID-19 sectors (n = 1319, 1108/201) | 0.045 | ||||
| No COVID-19 patients | 782 (59.3) | 680 (86.0) | 102 (13.0) | Ref. | |
| Some COVID-19 patients | 288 (21.8) | 236 (81.9) | 52 (18.1) | 1.5 [1.0–2.1] | |
| Dedicated to COVID-19 patients | 249 (18.9) | 202 (81.1) | 47 (18.9) | 1.6 [1.1–2.3] | |
| COVID-19 patient burden, median [IQR] (n = 579, 482/97) g | |||||
| Number of COVID-19 patients | 4 [1–15] | 4 [1–11] | 8 [3–29] | <0.001 | 1.4 [1.1–1.8] |
| Proportion of COVID-19 patients | 6.1 [2–31] | 6 [2–27] | 19.1 [5–51] | <0.001 | 1.5 [1.2–1.9] |
| Working in a sector with COVID-19 clusters (n = 1164, 1156/213) | <0.001 | ||||
| No | 981 (71.7) | 863 (88.0) | 118 (12.0) | Ref. | |
| Cluster among the patients | 29 (2.1) | 18 (62.1) | 11 (37.9) | 2.1 [1.4–3.1] | |
| Cluster among the staff | 67 (4.9) | 55 (82.1) | 12 (17.9) | ||
| Cluster among the patients and staff | 87 (6.4) | 69 (73.9) | 18 (20.7) | ||
| Systematic use of a mask in hospital (n = 1334, 1127/207) | 0.59 | ||||
| No | 143 (10.7) | 123 (86.0) | 20 (14.0) | - | |
| Yes | 1191 (89.3) | 1004 (84.3) | 187 (15.7) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastuji-Garin, S.; Brouard, L.; Bourgeon-Ghittori, I.; Zebachi, S.; Boutin, E.; Hemery, F.; Fourreau, F.; Oubaya, N.; De Roux, Q.; Mongardon, N.; et al. The Relative Contributions of Occupational and Community Risk Factors for COVID-19 among Hospital Workers: The HOP-COVID Cohort Study. J. Clin. Med. 2023, 12, 1208. https://doi.org/10.3390/jcm12031208
Bastuji-Garin S, Brouard L, Bourgeon-Ghittori I, Zebachi S, Boutin E, Hemery F, Fourreau F, Oubaya N, De Roux Q, Mongardon N, et al. The Relative Contributions of Occupational and Community Risk Factors for COVID-19 among Hospital Workers: The HOP-COVID Cohort Study. Journal of Clinical Medicine. 2023; 12(3):1208. https://doi.org/10.3390/jcm12031208
Chicago/Turabian StyleBastuji-Garin, Sylvie, Ludivine Brouard, Irma Bourgeon-Ghittori, Sonia Zebachi, Emmanuelle Boutin, Francois Hemery, Frédéric Fourreau, Nadia Oubaya, Quentin De Roux, Nicolas Mongardon, and et al. 2023. "The Relative Contributions of Occupational and Community Risk Factors for COVID-19 among Hospital Workers: The HOP-COVID Cohort Study" Journal of Clinical Medicine 12, no. 3: 1208. https://doi.org/10.3390/jcm12031208
APA StyleBastuji-Garin, S., Brouard, L., Bourgeon-Ghittori, I., Zebachi, S., Boutin, E., Hemery, F., Fourreau, F., Oubaya, N., De Roux, Q., Mongardon, N., Fourati, S., & Decousser, J.-W. (2023). The Relative Contributions of Occupational and Community Risk Factors for COVID-19 among Hospital Workers: The HOP-COVID Cohort Study. Journal of Clinical Medicine, 12(3), 1208. https://doi.org/10.3390/jcm12031208







