Risk of New-Onset Diabetes Mellitus as a Post-COVID-19 Condition and Possible Mechanisms: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Definitions
- Development of diabetes mellitus at least four weeks after the initial COVID-19 infection.
- Age ≥ 18 years.
- Patients with a pre-existing diagnosis of diabetes or diagnosis of diabetes earlier than four weeks after the initial COVID-19 infection.
- Exclusion criteria were kept to a minimum to review more relevant articles.
2.2. Data Sources and Search Strategy
2.3. Data Extraction
2.4. Data Analysis
3. Results
3.1. Literature Search Results
3.2. Study Characteristics
4. Discussion
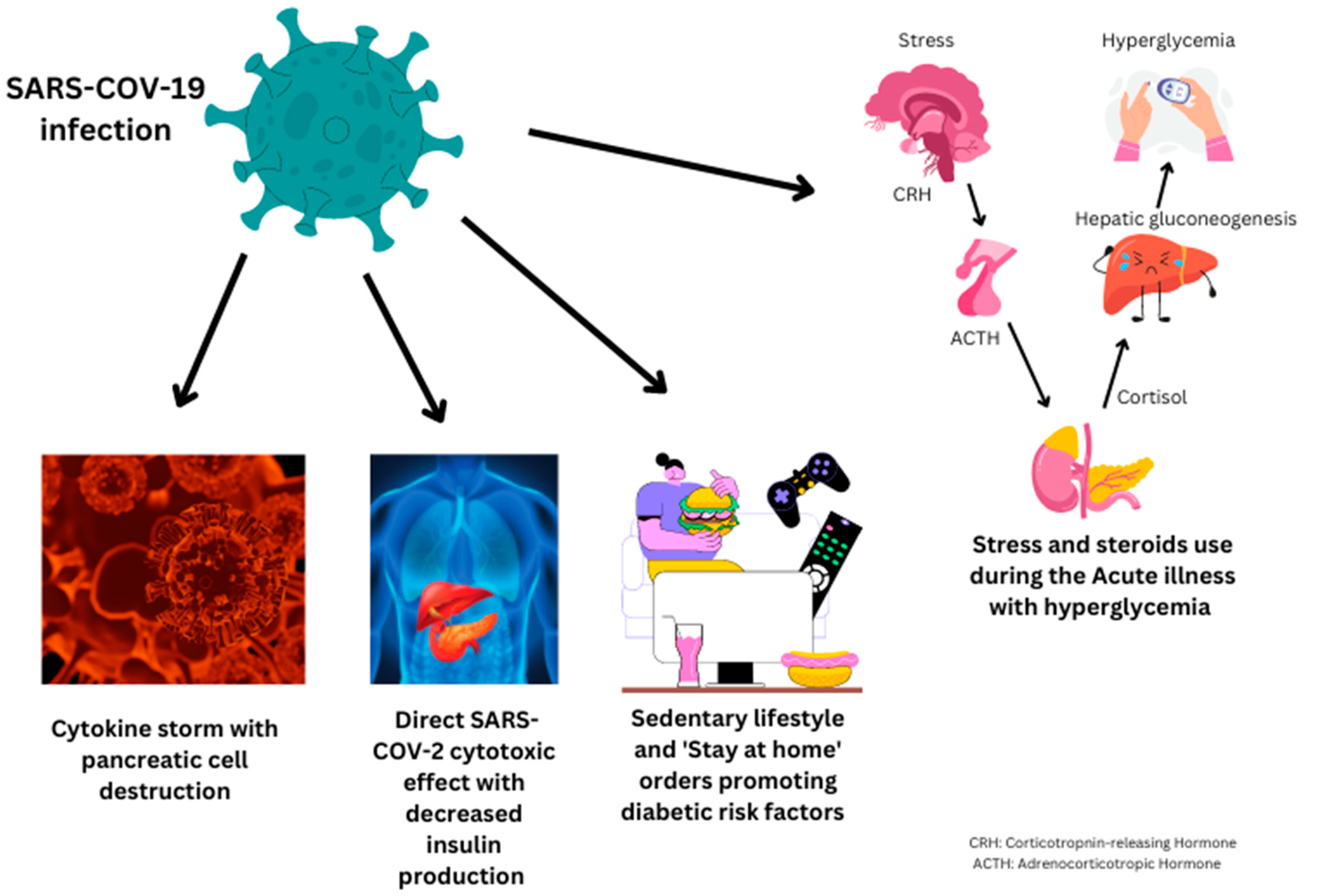
- A.
- Undiagnosed diabetes mellitus:
- B.
- SARS-CoV-2 virus affecting the pancreas:
- C.
- Hyperglycemia due to stress from acute COVID-19 infection:
4.1. Limitations
4.2. Way Forward
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/ (accessed on 8 October 2022).
- Goyal, L.; Zapata, M.; Ajmera, K.; Chaurasia, P.; Pandit, R.; Pandit, T. A Hitchhiker’s Guide to Worldwide COVID-19 Vaccinations: A Detailed Review of Monovalent and Bivalent Vaccine Schedules, COVID-19 Vaccine Side Effects, and Effectiveness Against Omicron and Delta Variants. Cureus 2022, 14, e29837. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Long COVID or Post-COVID Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 4 October 2022).
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Pinelli, K.; Vacca, M.; Raspanti, M.; Argano, C. Type 2 Diabetes Mellitus and COVID-19: A Narrative Review. Front. Endocrinol. 2021, 12, 609470. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.A.; Mehta, P.; Johnson, B.S.; Nagarjuna, A.; Snyder, M.P. COVID-19-Induced New-Onset Diabetes: Trends and Technologies. Diabetes 2021, 70, 2733–2744. [Google Scholar] [CrossRef]
- Khunti, K.; Del Prato, S.; Mathieu, C.; Kahn, S.E.; Gabbay, R.A.; Buse, J.B. COVID-19, Hyperglycemia, and New-Onset Diabetes. Diabetes Care 2021, 44, 2645–2655. [Google Scholar] [CrossRef]
- American Diabetes Association (ADA). Diagnosis. Available online: https://diabetes.org/diabetes/a1c/diagnosis (accessed on 12 October 2022).
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef]
- Estiri, H.; Strasser, Z.H.; Brat, G.A.; Semenov, Y.R.; Consortium for Characterization of COVID-19 by EHR (4CE); Patel, C.J.; Murphy, S.N. Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med. 2021, 19, 249. [Google Scholar] [CrossRef]
- Daugherty, S.E.; Guo, Y.; Heath, K.; Dasmariñas, M.C.; Jubilo, K.G.; Samranvedhya, J.; Lipsitch, M.; Cohen, K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ 2021, 373, n1098. [Google Scholar] [CrossRef]
- Birabaharan, M.; Kaelber, D.C.; Pettus, J.H.; Smith, D.M. Risk of new-onset type 2 diabetes in 600 055 people after COVID-19: A cohort study. Diabetes Obes. Metab. 2022, 24, 1176–1179. [Google Scholar] [CrossRef]
- Rathmann, W.; Kuss, O.; Kostev, K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia 2022, 65, 949–954. [Google Scholar] [CrossRef]
- Rezel-Potts, E.; Douiri, A.; Sun, X.; Chowienczyk, P.J.; Shah, A.M.; Gulliford, M.C. Cardiometabolic outcomes up to 12 months after COVID-19 infection. A matched cohort study in the UK. PLoS Med. 2022, 19, e1004052. [Google Scholar] [CrossRef]
- Montefusco, L.; Ben Nasr, M.; D’Addio, F.; Loretelli, C.; Rossi, A.; Pastore, I.; Daniele, G.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021, 3, 774–785. [Google Scholar] [CrossRef]
- Laurenzi, A.; Caretto, A.; Molinari, C.; Mercalli, A.; Melzi, R.; Nano, R.; Tresoldi, C.; Rovere Querini, P.; Ciceri, F.; Lampasona, V.; et al. No Evidence of Long-Term Disruption of Glycometabolic Control After SARS-CoV-2 Infection. J. Clin. Endocrinol. Metab. 2022, 107, e1009–e1019. [Google Scholar] [CrossRef]
- Maestre-Muñiz, M.M.; Arias, Á.; Mata-Vázquez, E.; Martín-Toledano, M.; López-Larramona, G.; Ruiz-Chicote, A.M.; Nieto-Sandoval, B.; Lucendo, A.J. Long-Term Outcomes of Patients with Coronavirus Disease 2019 at One Year after Hospital Discharge. J. Clin. Med. 2021, 10, 2945. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, T.; Zhu, R.; Yang, F.; Zhang, B.; Lai, X. The Long-Term Effect of COVID-19 Disease Severity on Risk of Diabetes Incidence and the Near 1-Year Follow-Up Outcomes among Postdischarge Patients in Wuhan. J. Clin. Med. 2022, 11, 3094. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Variants of the Virus. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/index.html (accessed on 14 October 2022).
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Guijarro, C.; Torres-Macho, J.; Velasco-Arribas, M.; Plaza-Canteli, S.; Hernández-Barrera, V.; Arias-Navalón, J.A. Diabetes and the Risk of Long-term Post-COVID Symptoms. Diabetes 2021, 70, 2917–2921. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, W.; Xia, P.; Xu, Y.; Li, L.; Li, Q.; Yang, L.; Wei, Q.; Wang, H.; Li, H.; et al. Impaired fasting glucose and diabetes are related to higher risks of complications and mortality among patients with coronavirus disease 2019. Front. Endocrinol. 2020, 11, 525. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, R.; Wang, J.; Cheng, Q.; Zhang, R.; Zhang, S.; Le, Y.; Wang, H.; Xiao, W.; Gao, H.; et al. Diabetes, even newly defined by HbA1c testing, is associated with an increased risk of in-hospital death in adults with COVID-19. BMC Endocr. Disord. 2021, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-K.; Zhao, M.-M.; Jin, J.-M.; Liu, S.; Bai, P.; He, W.; Wu, F.; Liu, X.-F.; Chai, Z.-L.; Han, D.-M. New-onset COVID-19-related diabetes: An early indicator of multi-organ injury and mortally of SARS-CoV-2 infection. Curr. Med. 2022, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar Joshi, S.; Pozzilli, P. COVID-19 induced Diabetes: A novel presentation. Diabetes Res. Clin. Pract. 2022, 191, 110034. [Google Scholar] [CrossRef] [PubMed]
- Sathish, T.; Kapoor, N.; Cao, Y.; Tapp, R.J.; Zimmet, P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes. Metab. 2021, 23, 870–874. [Google Scholar] [CrossRef]
- Shrestha, D.B.; Budhathoki, P.; Raut, S.; Adhikari, S.; Ghimire, P.; Thapaliya, S.; Rabaan, A.A.; Karki, B.J. New-onset diabetes in COVID-19 and clinical outcomes: A systematic review and meta-analysis. World J. Virol. 2021, 10, 275–287. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Takes Key Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 (accessed on 6 December 2022).
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.-H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccin. Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef]
- Hayden, M.R. An Immediate and Long-Term Complication of COVID-19 May Be Type 2 Diabetes Mellitus: The Central Role of β-Cell Dysfunction, Apoptosis and Exploration of Possible Mechanisms. Cells 2020, 9, 2475. [Google Scholar] [CrossRef]
- Rubino, F.; Amiel, S.A.; Zimmet, P.; Alberti, G.; Bornstein, S.; Eckel, R.H.; Mingrone, G.; Boehm, B.; Cooper, M.E.; Chai, Z.; et al. New-Onset Diabetes in Covid-19. N. Engl. J. Med. 2020, 383, 789–790. [Google Scholar] [CrossRef]
- Babajide, O.I.; Ogbon, E.O.; Adelodun, A.; Agbalajobi, O.; Ogunsesan, Y. COVID-19 and acute pancreatitis: A systematic review. JGH Open 2022, 6, 231–235. [Google Scholar] [CrossRef]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. Washington State 2019-nCoV Case Investigation Team First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef]
- Mamtani, M.; Athavale, A.M.; Abraham, M.; Vernik, J.; Amarah, A.R.; Ruiz, J.P.; Joshi, A.J.; Itteera, M.; Zhukovski, S.D.; Madaiah, R.P.; et al. Association of hyperglycaemia with hospital mortality in nondiabetic COVID-19 patients: A cohort study. Diabetes Metab. 2021, 47, 101254. [Google Scholar] [CrossRef]
- Tamez-Pérez, H.E.; Quintanilla-Flores, D.L.; Rodríguez-Gutiérrez, R.; González-González, J.G.; Tamez-Peña, A.L. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J. Diabetes 2015, 6, 1073–1081. [Google Scholar] [CrossRef]
- Ruzzin, J.; Wagman, A.S.; Jensen, J. Glucocorticoid-induced insulin resistance in skeletal muscles: Defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia 2005, 48, 2119–2130. [Google Scholar] [CrossRef]
- Clore, J.N.; Thurby-Hay, L. Glucocorticoid-induced hyperglycemia. Endocr. Pract. 2009, 15, 469–474. [Google Scholar] [CrossRef]
| Serial Number | Year | Author ID | Country | Study Design | Patient Population | Follow-Up (in Days) | Sample Size | Mean Age/Gender (Female %) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2020 | Ayoubkhani, D., et al. [20] | England | RCS with matched control | Post hospital discharge patients | 140 (mean) | 47,780 patients | 64.5/45% |
| 2 | 2021 | Daugherty, S.E., et al. [12] | USA | RCS | 3 databases:
| 87 (median) | 266,586 COVID-19 patients, 100% matched primary and secondary comparison groups | 42.4/49.8% |
| 3 | 2021 | Maestre-Muñiz, M.M., et al. [18] | Spain | Cross-sectional study | Post hospital discharge patients | 365 (mean) | 543 patients | 65.1/49.3% |
| 4 | 2021 | Rathmann, W., et al. [14] | Germany | RCS | Outpatients with non-exposed control group with AURI | 119 (median) | 35,865 patients in both groups | 42.6/45.6% |
| 5 | 2021 | Estiri, H., et al. [11] | USA | RCS | Non-hospitalized patients | 3–6 months and 6–9 months after COVID | 96,025 patients | NA |
| 6 | 2021 | Montefusco, L., et al. [16] | Italy | Cohort study | Post hospital discharge patients | ~60 (mean) | 551 patients | 61/38% |
| 7 | 2022 | Xie, Y., et al. [10] | USA | Cohort study | Inpatients including ICU patients and outpatients | 352 (median) | COVID-19 patients—181,280 patients, contemporary control 4,118,441 patients, historical control 4,286,911 patients | 60.92/11.9% |
| 8 | 2022 | Zhang, J., et al. [19] | China | Longitudinal prospective study | Post hospital discharge patients | ~365 (mean) | 248 patients | 61 (median)/54.8% |
| 9 | 2022 | Rezel-Potts, E., et al. [15] | England | Cohort study | Outpatients | <4 weeks, 5–12 weeks, and 13 to 52 weeks from index date | 428,650 patients in case and control group each | 35/56% |
| 10 | 2022 | Laurenzi, A., et al. [17] | Italy | Prospective cohort study | Post hospital discharge patients | 215 (median) | 660 patients | 64/33.3% |
| 11 | 2022 | Birabaharan, et al. [13] | USA and outside USA | RCS | Outpatients and inpatients. Control population included influenza patients | 180 days | 600,055 COVID-19 patients and 394,667 influenza patients | NA |
| Serial Number | Author ID | Comparison Groups | Key Findings |
|---|---|---|---|
| 1 | Ayoubkhani, D., et al. [20] | Patients with COVID-19 infection vs. patients with no COVID-19 infection | Diabetes was diagnosed after discharge in 4.9% of patients with COVID-19 with rate of 127 (122 to 132) diagnoses per 1000 person years. New-onset diabetes was diagnosed 29 (26 to 32) diagnoses per 1000 person years. |
| 2 | Daugherty, S.E., et al. [12] | COVID-19 vs. non-COVID-19 (cases vs. matched controls)—3 comparator groups: (1) 2019 comparator group (2) 2020 with adult patients with no diagnosis of COVID-19 (3) Viral lower respiratory tract illness comparator group | Hazard ratios for patients with COVID-19 infection and 2020 comparator group after the acute infection were highest in the first month from the index date and elevated for up to 6 months for diabetes mellitus (2.47, 95% CI 1.14–5.38). Diabetes mellitus was increased after COVID-19 infection with all comparison groups (2020, 2019, and viral lower respiratory tract illness group). Excess risk for new clinical sequelae after acute COVID-19 did not differ significantly between male and females. |
| 3 | Maestre-Muñiz, M.M., et al. [18] | No | Described incidence of DM2 in 1.3% (7) patients |
| 4 | Rathmann, W., et al. [14] | Patients with COVID-19 infection vs. acute upper respiratory tract infection as control | Increased incidence of type 2 diabetes mellitus in patients with COVID-19 as compared to AURI group (15.8 vs. 12.3 per 1000 person years). IRR for type 2 diabetes was 1.28 (95% CI 1.05–1.57). IRR was not increased for other forms of diabetes (IRR 1.17, 95% CI 0.80–1.71). |
| 5 | Estiri, H., et al. [11] | No | Increased risk of diabetes mellitus in both 3–6- and 6–9-month time windows (OR 1.41, 95% CI 1.22–1.64). |
| 6 | Montefusco, L., et al. [16] | Continuous glucose monitoring (CGM) was performed in COVID-19 or in patients recovering from COVID-19 with normal fasting glucose levels, in healthy controls and in patients with type 2 diabetes (during hospitalization) | 46% of patients were hyperglycemic, whereas 27% were normoglycemic in post-discharge follow-up. In subgroup with CGM, COVID-19 was associated with impaired glycemic profile in normoglycemic patients, significantly higher glycemic area under the curve (AUC) above 140 m/dL, higher postprandial glycemic at 60 minutes, higher glycemic variability, higher standard deviation as compared to healthy controls. |
| 7 | Xie, Y., et al. [10] | COVID-19 patients with contemporary control and historical control | In post-acute phase, compared with contemporary control group, COVID-19 patients had increased risk (1.40, 95% CI 1.36–1.44) and excess burden (12.35, 11.36–13.38, per 1000 people at 12 months) of diabetes compared with contemporary control group, increased risk (1.85, (1.78–1.92)) and excess burden (12.35, 11.36–13.38) of incident anti-hyperglycemic use, increased risk of a composite endpoint of incident diabetes or anti hyperglycemic (HR of 1·46 (95% CI 1.43–1.50)) and excess burden of 18.03 (95% CI 16.59–19.51) per 1000 people at 12 months. Risk and burden of post-acute outcomes increased in a graded fashion based on severity of acute infection (non-hospitalized, hospitalized, admission to ICU). |
| 8 | Zhang, J., et al. [19] | Categorized into two groups according to whether they were critically ill or not during their initial hospital stay | Critical illness was associated with a higher risk of diabetes incidence one year after discharge as compared to mild/moderate risk COVID-19 infection. Association was more evident in males (OR = 5.70, 95% CI: 1.46, 22.15). Limitation: (1) No control group, OR was between severely ill vs. not severely ill. (2) Critical illness was defined by the following criteria: (1) respiratory failure requiring mechanical ventilation, (2) shock, (3) complications with other organ failures that required monitoring and treatment in an intensive care unit (ICU). |
| 9 | Rezel-Potts, E., et al. [15] | Patients with COVID-19 infection vs. patients without COVID-19 infection | Diabetes mellitus net incidence increased in the first 4 weeks after COVID-19 infection (relative risk 1.81, 95% CI 1.51 to 2.19). Diabetes mellitus net incidence continued to be elevated from weeks 5 to 12 after acute COVID-19 infection (relative risk 1.27, 95% CI 1.51–2.19). Diabetes mellitus net incidence was not increased from weeks 13 to 52 from acute COVID-19 infection (relative risk 10.07, 95% CI 0.99–1.16). |
| 10 | Laurenzi, A., et al. [17] | Patients with COVID-19 vs. patients without COVID-19 infection | A significant increase in FBG was noted during hospitalization (from 95 to 102 mg/d, p = 0.003) with return to pre-infection levels during follow-up (97.5 mg/dL p = 0.24 vs. pre-infection levels, p = 0.004 vs. hospitalization). After resolution of the infection, prevalence of dysglycemia reverted to preadmission frequency. |
| 11 | Birabaharan, et al. [13] | Patients with COVID-19 infection vs. patients with influenza | In patients with mild COVID-19 disease, estimated risk of new-onset type 2 diabetes mellitus within 180 days was 1.1%, estimated rate per 1000 person years was 23 and had relative risk 1.54 (95% CI 1.46–1.62) times higher than mild influenza controls. In patients with moderate/severe COVID-19 disease, estimated risk of new-onset type 2 diabetes mellitus within 180 days was 4.1%, estimated rate per 1000 person years was 83 and had relative risk 1.46 (95% CI 1.26–1.69) times higher than moderate/severe influenza controls. In subgroup analysis after excluding steroid use, risk of new-onset type 2 diabetes was less in patients with mild COVID-19 (RR 1.22, 95% CI 1.14–1.29) and remained same in patients with moderate/severe COVID-19(RR 1.42 (95% CI 1.13–1.80). |
| Serial Number | Author ID | Reported Events of Incident Diabetes Reported (COVID-19 vs. Control) | Adjusted HR/RR (95% CI) of Study Outcomes |
|---|---|---|---|
| 1 | Ayoubkhani, D., et al. [20] | 28.7 (CI 26–31.7) vs. 8.2 (CI 6.9–9.8) per 1000 person years | 1.50 (1.40, 1.61) |
| 2 | Daugherty, S.E., et al. [12] | Cumulative incidence 1.04 vs. 0.57 events per 100 person years | 1.82 (1.69, 1.96) |
| 3 | Maestre-Muñiz, M.M., et al. [18] | NA | NA |
| 4 | Rathmann, W., et al. [14] | 20.5 vs. 13.6 events per 1000 person years | 1.26 (0.93, 1.71) |
| 5 | Estiri, H., et al. [11] | NA | NA |
| 6 | Montefusco, L., et al. [16] | 65 patients vs. 147 patients with normal blood glucose levels | NA |
| 7 | Xie, Y., et al. [10] | 48.38 (CI 47.04–49.76) vs. 34.9 (CI 34.7–35.1) events per 1000 person years at 12 months | 1.40 (1.36, 1.44) |
| 8 | Zhang, J., et al. [19] | 36 diabetic cases vs. 166 normal blood sugar level | 3.53 (1.48–8.40) |
| 9 | Rezel-Potts, E., et al. [15] | 681 vs. 384 cases of diabetes; 19.54 (95% CI: 18.10 to 21.06) vs. 11.10 (95% CI 10.01 to 12.26) per 100,000 patient weeks | 1.27 (1.11 to 1.46) |
| 10 | Laurenzi, A., et al. [17] | 154 diabetes vs. 176 normoglycemia | NA |
| 11 | Birabaharan, et al. [13] | 83 vs. 56 events per 1000 person years 63 vs. 44 events per 1000 person years | 1.46 (1.26, 1.69) 1.42 (1.13. 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chourasia, P.; Goyal, L.; Kansal, D.; Roy, S.; Singh, R.; Mahata, I.; Sheikh, A.B.; Shekhar, R. Risk of New-Onset Diabetes Mellitus as a Post-COVID-19 Condition and Possible Mechanisms: A Scoping Review. J. Clin. Med. 2023, 12, 1159. https://doi.org/10.3390/jcm12031159
Chourasia P, Goyal L, Kansal D, Roy S, Singh R, Mahata I, Sheikh AB, Shekhar R. Risk of New-Onset Diabetes Mellitus as a Post-COVID-19 Condition and Possible Mechanisms: A Scoping Review. Journal of Clinical Medicine. 2023; 12(3):1159. https://doi.org/10.3390/jcm12031159
Chicago/Turabian StyleChourasia, Prabal, Lokesh Goyal, Dhruv Kansal, Sasmit Roy, Rohit Singh, Indrajeet Mahata, Abu Baker Sheikh, and Rahul Shekhar. 2023. "Risk of New-Onset Diabetes Mellitus as a Post-COVID-19 Condition and Possible Mechanisms: A Scoping Review" Journal of Clinical Medicine 12, no. 3: 1159. https://doi.org/10.3390/jcm12031159
APA StyleChourasia, P., Goyal, L., Kansal, D., Roy, S., Singh, R., Mahata, I., Sheikh, A. B., & Shekhar, R. (2023). Risk of New-Onset Diabetes Mellitus as a Post-COVID-19 Condition and Possible Mechanisms: A Scoping Review. Journal of Clinical Medicine, 12(3), 1159. https://doi.org/10.3390/jcm12031159







