Greater Tuberosity Fractures after RTSA: A Matched Group Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Technique
2.3. Clinical and Radiological Evaluation
2.4. Statistical Analysis and Data Collection
3. Results
3.1. Incidence and Indication for RTSA
3.2. Clinical Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grammont, P. Etude et réalisation d’une nouvelle prothèse d’épaule. Rheumatologie 1987, 39, 27–38. [Google Scholar]
- Hao, K.A.; Wright, T.W.; Schoch, B.S.; Wright, J.O.; Dean, E.W.; Struk, A.M.; King, J.J. Rate of improvement in shoulder strength after anatomic and reverse total shoulder arthroplasty. JSES Int. 2021, 6, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Weber, S.; Frima, H.; Hutter, R.; Grehn, H.; Sommer, C. Proximal humeral fracture-dislocation: Outcome analysis in osteosynthesis and arthroplasties. Eur. J. Orthop. Surg. Traumatol. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karademir, G.; Tunalı, O.; Erşen, A.; Akpınar, S.; Atalar, A.C. Reverse total shoulder arthroplasty for failed treatment of proximal humerus fractures. reconstruction 2021, 6, 8. [Google Scholar] [CrossRef]
- Ferlauto, H.R.; Wickman, J.R.; Lazarides, A.L.; Hendren, S.; Visgauss, J.D.; Brigman, B.E.; Anakwenze, O.A.; Klifto, C.S.; Eward, W.C. Reverse total shoulder arthroplasty for oncologic reconstruction of the proximal humerus: A systemat-ic review. J. Shoulder Elb. Surg. 2021, 30, e647–e658. [Google Scholar] [CrossRef] [PubMed]
- Westermann, R.W.; Pugely, A.J.; Martin, C.T.; Gao, Y.; Wolf, B.R.; Hettrich, C.M. Reverse Shoulder Arthroplasty in the United States: A Comparison of National Volume, Patient Demographics, Complications, and Surgical Indications. Iowa Orthop. J. 2015, 35, 1–7. [Google Scholar]
- Schairer, W.W.; Nwachukwu, B.U.; Lyman, S.; Craig, E.V.; Gulotta, L.V. National utilization of reverse total shoulder arthroplasty in the United States. J. Shoulder Elb. Surg. 2015, 24, 91–97. [Google Scholar] [CrossRef]
- Farshad, M.; Gerber, C. Reverse total shoulder arthroplasty—From the most to the least common complication. Int. Orthop. 2010, 34, 1075–1082. [Google Scholar] [CrossRef]
- Shah, S.S.; Gaal, B.T.; Roche, A.M.; Namdari, S.; Grawe, B.M.; Lawler, M.; Dalton, S.; King, J.J.; Helmkamp, J.; Garrigues, G.E.; et al. The modern reverse shoulder arthroplasty and an updated systematic review for each complication: Part I. JSES Int. 2020, 4, 929–943. [Google Scholar] [CrossRef]
- Bohsali, K.I.; Wirth, M.A.; Rockwood, C.A., Jr. Complications of total shoulder arthroplasty. JBJS 2006, 88, 2279–2292. [Google Scholar]
- Boileau, P. Complications and revision of reverse total shoulder arthroplasty. Orthop. Traumatol. Surg. Res. 2016, 102, S33–S43. [Google Scholar] [CrossRef] [PubMed]
- Molé, D.; Favard, L. Excentered scapulohumeral osteoarthritis. Revue de Chirurgie Orthopedique et Reparatrice de l’ap-pareil Moteur 2007, 93 (Suppl. 6), 37–94. [Google Scholar] [CrossRef] [PubMed]
- Zumstein, M.A.; Pinedo, M.; Old, J.; Boileau, P. Problems, complications, reoperations, and revisions in reverse total shoulder arthroplasty: A sys-tematic review. J. Shoulder Elb. Surg. 2011, 20, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-H.; Rhee, Y.G.; Yoo, J.C.; Ji, J.H.; Kim, D.-S.; Kim, Y.-S.; Rhee, S.-M.; Kim, D.-H. Incidence and risk factors of acromial fracture following reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2020, 30, 57–64. [Google Scholar] [CrossRef]
- Kriechling, P.; Zaleski, M.; Loucas, R.; Loucas, M.; Fleischmann, M.; Wieser, K. Complications and further surgery after reverse total shoulder arthroplasty: Report of 854 primary cases. Bone Joint J. 2022, 104, 401–407. [Google Scholar] [CrossRef]
- Moverman, M.A.; Menendez, M.E.; Mahendraraj, K.A.; Polisetty, T.; Jawa, A.; Levy, J.C. Patient risk factors for acromial stress fractures after reverse shoulder arthroplasty: A multicenter study. J. Shoulder Elb. Surg. 2020, 30, 1619–1625. [Google Scholar] [CrossRef]
- Mahendraraj, K.A.; Abboud, J.; Armstrong, A.; Austin, L.; Brolin, T.; Entezari, V.; Friedman, L.; Garrigues, G.E.; Grawe, B.; Gulotta, L.; et al. Predictors of acromial and scapular stress fracture after reverse shoulder arthroplasty: A study by the ASES Complications of RSA Multicenter Research Group. J. Shoulder Elb. Surg. 2021, 30, 2296–2305. [Google Scholar] [CrossRef]
- Werthel, J.-D.; Schoch, B.S.; van Veen, S.C.; Elhassan, B.T.; An, K.-N.; Cofield, R.H.; Sperling, J.W. Acromial Fractures in Reverse Shoulder Arthroplasty: A Clinical and Radiographic Analysis. J. Shoulder Elb. Arthroplast. 2018, 2, 2471549218777628. [Google Scholar] [CrossRef]
- Otto, R.J.; Virani, N.A.; Levy, J.C.; Nigro, P.T.; Cuff, D.J.; Frankle, M.A. Scapular fractures after reverse shoulder arthroplasty: Evaluation of risk factors and the reliability of a proposed classification. J. Shoulder Elb. Surg. 2013, 22, 1514–1521. [Google Scholar] [CrossRef]
- LaCoste, K.L.; Arguello, A.M.; Ponce, B.A. Physical Therapy-Induced Fracture After Reverse Shoulder Arthroplasty: A Case Report. JBJS Case Connect 2021, 11, e20. [Google Scholar] [CrossRef]
- Ernstbrunner, L.; Rahm, S.; Suter, A.; Imam, M.A.; Catanzaro, S.; Grubhofer, F.; Gerber, C. Salvage reverse total shoulder arthroplasty for failed operative treatment of proximal humeral fractures in patients younger than 60 years: Long-term results. J. Shoulder Elb. Surg. 2019, 29, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Gallinet, D.; Adam, A.; Gasse, N.; Rochet, S.; Obert, L. Improvement in shoulder rotation in complex shoulder fractures treated by reverse shoulder arthro-plasty. J. Shoulder Elb. Surg. 2013, 22, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Simovitch, R.W.; Roche, C.P.; Jones, R.B.; Routman, H.D.; Marczuk, Y.; Wright, T.W.; Zuckerman, J. Effect of Tuberosity Healing on Clinical Outcomes in Elderly Patients Treated With a Reverse Shoulder Arthroplasty for 3- and 4-Part Proximal Humerus Fractures. J. Orthop. Trauma 2019, 33, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, B.; Hasler, A.; Hasler, J.; Kriechling, P.; Borbas, P.; Gerber, C. Factors influencing functional internal rotation after reverse total shoulder arthroplasty. JSES Int. 2021, 5, 679–687. [Google Scholar] [CrossRef]
- Nyffeler, R.W.; Seidel, A.; Werlen, S.; Bergmann, M. Radiological and biomechanical assessment of displaced greater tuberosity fractures. Int. Orthop. 2018, 43, 1479–1486. [Google Scholar] [CrossRef]
- Kriechling, P.; Loucas, R.; Loucas, M.; Künzler, T.; Gerber, C.; Wieser, K. Primary reverse total shoulder arthroplasty in patients older than 80 years: Clinical and radiologic outcome measures. J. Shoulder Elb. Surg. 2021, 30, 877–883. [Google Scholar] [CrossRef]
- Constant, C.R.; Murley, A.H. A clinical method of functional assessment of the shoulder. Clin. Orthop. Relat. Res. 1987, 160–164. [Google Scholar] [CrossRef]
- Gilbart, M.K.; Gerber, C. Comparison of the subjective shoulder value and the Constant score. J. Shoulder Elb. Surg. 2007, 16, 717–721. [Google Scholar] [CrossRef]
- Torrens, C.; Guirro, P.; Santana, F. The minimal clinically important difference for function and strength in patients undergoing reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2015, 25, 262–268. [Google Scholar] [CrossRef]
- Simovitch, R.; Flurin, P.-H.; Wright, T.; Zuckerman, J.D.; Roche, C.P. Quantifying success after total shoulder arthroplasty: The minimal clinically important difference. J. Shoulder Elb. Surg. 2018, 27, 298–305. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Cazeneuve, J.-F.; Cristofari, D.-J. Grammont reversed prosthesis for acute complex fracture of the proximal humerus in an elderly population with 5 to 12 years follow-up. Orthop. Traumatol. Surg. Res. 2014, 100, 93–97. [Google Scholar] [CrossRef]
- Grubhofer, F.; Wieser, K.; Meyer, D.C.; Catanzaro, S.; Beeler, S.; Riede, U.; Gerber, C. Reverse total shoulder arthroplasty for acute head-splitting, 3- and 4-part fractures of the proximal humerus in the elderly. J. Shoulder Elbow Surg. 2016, 25, 1690–1698. [Google Scholar] [CrossRef]
- Grubhofer, F.; Wieser, K.; Meyer, D.C.; Catanzaro, S.; Schürholz, K.; Gerber, C. Reverse total shoulder arthroplasty for failed open reduction and internal fixation of fractures of the proximal humerus. J. Shoulder Elb. Surg. 2017, 26, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ohl, X.; Bonnevialle, N.; Gallinet, D.; Ramdane, N.; Valenti, P.; Decroocq, L.; Boileau, P. How the greater tuberosity affects clinical outcomes after reverse shoulder arthroplasty for proximal humeral fractures. J. Shoulder Elb. Surg. 2018, 27, 2139–2144. [Google Scholar] [CrossRef] [PubMed]
- Gallinet, D.; Cazeneuve, J.-F.; Boyer, E.; Menu, G.; Obert, L.; Ohl, X.; Bonnevialle, N.; Valenti, P.; Boileau, P. Reverse shoulder arthroplasty for recent proximal humerus fractures: Outcomes in 422 cases. Orthop. Traumatol. Surg. Res. 2019, 105, 805–811. [Google Scholar] [CrossRef]
- Boileau, P.; Alta, T.D.; Decroocq, L.; Sirveaux, F.; Clavert, P.; Favard, L.; Chelli, M. Reverse shoulder arthroplasty for acute fractures in the elderly: Is it worth reattaching the tuberosities? J. Shoulder Elb. Surg. 2019, 28, 437–444. [Google Scholar] [CrossRef]
- Gunst, S.; Louboutin, L.; Swan, J.; Lustig, S.; Servien, E.; Nove-Josserand, L. Does healing of both greater and lesser tuberosities improve functional outcome after reverse shoulder arthroplasty for fracture? A retrospective study of twenty-eight cases with a computed tomography scan at a minimum of one-year follow-up. Int. Orthop. 2021, 45, 681–687. [Google Scholar] [CrossRef]
- Ek, E.T.; Neukom, L.; Catanzaro, S.; Gerber, C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: Results after five to fifteen years. J. Shoulder Elb. Surg. 2013, 22, 1199–1208. [Google Scholar] [CrossRef]
- Hasler, A.; Fornaciari, P.; Jungwirth-Weinberger, A.; Jentzsch, T.; Wieser, K.; Gerber, C. Reverse shoulder arthroplasty in the treatment of glenohumeral instability. J. Shoulder Elb. Surg. 2019, 28, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Hasler, A.; Kriechling, P.; Passaplan, C.; Wieser, K. Inadvertent, intraoperative, non- to minimally displaced periprosthetic humeral shaft fractures in RTSA do not affect the clinical and radiographic short-term outcome. Arch. Orthop. Trauma. Surg. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sebastiá-Forcada, E.; Cebrián-Gómez, R.; Lizaur-Utrilla, A.; Gil-Guillén, V. Reverse shoulder arthroplasty versus hemiarthroplasty for acute proximal humeral fractures. A blinded, randomized, controlled, prospective study. J. Shoulder Elb. Surg. 2014, 23, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Boileau, P.; Krishnan, S.; Tinsi, L.; Walch, G.; Coste, J.; Molé, D. Tuberosity malposition and migration: Reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J. Shoulder Elb. Surg. 2002, 11, 401–412. [Google Scholar] [CrossRef]
| Characteristic | Fracture | Control | p-Value |
|---|---|---|---|
| Patients, n | 17 | 34 | |
| Mean age, years (SD) | 69 (9) | 69 (10) | 0.984 * |
| Male, n (%) | 4 (24) | 6 (17) | 0.618 # |
| Right side, n (%) | 13 (76) | 17 (50) | 0.070 # |
| ASA grade, n (%) | 0.077 * | ||
| I | 0 | 1 | |
| II | 9 | 25 | |
| III | 8 | 8 | |
| Indication | 1.000 # | ||
| CTA | 1 | 2 | |
| MRCT without osteoarthritis | 1 | 2 | |
| MRCT with osteoarthritis | 1 | 2 | |
| Osteoarthritis | 4 | 8 | |
| Instability | 1 | 2 | |
| Fracture | 3 | 6 | |
| Arthroplasty revision | 6 | 12 | |
| Mean BMI, kg/m2 (SD) | 29 (7) | 28 (6) | 0.704 * |
| Mean FU, moths (SD) | 72 (37) | 84 (35) | 0.263 * |
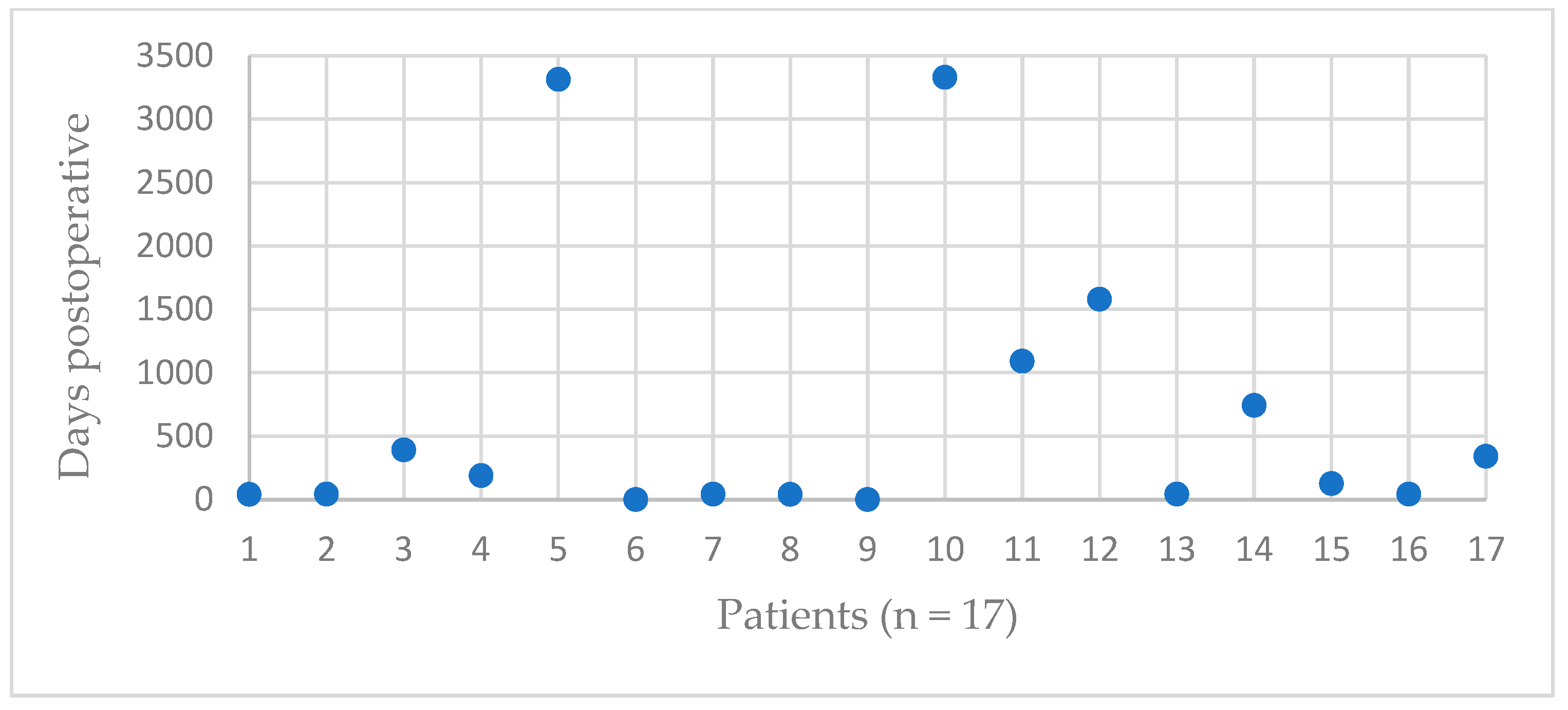
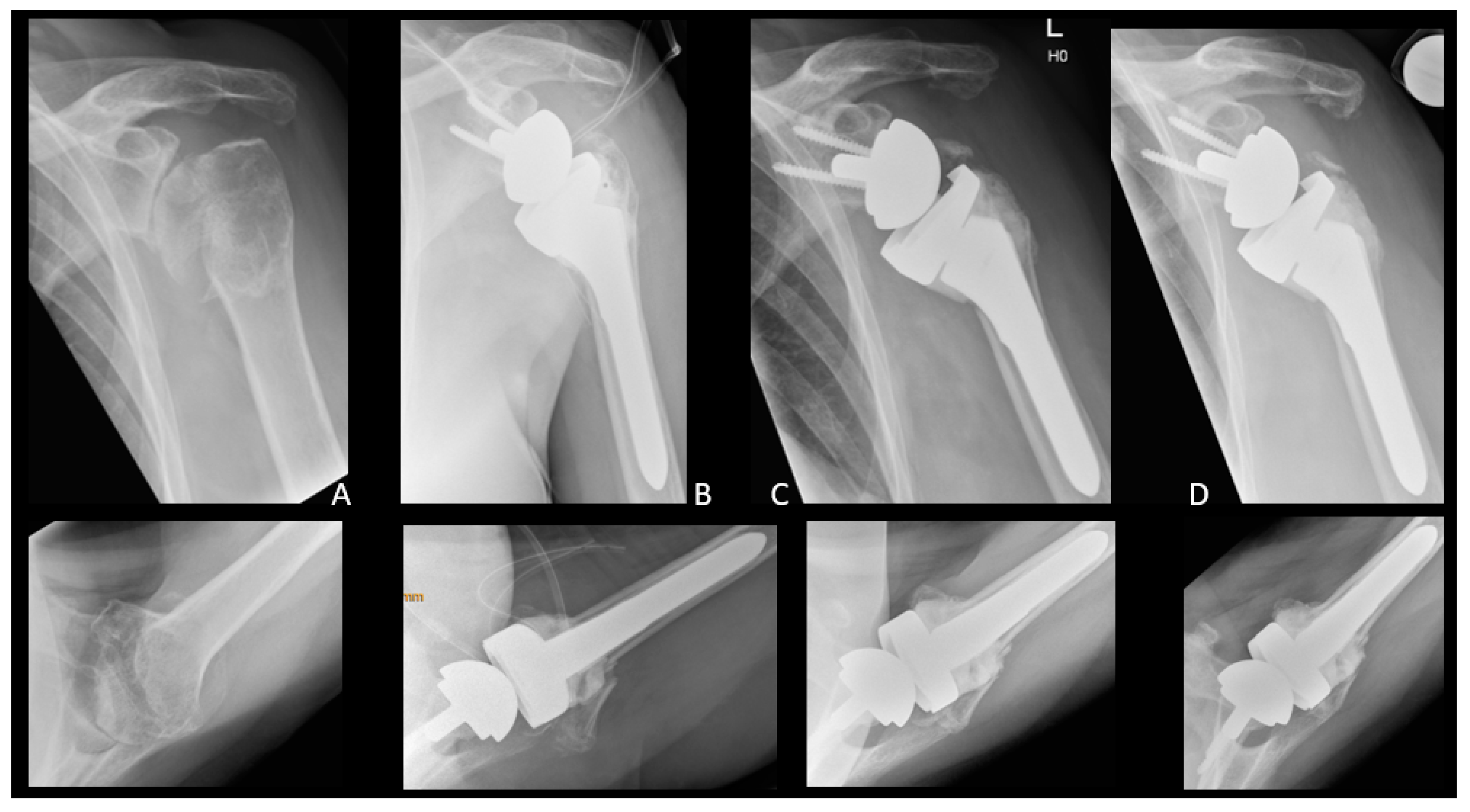
| Patient | Occurrence of GTF [Days after RTSA] | Trauma | Dislocation | Treatment | Further Dislocation of GTF | Radiological Consolidation of GTF |
|---|---|---|---|---|---|---|
| 1 | 41 | No | Slightly | Operative | No | Yes |
| 2 | 43 | No | No | Operative | Yes | Yes, after 2nd refixation |
| 3 | 391 | No | Slightly | Conservative | No | No |
| 4 | 188 | No | No | Conservative | No | No |
| 5 | 3313 | No | Yes | Conservative | No | Partial |
| 6 | 0 | No | Yes | Conservative | No | No |
| 7 | 43 | No | No | Conservative | No | Yes |
| 8 | 41 | No | Yes | Conservative | No | Yes |
| 9 | 0 | No | Yes | Operative | Yes | No |
| 10 | 3329 | No | Slightly | Conservative | No | Yes |
| 11 | 1091 | No | No | Conservative | No | Yes |
| 12 | 1580 | No | No | Operative | No | Yes |
| 13 | 43 | No | Slightly | Operative | No | No |
| 14 | 743 | No | Yes | Conservative | No | No |
| 15 | 125 | Yes | Yes | Conservative | No | No |
| 16 | 43 | No | No | Conservative | No | No |
| 17 | 340 | No | Slightly | Conservative | No | Yes |
| Fracture | Control | p-Value 2 | ||
|---|---|---|---|---|
| Number | 17 | 34 | ||
| Mean CS absolute, points (SD) | Preop | 30 (17) | 32 (13) | 0.508 |
| Postop | 50 (19) | 62 (21) | 0.032 | |
| p-Value 1 | <0.001 | <0.001 | ||
| Mean CS relative, % (SD) | Preop | 36 (20) | 40 (15) | 0.241 |
| Postop | 62 (21) | 75 (20) | 0.015 | |
| p-Value 1 | <0.001 | <0.001 | ||
| Mean SSV, % (SD) | Preop | 26 (16) | 36 (17) | 0.101 |
| Postop | 63 (26) | 77 (29) | 0.022 | |
| p-Value 1 | <0.001 | <0.001 | ||
| Mean CS pain, points (SD) | Preop | 8 (4) | 7 (4) | 0.367 |
| Postop | 12 (4) | 14 (2) | 0.271 | |
| p-Value 1 | <0.001 | <0.001 | ||
| Mean flexion, ° (SD) | Preop | 67 (42) | 80 (37) | 0.146 |
| Postop | 102 (28) | 114 (27) | 0.160 | |
| p-Value 1 | <0.001 | <0.001 | ||
| Mean abduction, ° (SD) | Preop | 54 (31) | 69 (35) | 0.076 |
| Postop | 109 (42) | 120 (39) | 0.317 | |
| p-Value 1 | <0.001 | <0.001 | ||
| Mean ER, ° (SD) | Preop | 32 (26) | 28 (21) | 0.822 |
| Postop | 17 (19) | 30 (19) | 0.029 | |
| p-Value 1 | 0.010 | 0.770 | ||
| Mean IR, ° (SD) | Preop | 4 (2) | 4 (3) | 0.965 |
| Postop | 4 (2) | 5 (3) | 0.138 | |
| p-Value 1 | 0.627 | 0.137 | ||
| Mean force, kg (SD) | Preop | 1 (1) | 0 (1) | 0.155 |
| Postop | 1 (2) | 2 (2) | 0.044 | |
| p-Value 1 | 0.441 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selman, F.; Kriechling, P.; Ernstbrunner, L.; Wieser, K.; Borbas, P. Greater Tuberosity Fractures after RTSA: A Matched Group Analysis. J. Clin. Med. 2023, 12, 1153. https://doi.org/10.3390/jcm12031153
Selman F, Kriechling P, Ernstbrunner L, Wieser K, Borbas P. Greater Tuberosity Fractures after RTSA: A Matched Group Analysis. Journal of Clinical Medicine. 2023; 12(3):1153. https://doi.org/10.3390/jcm12031153
Chicago/Turabian StyleSelman, Farah, Philipp Kriechling, Lukas Ernstbrunner, Karl Wieser, and Paul Borbas. 2023. "Greater Tuberosity Fractures after RTSA: A Matched Group Analysis" Journal of Clinical Medicine 12, no. 3: 1153. https://doi.org/10.3390/jcm12031153
APA StyleSelman, F., Kriechling, P., Ernstbrunner, L., Wieser, K., & Borbas, P. (2023). Greater Tuberosity Fractures after RTSA: A Matched Group Analysis. Journal of Clinical Medicine, 12(3), 1153. https://doi.org/10.3390/jcm12031153






