Use of a Fibula Free Flap for Mandibular Reconstruction in Severe Craniofacial Microsomia in Children with Obstructive Sleep Apnea
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birgfeld, C.; Heike, C. Craniofacial Microsomia. Clin. Plast. Surg. 2019, 46, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Wink, J.D.; Goldstein, J.A.; Paliga, J.T.; Taylor, J.A.; Bartlett, S.P. The mandibular deformity in hemifacial microsomia: A reassessment of the pruzansky and kaban classification. Plast. Reconstr. Surg. 2014, 133, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Heike, C.L.; Wallace, E.; Speltz, M.L.; Siebold, B.; Werler, M.M.; Hing, A.V.; Birgfeld, C.B.; Collett, B.R.; Leroux, B.G.; Luquetti, D.V. Characterizing facial features in individuals with craniofacial microsomia: A systematic approach for clinical research. Birth Defects Res. Part A Clin. Mol. Teratol. 2016, 106, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Heike, C.L.; Hing, A.V.; Aspinall, C.A.; Bartlett, S.P.; Birgfeld, C.B.; Drake, A.F.; Pimenta, L.A.; Sie, K.C.; Urata, M.M.; Vivaldi, D.; et al. Clinical care in craniofacial microsomia: A review of current management recommendations and opportunities to advance research. Am. J. Med. Genet. Part C Semin. Med. Genet. 2013, 163, 271–282. [Google Scholar] [CrossRef]
- Padwa, B. Indications for neonatal and pediatric distraction. Int. J. Oral Maxillofac. Surg. 2011, 40, 1011. [Google Scholar] [CrossRef]
- Brevi, B.C.; Leporati, M.; Sesenna, E. Neonatal mandibular distraction in a patient with Treacher Collins syndrome. J. Craniofacial Surg. 2015, 26, e44–e48. [Google Scholar] [CrossRef]
- Pluijmers, B.I.; Caron, C.J.J.M.; Dunaway, D.J.; Wolvius, E.B.; Koudstaal, M.J. Mandibular reconstruction in the growing patient with unilateral craniofacial microsomia: A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 286–295. [Google Scholar] [CrossRef]
- Hidalgo, D.A. Fibula free flap: A new method of mandible reconstruction. Plast. Reconstr. Surg. 1989, 84, 71–79. [Google Scholar] [CrossRef]
- Aboelatta, Y.A.; Aly, H.M. Free Tissue Transfer and Replantation in Pediatric Patients: Technical Feasibility and Outcome in a Series of 28 Patients. J. Hand Microsurg. 2016, 5, 74–80. [Google Scholar] [CrossRef]
- Temiz, G.; Bilkay, U.; Tiftikçioğlu, Y.Ö.; Mezili, C.T.; Songür, E. The evaluation of flap growth and long-term results of pediatric mandible reconstructions using free fibular flaps. Microsurgery 2015, 35, 253–261. [Google Scholar] [CrossRef]
- Cleveland, E.C.; Zampell, J.; Avraham, T.; Lee, Z.-H.; Hirsch, D.; Levine, J.P. Reconstruction of congenital mandibular hypoplasia with microvascular free fibula flaps in the pediatric population: A paradigm shift. J. Craniofacial Surg. 2017, 28, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Caron, C.; Pluijmers, B.; Maas, B.; Klazen, Y.; Katz, E.; Abel, F.; van der Schroeff, M.; Mathijssen, I.; Dunaway, D.; Mills, C.; et al. Obstructive sleep apnoea in craniofacial microsomia: Analysis of 755 patients. Int. J. Oral Maxillofac. Surg. 2017, 46, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.-C.; Demirkan, F.; Chen, H.-C.; Chen, I.-H. Double free flaps in reconstruction of extensive composite mandibular defects in head and neck cancer. Plast. Reconstr. Surg. 1999, 103, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.R.; Tibesar, R.J.; Sidman, J.D. Pierre Robin Sequence. Evaluation, Management, Indications for Surgery, and Pitfalls. Otolaryngol. Clin. N. Am. 2012, 45, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Cielo, C.M.; Marcus, C.L. Obstructive sleep apnoea in children with craniofacial syndromes. Paediatr. Respir. Rev. 2015, 16, 189–196. [Google Scholar] [CrossRef]
- Katz, E.S.; D’Ambrosio, C.M. Pediatric obstructive sleep apnea syndrome. Clin. Chest Med. 2010, 31, 221–234. [Google Scholar] [CrossRef]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef]
- Damlar, İ.; Altan, A.; Turgay, B.; Kiliç, S. Management of obstructive sleep apnea in a Treacher Collins syndrome patient using distraction osteogenesis of the mandible. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 388. [Google Scholar] [CrossRef]
- Uliel, S.; Tauman, R.; Greenfeld, M.; Sivan, Y. Normal polysomnographic respiratory values in children and adolescents. Chest 2004, 125, 872–878. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Su, Y.-X.; Wu, Y.-C.; Chang, J.Z.-C.; Tu, Y.-K. Management of paediatric obstructive sleep apnoea: A systematic review and network meta-analysis. Int. J. Paediatr. Dent. 2020, 30, 156–170. [Google Scholar] [CrossRef]
- Marcus, C.L.; Ward, S.L.D.; Mallory, G.B.; Rosen, C.L.; Beckerman, R.C.; Weese-Mayer, D.E.; Brouillette, R.T.; Trang, H.T.; Brooks, L.J. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J. Pediatr. 1995, 127, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Tahiri, Y.; Chang, C.S.; Tuin, J.; Paliga, J.T.; Lowe, K.M.; Taylor, J.A.; Bartlett, S.P. Costochondral grafting in craniofacial microsomia. Plast. Reconstr. Surg. 2015, 135, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.; Fattahi, T.; Steinberg, B. Costochondral Rib Grafts in Mandibular Reconstruction. Atlas Oral Maxillofac. Surg. Clin. 2006, 14, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Merkx, M.A.W.; Freihofer, H.P.M. Fracture of costochondral graft in temporomandibular joint reconstructive surgery: An unexpected complication. Int. J. Oral Maxillofac. Surg. 1995, 24, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fan, H.; Du, W.; Li, J.; Hu, J.; Luo, E. Overgrowth of costochondral grafts in craniomaxillofacial reconstruction: Rare complication and literature review. J. Cranio-Maxillofac. Surg. 2015, 43, 803–812. [Google Scholar] [CrossRef]
- Chan, C.; Włodarczyk, J.R.; Wolfswinkel, E.M.; Odono, L.T.; Urata, M.M.; Hammoudeh, J.A. Establishing a Novel Treatment Algorithm for Pediatric Mandibular Tumor Reconstruction. J. Craniofacial Surg. 2022, 33, 744–749. [Google Scholar] [CrossRef]
- Acosta, H.L.; Stelnicki, E.J.; Boyd, J.B.; Barnavon, Y.; Uecker, C. Vertical mesenchymal distraction and bilateral free fibula transfer for severe Treacher Collins syndrome. Plast. Reconstr. Surg. 2004, 113, 1209–1217. [Google Scholar] [CrossRef]
- Shokri, T.; Stahl Bs, L.E.; Kanekar, S.G.; Goyal, N. Osseous Changes Over Time in Free Fibular Flap Reconstruction. Laryngoscope 2019, 129, 1113–1116. [Google Scholar] [CrossRef]
- Warren, S.M.; Borud, L.J.; Brecht, L.E.; Longaker, M.T.; Siebert, J.W. Microvascular reconstruction of the pediatric mandible. Plast. Reconstr. Surg. 2007, 119, 649–661. [Google Scholar] [CrossRef]
- Dowgierd, K.; Pokrowiecki, R.; Borowiec, M.; Kozakiewicz, M.; Smyczek, D.; Krakowczyk, Ł. A protocol for the use of a combined microvascular free flap with custom-made 3d-printed total temporomandibular joint (TMJ) prosthesis for mandible reconstruction in children. Appl. Sci. 2021, 11, 2176. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Liang, T.; Peng, X. Mandibular growth after paediatric mandibular reconstruction with the vascularized free fibula flap: A systematic review. Int. J. Oral Maxillofac. Surg. 2016, 45, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Krakowczyk, Ł.; Piotrowska-Seweryn, A.; Szymczyk, C.; Wierzgoń, J.; Oleś, K.; Ulczok, R.; Donocik, K.; Dowgierd, K.; Maciejewski, A. Virtual surgical planning and cone beam computed tomography in reconstruction of head and neck tumors—Pilot study. Otolaryngol. Pol. 2020, 74, 28–33. [Google Scholar] [CrossRef] [PubMed]
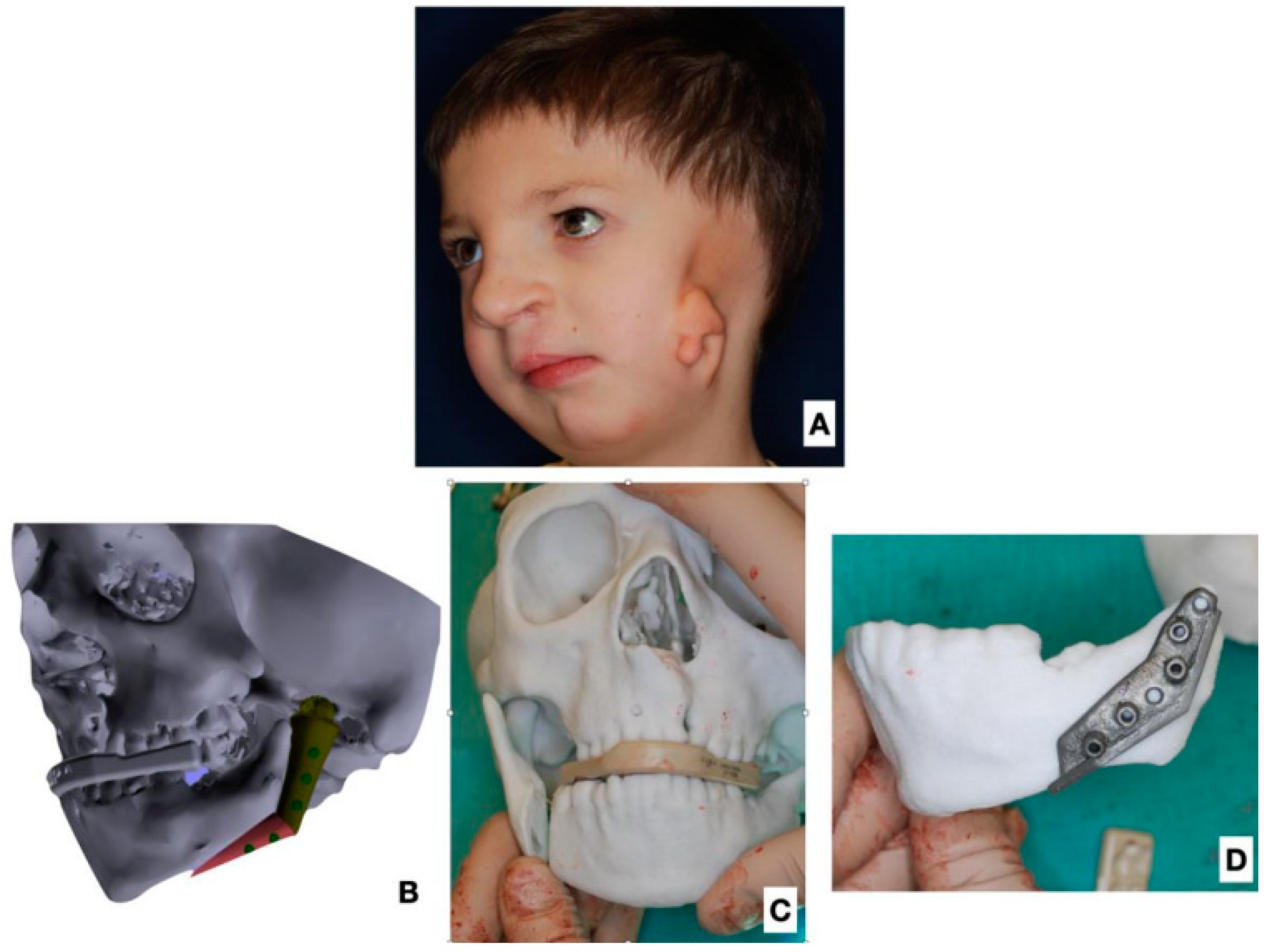


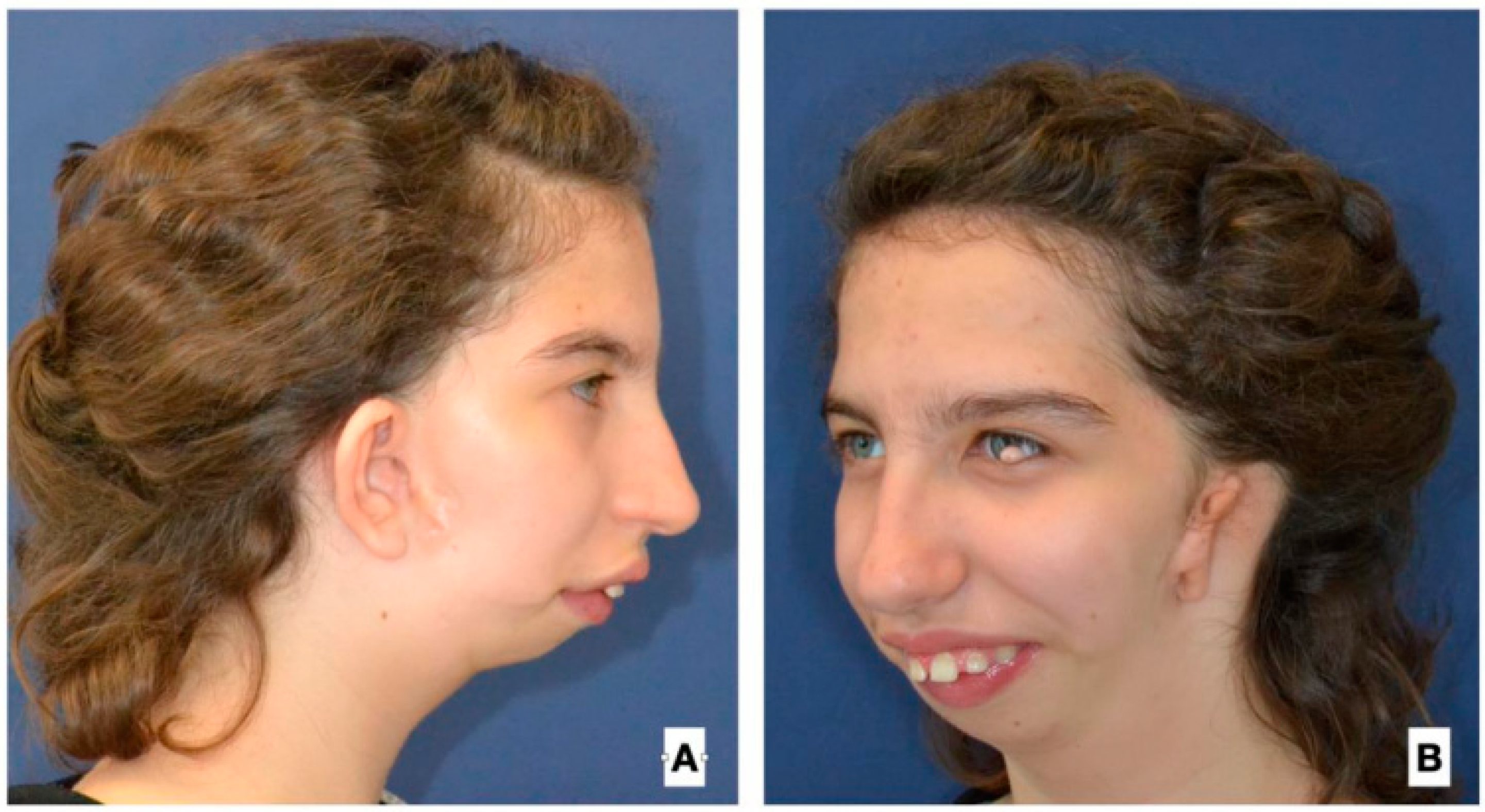
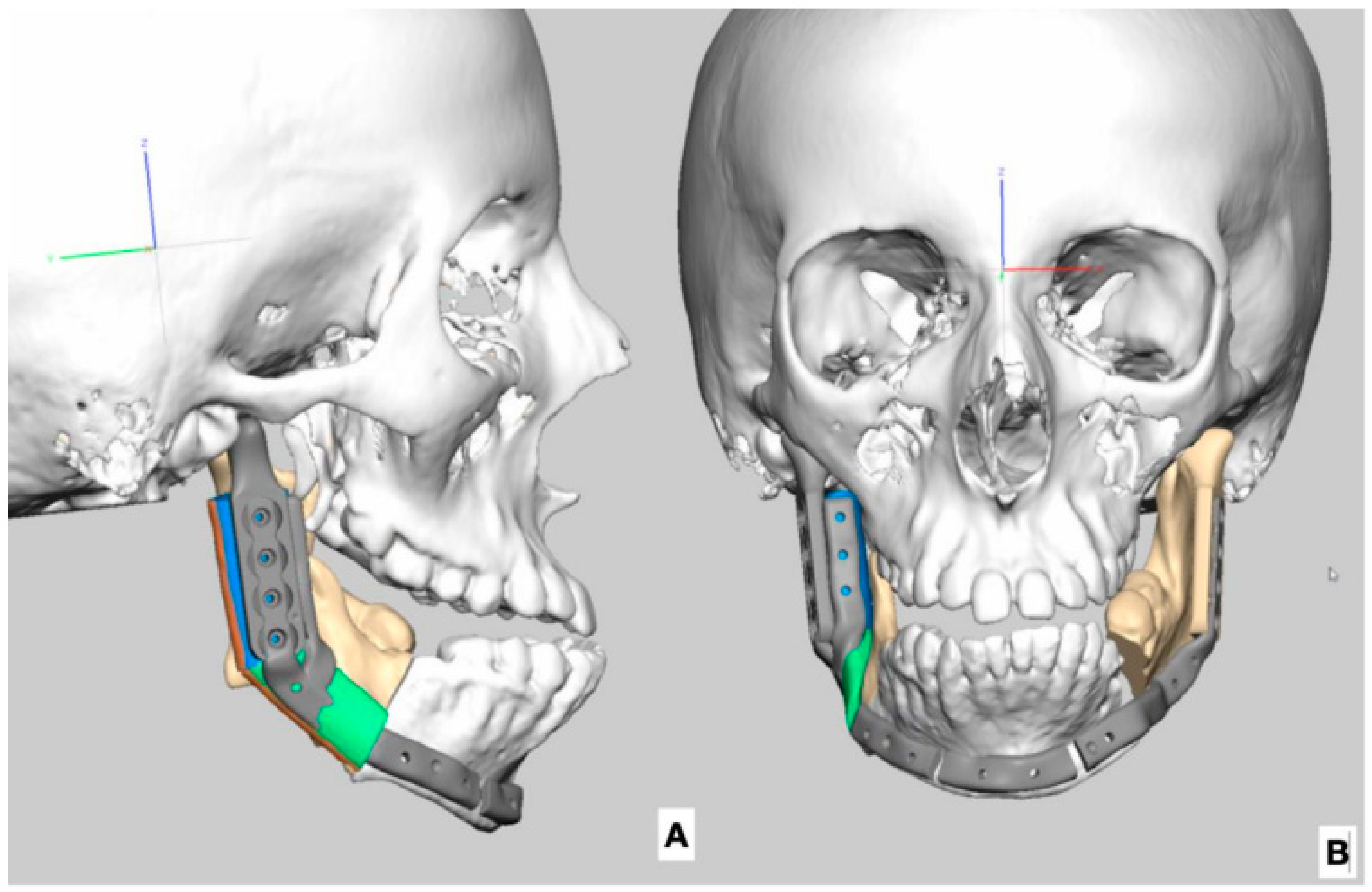
| Age at 1st Operation y.o. | Gender | Side | Pruzansky | Additinal Deformations of Face | Other Deformations |
|---|---|---|---|---|---|
| 4 | Female | Left | III | Soft tissue atrophy | |
| 4 | Male | Left | III | Microtia, atresia, VII palsy, soft tissue atrophy | |
| 4 | Female | Left | III | Microtia, atresia, VII palsy, soft tissue atrophy, orbital dystopia, cervical spinal deformation | |
| 5 | Male | Left | III | Cleft LP orbital dystopia anoftalmia, mictai, VII palsy, soft tissue atrophy | Cervical spinal deformation, scoliosis |
| 5 | Female | Right | III | Microtia, atresia, VII palsy, soft tissue atrophy | |
| 5 | Male | Left | IIB | Microtia, atresia, VII palsy, soft tissue atrophy | Forearm deformation, cervical spinal deformation |
| 5 | Female | Right | IIB | Microtia, atresia, VII palsy, soft tissue atrophy | Forearm deformation |
| 7 | Male | Right | III | Microtia, atresia, VII palsy, soft tissue atrophy, orbital dystopia, deformation Cleft Tessier 11/4 | Cervical spinal deformation |
| 8 | Male | Left | III | Microtia, atresia, VII palsy, soft tissue atrophy, orbital dystopia, cervical spinal deformation | |
| 9 | Male | Left | IIB | Microtia, atresia, VII palsy, soft tissue atrophy | |
| 14 | Male | Right | IIB | Microtia, atresia, VII palsy, soft tissue atrophy | |
| 15 | Female | Right | Bilat III/II B | Microtia, atresia, soft tissue atrophy | |
| 17 | Male | Right | III | Microtia, atresia, VII palsy, soft tissue atrophy |
| Age before Treatment y.o. | Previous Procedures | Side | Type of Flap | Number of Pieces | Soft Tissue Island | Complications | VSP and IPS | Time of Observation in Months |
|---|---|---|---|---|---|---|---|---|
| 4 | left | FFF | 2 | Yes | No | No | 59 | |
| 4 | right | FFF | 1 | No | Bone resorption | No | 77 | |
| 4 | right | FFF | 1 | Yes | No | Yes | 15 | |
| 5 | left | FFF | 2 | Yes | No | Yes | 9 | |
| 5 | left | FFF | 1 | Yes | No | Yes | 17 | |
| 5 | CCG | left | FFF | 2 | No | No | Yes | 24 |
| 5 | right | FFF | 2 | Yes | Partial bone resorption | Yes | 24 | |
| 7 | right | FFF | 1 | No | No | Yes | 33 | |
| 8 | left | FFF | 2 | Yes | No | Yes | 37 | |
| 9 | left | FFF | 2 | Yes | No | Yes | 43 | |
| 14 | left | FFF | 2 | Yes | No | Yes | 47 | |
| 15 | right | FFF | 2 | Yes | No | Yes | 53 | |
| 17 | CCG | right | FFF | 2 | No | No | Yes | 69 |
| Age before Treatment y.o. | TRACHEO before FFFR | Tracheostomy Removal | AHI before FFFR | AHI after FFFR | AHI before DO | Age during DO y.o. | AHI after DO | Additional Surgery |
|---|---|---|---|---|---|---|---|---|
| 4 | n | n | 26 | 16 | 22 | 6 | 10 | |
| 4 | n | n | 24 | 15 | 23 | 6 | 13 | |
| 4 | y | y | TRACHEO | 16 | 22 | 6 | 12 | |
| 5 | n | n | 23 | 11 | 18 | 8 | 16 | |
| 5 | y | n | TRACHEO | 21 | 24 | 8 | 12 | |
| 5 | n | n | 20 | 19 | 18 | No DO | No DO/9 | |
| 5 | y | y | TRACHEO | 13 | 17 | No DO | No DO/10 | |
| 7 | y | n | TRACHEO | TRACHO | TRACHO | No DO | TRACHEO | |
| 8 | n | n | 20 | 12 | 20 | 13 | 12 | |
| 9 | n | n | 17 | 10 | 15 | No DO | No DO/9 | |
| 14 | n | n | 16 | 12 | 18 | No DO | No DO/11 | TMR/BIMAX |
| 15 | y | y | 27 | 11 | 16 | No DO | No DO/11 | TMR/BIMAX |
| 17 | n | n | 20 | 12 | 16 | No DO | No DO/11 | TMR/BIMAX |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dowgierd, K.; Pokrowiecki, R.; Myśliwiec, A.; Krakowczyk, Ł. Use of a Fibula Free Flap for Mandibular Reconstruction in Severe Craniofacial Microsomia in Children with Obstructive Sleep Apnea. J. Clin. Med. 2023, 12, 1124. https://doi.org/10.3390/jcm12031124
Dowgierd K, Pokrowiecki R, Myśliwiec A, Krakowczyk Ł. Use of a Fibula Free Flap for Mandibular Reconstruction in Severe Craniofacial Microsomia in Children with Obstructive Sleep Apnea. Journal of Clinical Medicine. 2023; 12(3):1124. https://doi.org/10.3390/jcm12031124
Chicago/Turabian StyleDowgierd, Krzysztof, Rafał Pokrowiecki, Andrzej Myśliwiec, and Łukasz Krakowczyk. 2023. "Use of a Fibula Free Flap for Mandibular Reconstruction in Severe Craniofacial Microsomia in Children with Obstructive Sleep Apnea" Journal of Clinical Medicine 12, no. 3: 1124. https://doi.org/10.3390/jcm12031124
APA StyleDowgierd, K., Pokrowiecki, R., Myśliwiec, A., & Krakowczyk, Ł. (2023). Use of a Fibula Free Flap for Mandibular Reconstruction in Severe Craniofacial Microsomia in Children with Obstructive Sleep Apnea. Journal of Clinical Medicine, 12(3), 1124. https://doi.org/10.3390/jcm12031124






