The Response of Macrophages in Sepsis-Induced Acute Kidney Injury
Abstract
1. Introduction
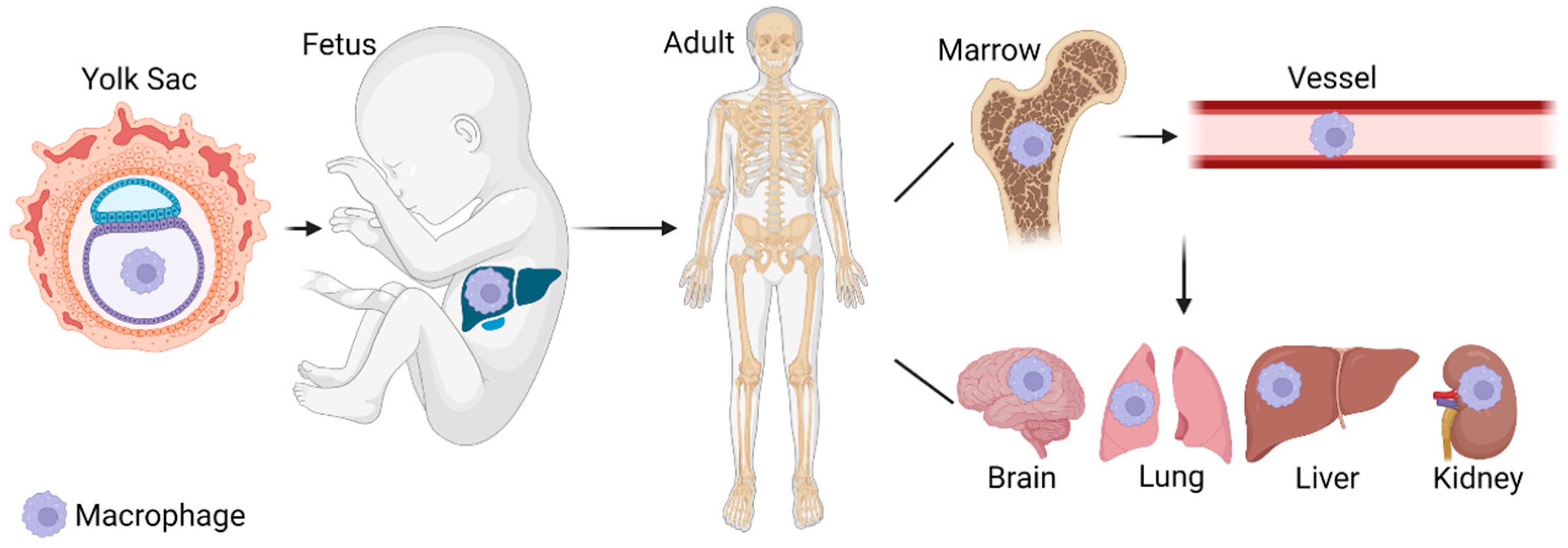
2. Macrophage Origins
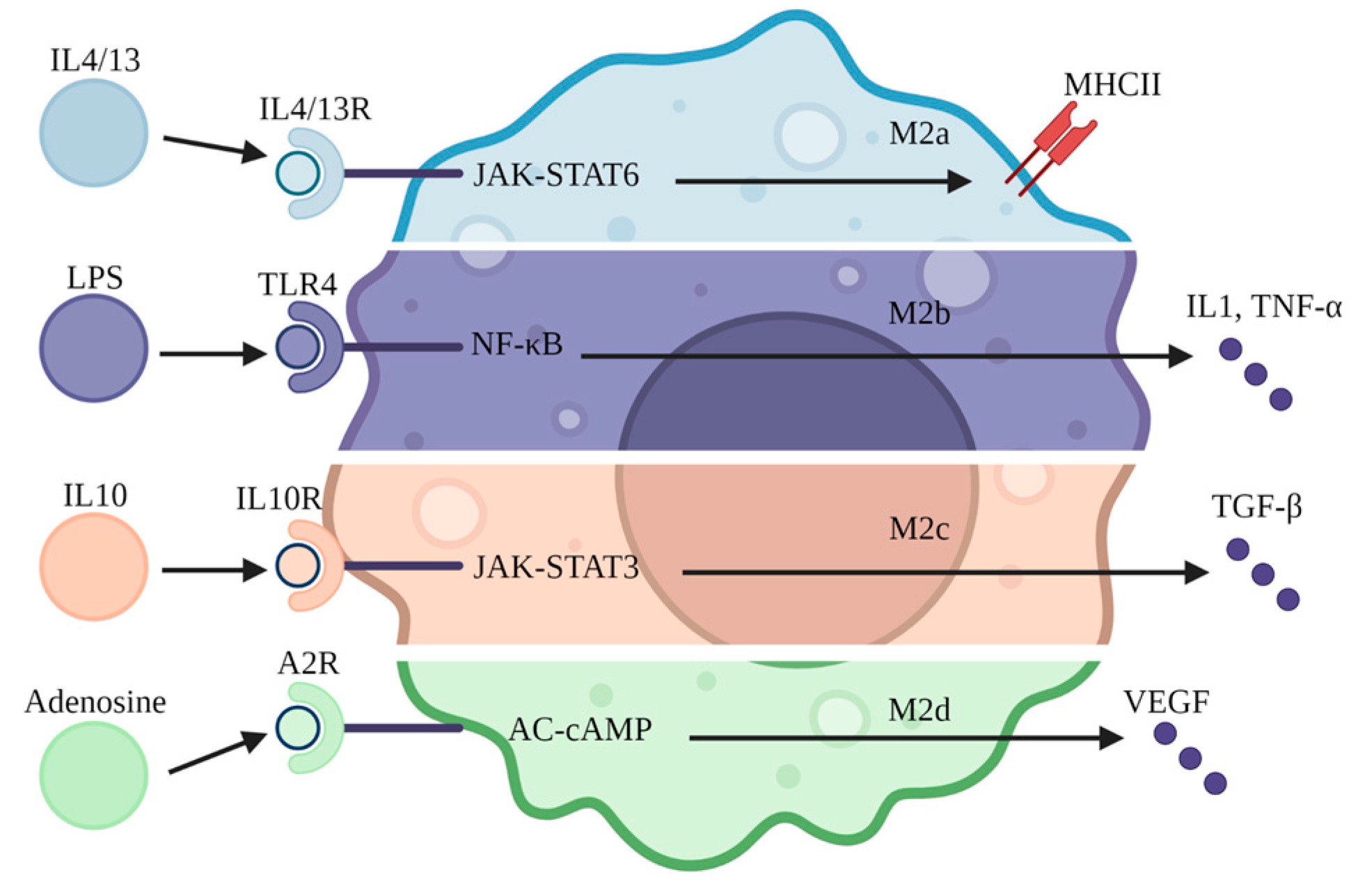
3. Macrophage Subtypes
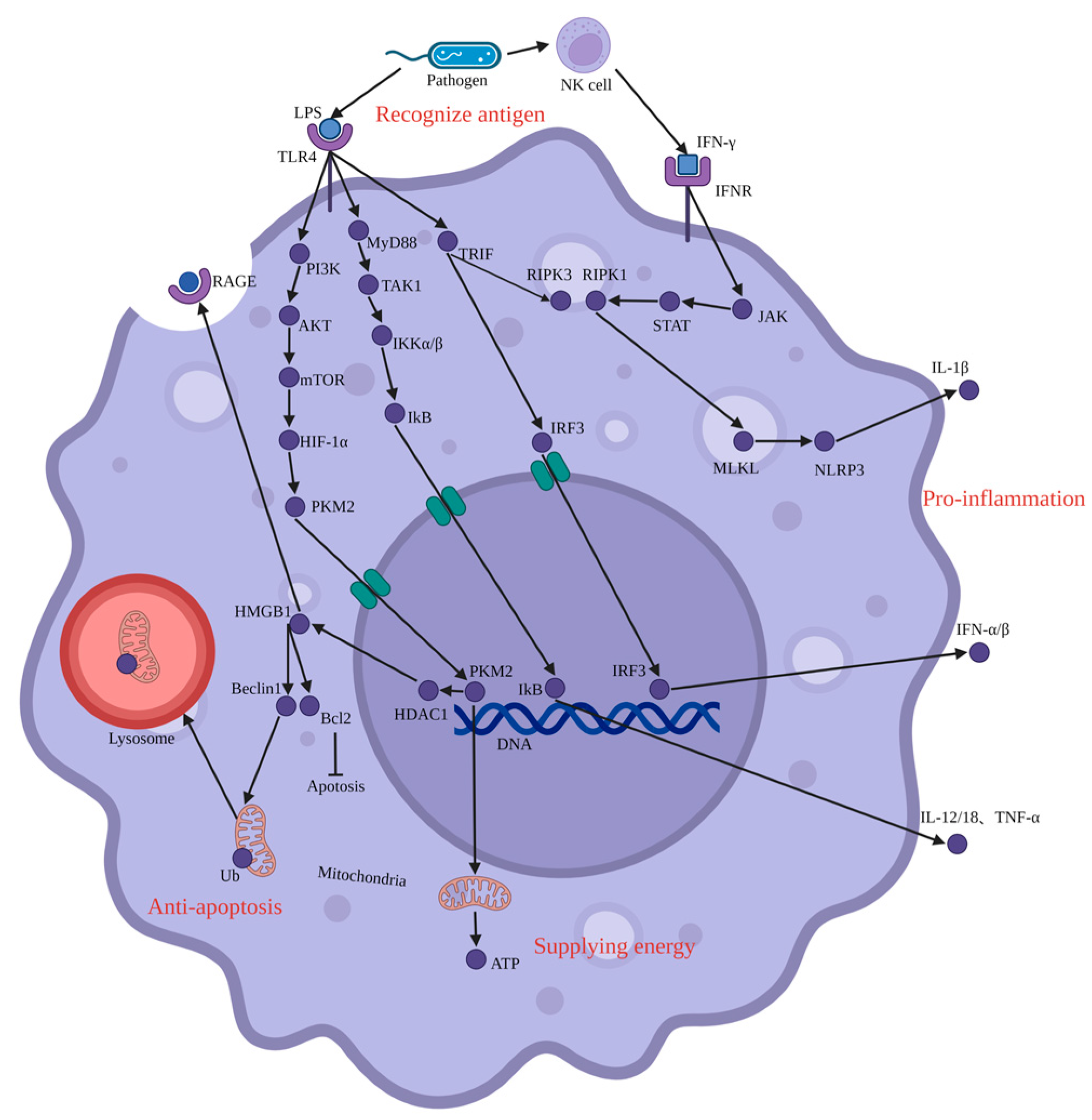
4. Pro-Inflammation and Injury
4.1. JAK-STAT
4.2. NF-κB
4.3. IRF3-IFN
4.4. PI3K-AKT-mTOR
5. Macrophage Transformation
6. Anti-Inflammation and Repair
7. Targeting Macrophages to Treat SAKI
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Poston, J.T.; Koyner, J.L. Sepsis associated acute kidney injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, S.; Verheijden, S.; Aguilera-Lizarraga, J.; Viola, M.F.; Boesmans, W.; Stakenborg, N.; Voytyuk, I.; Schmidt, I.; Boeckx, B.; de Casterlé, I.D.; et al. Self-Maintaining Gut Macrophages Are Essential for Intestinal Homeostasis. Cell 2018, 175, 400–415. [Google Scholar] [CrossRef]
- Nelson, P.J.; Rees, A.J.; Griffin, M.D.; Hughes, J.; Kurts, C.; Duffield, J. The renal mononuclear phagocytic system. J. Am. Soc. Nephrol. 2012, 23, 194–203. [Google Scholar] [CrossRef]
- Rogers, N.M.; Ferenbach, D.A.; Isenberg, J.S.; Thomson, A.W.; Hughes, J. Dendritic cells and macrophages in the kidney: A spectrum of good and evil. Nat. Rev. Nephrol. 2014, 10, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Varol, C.; Mildner, A.; Jung, S. Macrophages: Development and tissue specialization. Annu. Rev. Immunol. 2015, 33, 643–675. [Google Scholar] [CrossRef]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef]
- A-Gonzalez, N.; Quintana, J.A.; García-Silva, S.; Mazariegos, M.; de la Aleja, A.G.; Nicolás-Ávila, J.A.; Walter, W.; Adrover, J.M.; Crainiciuc, G.; Kuchroo, V.K.; et al. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med. 2017, 214, 1281–1296. [Google Scholar] [CrossRef]
- Salei, N.; Rambichler, S.; Salvermoser, J.; Papaioannou, N.E.; Schuchert, R.; Pakalniškytė, D.; Li, N.; Marschner, J.A.; Lichtnekert, J.; Stremmel, C.; et al. The Kidney Contains Ontogenetically Distinct Dendritic Cell and Macrophage Subtypes throughout Development That Differ in Their Inflammatory Properties. J. Am. Soc. Nephrol. 2020, 31, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.M.; Hull, T.D.; Boddu, R.; Pepin, M.E.; Black, L.M.; Adedoyin, O.O.; Yang, Z.; Traylor, A.M.; Jiang, Y.; Li, Z.; et al. Resident macrophages reprogram toward a developmental state after acute kidney injury. JCI Insight 2019, 4, e125503. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.-K.; Nikolic-Paterson, D.J.; Lan, H.-Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Ma, R.-Y.; Black, A.; Qian, B.-Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022, 43, 546–563. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Lichtnekert, J.; Thompson, L.J.; Karna, P.; Bouabe, H.; Hohl, T.M.; Heinecke, J.W.; Ziegler, S.F.; Nelson, P.J.; Duffield, J.S. Resident renal mononuclear phagocytes comprise five discrete populations with distinct phenotypes and functions. J. Immunol. 2013, 191, 3358–3372. [Google Scholar] [CrossRef]
- Nordlohne, J.; Hulsmann, I.; Schwafertz, S.; Zgrajek, J.; Grundmann, M.; von Vietinghoff, S.; Eitner, F.; Becker, M.S. A flow cytometry approach reveals heterogeneity in conventional subsets of murine renal mononuclear phagocytes. Sci. Rep. 2021, 11, 13251. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, K.A.; Bentley, M.R.; Lever, J.M.; Li, Z.; Crossman, D.K.; Song, C.J.; Liu, S.; Crowley, M.R.; George, J.F.; Mrug, M. Single-Cell RNA Sequencing Identifies Candidate Renal Resident Macrophage Gene Expression Signatures across Species. J. Am. Soc. Nephrol. 2019, 30, 767–781. [Google Scholar] [CrossRef]
- Berry, M.R.; Mathews, R.; Ferdinand, J.; Jing, C.; Loudon, K.W.; Wlodek, E.; Dennison, T.; Kuper, C.; Neuhofer, W.; Clatworthy, M. Renal Sodium Gradient Orchestrates a Dynamic Antibacterial Defense Zone. Cell 2017, 170, 860–874. [Google Scholar] [CrossRef]
- Potter, S.S. Single-cell RNA sequencing for the study of development, physiology and disease. Nat. Rev. Nephrol. 2018, 14, 479–492. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Stamatiades, E.G.; Tremblay, M.-E.; Bohm, M.; Crozet, L.; Bisht, K.; Kao, D.; Coelho, C.; Fan, X.; Yewdell, W.T.; Davidson, A.; et al. Immune Monitoring of Trans-endothelial Transport by Kidney-Resident Macrophages. Cell 2016, 166, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guo, Z.; Lai, S.; Chen, H. Interference with miR-210 Alleviated Renal Injury in Septic Rats by Inhibiting JAK-STAT Pathway. Inflammation 2020, 43, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Fusella, F.; Seclì, L.; Cannata, C.; Brancaccio, M. The one thousand and one chaperones of the NF-κB pathway. Cell. Mol. Life Sci. 2020, 77, 2275–2288. [Google Scholar] [CrossRef]
- Maciag, K.; Raychowdhury, R.; Smith, K.; Schneider, A.M.; Coers, J.; Mumbach, M.R.; Schwartz, S.; Hacohen, N. IRF3 inhibits IFN-γ-mediated restriction of intracellular pathogens in macrophages independently of IFNAR. J. Leukoc. Biol. 2022, 112, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Martin-Sanchez, D.; Guerrero-Mauvecin, J.; Fontecha-Barriuso, M.; Mendez-Barbero, N.; Saiz, M.L.; Lopez-Diaz, A.M.; Sanchez-Niño, M.D.; Carrasco, S.; Cannata-Ortiz, P.; Ruiz-Ortega, M.; et al. Bone Marrow-Derived RIPK3 Mediates Kidney Inflammation in Acute Kidney Injury. J. Am. Soc. Nephrol. 2022, 33, 357–373. [Google Scholar] [CrossRef]
- Aaronson, D.S.; Horvath, C.M. A road map for those who don’t know JAK-STAT. Science 2002, 296, 1653–1655. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.-H.; Kim, S.-H.; Jeon, S.-H.; Kang, M.-J.; Choi, J.-M. Macrophage-preferable delivery of the leucine-rich repeat domain of NLRX1 ameliorates lethal sepsis by regulating NF-κB and inflammasome signaling activation. Biomaterials 2021, 274, 120845. [Google Scholar] [CrossRef]
- Martinon, F. Detection of immune danger signals by NALP3. J. Leukoc. Biol. 2008, 83, 507–511. [Google Scholar] [CrossRef]
- Smith, E.M.; Gregg, M.; Hashemi, F.; Schott, L.; Hughes, T.K. Corticotropin Releasing Factor (CRF) activation of NF-kappaB-directed transcription in leukocytes. Cell. Mol. Neurobiol. 2006, 26, 1021–1036. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef]
- Linton, M.F.; Moslehi, J.J.; Babaev, V.R. Akt Signaling in Macrophage Polarization, Survival, and Atherosclerosis. Int. J. Mol. Sci. 2019, 20, 2703. [Google Scholar] [CrossRef] [PubMed]
- Hiscott, J.; Pitha, P.; Genin, P.; Nguyen, H.; Heylbroeck, C.; Mamane, Y.; Algarte, M.; Lin, R. Triggering the interferon response: The role of IRF-3 transcription factor. J. Interferon Cytokine Res. 1999, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Vance, R.E. STING and the innate immune response to nucleic acids in the cytosol. Nat. Immunol. 2013, 14, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, M.; Yang, M.; Yu, Y.; Zhu, S.; Hou, W.; Kang, R.; Lotze, M.T.; Billiar, T.R.; Wang, H.; et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 2014, 5, 4436. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, Y.; Wang, J.; Shi, X.; Liu, Q.; Liu, Z.; Li, Y.; Scott, M.J.; Xiao, G.; Li, S.; et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014, 21, 1229–1239. [Google Scholar] [CrossRef]
- Zhu, C.S.; Wang, W.; Qiang, X.; Chen, W.; Lan, X.; Li, J.; Wang, H. Endogenous Regulation and Pharmacological Modulation of Sepsis-Induced HMGB1 Release and Action: An Updated Review. Cells 2021, 10, 2220. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, X.; Zhang, Q.-J.; Zhu, M.; Liu, Z.-P.; Ci, B.; Xie, Y.; Carlson, D.; Rothermel, B.A.; Sun, Y.; et al. Beclin-1-Dependent Autophagy Protects the Heart During Sepsis. Circulation 2018, 138, 2247–2262. [Google Scholar] [CrossRef]
- Deng, C.; Zhao, L.; Yang, Z.; Shang, J.-J.; Wang, C.-Y.; Shen, M.-Z.; Jiang, S.; Li, T.; Di, W.-C.; Chen, Y.; et al. Targeting HMGB1 for the treatment of sepsis and sepsis-induced organ injury. Acta Pharmacol. Sin. 2022, 43, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Fan, M.; Wang, X.; Xu, J.; Wang, Y.; Tu, F.; Gill, P.S.; Ha, T.; Liu, L.; Williams, D.L.; et al. Lactate promotes macrophage HMGB1 lactylation, acetylation, and exosomal release in polymicrobial sepsis. Cell. Death Differ. 2022, 29, 133–146. [Google Scholar] [CrossRef]
- Kim, M.J.; Bae, S.H.; Ryu, J.C.; Kwon, Y.; Oh, J.H.; Kwon, J.; Moon, J.S.; Kim, K.; Miyawaki, A.; Lee, M.G. SESN2/sestrin2 suppresses sepsis by inducing mitophagy and inhibiting NLRP3 activation in macrophages. Autophagy 2016, 12, 1272–1291. [Google Scholar] [CrossRef]
- Patoli, D.; Mignotte, F.; Deckert, V.; Dusuel, A.; Dumont, A.; Rieu, A.; Jalil, A.; Van Dongen, K.; Bourgeois, T.; Gautier, T. Inhibition of mitophagy drives macrophage activation and antibacterial defense during sepsis. J. Clin. Investig. 2020, 130, 5858–5874. [Google Scholar] [CrossRef]
- Lv, L.L.; Wang, C.; Li, Z.L.; Cao, J.Y.; Zhong, X.; Feng, Y.; Chen, J.; Tang, T.T.; Ni, H.F.; Wu, Q.L.; et al. SAP130 released by damaged tubule drives necroinflammation via miRNA-219c/Mincle signaling in acute kidney injury. Cell. Death Dis. 2021, 12, 866. [Google Scholar] [CrossRef]
- Li, B.; Liu, C.; Tang, K.; Dong, X.; Xue, L.; Su, G.; Zhang, W.; Jin, Y. Aquaporin-1 attenuates macrophage-mediated inflammatory responses by inhibiting p38 mitogen-activated protein kinase activation in lipopolysaccharide-induced acute kidney injury. Inflamm. Res. 2019, 68, 1035–1047. [Google Scholar] [CrossRef]
- Gong, Q.; Jiang, Y.; Pan, X.; You, Y. Fractalkine aggravates LPS-induced macrophage activation and acute kidney injury via Wnt/β-catenin signalling pathway. J. Cell. Mol. Med. 2021, 25, 6963–6975. [Google Scholar] [CrossRef]
- Lv, L.-L.; Feng, Y.; Wu, M.; Wang, B.; Li, Z.-L.; Zhong, X.; Wu, W.-J.; Chen, J.; Ni, H.-F.; Tang, T.-T.; et al. Exosomal miRNA-19b-3p of tubular epithelial cells promotes M1 macrophage activation in kidney injury. Cell. Death Differ. 2020, 27, 210–226. [Google Scholar] [CrossRef]
- Lv, L.L.; Tang, P.M.K.; Li, C.J.; You, Y.K.; Li, J.; Huang, X.-R.; Ni, J.; Feng, M.; Liu, B.C.; Lan, H.-Y. The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int. 2017, 91, 587–602. [Google Scholar] [CrossRef]
- Cushing, L.; Winkler, A.; Jelinsky, S.A.; Lee, K.; Korver, W.; Hawtin, R.; Rao, V.R.; Fleming, M.; Lin, L.-L. IRAK4 kinase activity controls Toll-like receptor-induced inflammation through the transcription factor IRF5 in primary human monocytes. J. Biol. Chem. 2017, 292, 18689–18698. [Google Scholar] [CrossRef]
- Chen, X.S.; Wang, S.H.; Liu, C.Y.; Gao, Y.L.; Meng, X.L.; Wei, W.; Shou, S.T.; Liu, Y.C.; Chai, Y.F. Losartan attenuates sepsis-induced cardiomyopathy by regulating macrophage polarization via TLR4-mediated NF-κB and MAPK signaling. Pharmacol. Res. 2022, 185, 106473. [Google Scholar] [CrossRef]
- Liangliang, Z.; Mu, G.; Song, C.; Zhou, L.; He, L.; Jin, Q.; Lu, Z. Role of M2 Macrophages in Sepsis-Induced Acute Kidney Injury. Shock 2018, 50, 233–239. [Google Scholar]
- Hu, W.; Lin, J.; Lian, X.; Yu, F.; Liu, W.; Wu, Y.; Fang, X.; Liang, X.; Hao, W. M2a and M2b macrophages predominate in kidney tissues and M2 subpopulations were associated with the severity of disease of IgAN patients. Clin. Immunol. 2019, 205, 8–15. [Google Scholar] [CrossRef]
- Bianchini, R.; Roth-Walter, F.; Ohradanova-Repic, A.; Flicker, S.; Hufnagl, K.; Fischer, M.B.; Stockinger, H.; Jensen-Jarolim, E. IgG4 drives M2a macrophages to a regulatory M2b-like phenotype: Potential implication in immune tolerance. Allergy 2019, 74, 483–494. [Google Scholar] [CrossRef]
- Lu, J.; Cao, Q.; Zheng, D.; Sun, Y.; Wang, C.; Yu, X.; Wang, Y.; Lee, V.W.; Zheng, G.; Tan, T.K.; et al. Discrete functions of M2a and M2c macrophage subsets determine their relative efficacy in treating chronic kidney disease. Kidney Int. 2013, 84, 745–755. [Google Scholar] [CrossRef]
- Su, J.; Morgani, S.M.; David, C.J.; Wang, Q.; Er, E.E.; Huang, Y.-H.; Basnet, H.; Zou, Y.; Shu, W.; Soni, R.K.; et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature 2020, 577, 566–571. [Google Scholar] [CrossRef]
- Kumar, S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 2018, 93, 27–40. [Google Scholar] [CrossRef]
- Xu, M.J.; Feng, D.; Wang, H.; Guan, Y.; Yan, X.; Gao, B. IL-22 ameliorates renal ischemia-reperfusion injury by targeting proximal tubule epithelium. J. Am. Soc. Nephrol. 2014, 25, 967–977. [Google Scholar] [CrossRef]
- Molema, G.; Zijlstra, J.G.; van Meurs, M.; Kamps, J.A.A.M. Renal microvascular endothelial cell responses in sepsis-induced acute kidney injury. Nat. Rev. Nephrol. 2022, 18, 95–112. [Google Scholar] [CrossRef]
- Chung, S.; Overstreet, J.M.; Li, Y.; Wang, Y.; Niu, A.; Wang, S.; Fan, X.; Sasaki, K.; Jin, G.N.; Khodo, S.N. TGF-β promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 2018, 3, e123563. [Google Scholar] [CrossRef]
- Jongman, R.M.; Zwiers, P.J.; van de Sluis, B.; van der Laan, M.; Moser, J.; Zijlstra, J.G.; Dekker, D.; Huijkman, N.; Moorlag, H.E.; Popa, E.R. Partial Deletion of Tie2 Affects Microvascular Endothelial Responses to Critical Illness in A Vascular Bed and Organ-Specific Way. Shock 2019, 51, 757–769. [Google Scholar] [CrossRef]
- Wang, Q.; Ni, H.; Lan, L.; Wei, X.; Xiang, R.; Wang, Y. Fra-1 protooncogene regulates IL-6 expression in macrophages and promotes the generation of M2d macrophages. Cell. Res. 2010, 20, 701–712. [Google Scholar] [CrossRef]
- Gibier, J.-B.; Swierczewski, T.; Csanyi, M.; Hemon, B.; Glowacki, F.; Maboudou, P.; Van Seuningen, I.; Cauffiez, C.; Pottier, N.; Aubert, S.; et al. MUC1 Mitigates Renal Injury and Inflammation in Endotoxin-Induced Acute Kidney Injury by Inhibiting the TLR4-MD2 Axis and Reducing Pro-inflammatory Macrophages Infiltration. Shock 2021, 56, 629–638. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Zumbrun, E.E.; Guan, H.; Nagarkatti, P.S.; Nagarkatti, M. Resveratrol attenuates lipopolysaccharide-induced acute kidney injury by suppressing inflammation driven by macrophages. Mol. Nutr. Food Res. 2015, 59, 853–864. [Google Scholar] [CrossRef]
- Shu, B.; Feng, Y.; Gui, Y.; Lu, Q.; Wei, W.; Xue, X.; Sun, X.; He, W.; Yang, J.; Dai, C. Blockade of CD38 diminishes lipopolysaccharide-induced macrophage classical activation and acute kidney injury involving NF-κB signaling suppression. Cell. Signal. 2018, 42, 249–258. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, Q.; Zhu, P.; Ma, H.; Cui, S.; Li, J.; Hou, A.; Li, J. Rhodomeroterpene alleviates macrophage infiltration and the inflammatory response in renal tissue to improve acute kidney injury. FASEB J. 2021, 35, e21985. [Google Scholar] [CrossRef]
- Lee, J.; Son, W.; Hong, J.; Song, Y.; Yang, C.-S.; Kim, Y.-H. Down-regulation of TNF-α via macrophage-targeted RNAi system for the treatment of acute inflammatory sepsis. J. Control. Release 2021, 336, 344–353. [Google Scholar] [CrossRef]
- Li, Z.-L.; Yang, B.-C.; Gao, M.; Xiao, X.-F.; Zhao, S.-P.; Liu, Z.-L. Naringin improves sepsis-induced intestinal injury by modulating macrophage polarization via PPARγ/miR-21 axis. Mol. Ther. Nucleic Acids 2021, 25, 502–514. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, G.-H.; Cai, J.-J.; Zhang, R.; Zheng, Y.; Liu, J.-Q.; Yang, X.-H.; Yang, X.; Shen, Y.; Lai, J.-M.; et al. Tubule-mitophagic secretion of SerpinG1 reprograms macrophages to instruct anti-septic acute kidney injury efficacy of high-dose ascorbate mediated by NRF2 transactivation. Int. J. Biol. Sci. 2022, 18, 5168–5184. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Angsantikul, P.; Escajadillo, T.; Zhang, Q.; Olson, J.; Luk, B.T.; Zhang, S.; Fang, R.H.; Gao, W.; Nizet, V.; et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. USA 2017, 114, 11488–11493. [Google Scholar] [CrossRef]
- Lamontagne, F.; Masse, M.-H.; Menard, J.; Sprague, S.; Pinto, R.; Heyland, D.K.; Cook, D.J.; Battista, M.-C.; Day, A.G.; Guyatt, G.H.; et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. N. Engl. J. Med. 2022, 386, 2387–2398. [Google Scholar] [CrossRef]
- Lai, C.; Chadban, S.J.; Loh, Y.W.; Kwan, T.K.-T.; Wang, C.; Singer, J.; Niewold, P.; Ling, Z.; Spiteri, A.; Getts, D.; et al. Targeting inflammatory monocytes by immune-modifying nanoparticles prevents acute kidney allograft rejection. Kidney Int. 2022, 102, 1090–1102. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Zhao, W.; Zeng, C.; Deng, B.; McComb, D.W.; Du, S.; Zhang, C.; Li, W.; Dong, Y. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 2020, 15, 41–46. [Google Scholar] [CrossRef]
- Cao, H.; Gao, Y.; Jia, H.; Zhang, L.; Liu, J.; Mu, G.; Gui, H.; Wang, Y.; Yang, C.; Liu, J. Macrophage-Membrane-Camouflaged Nonviral Gene Vectors for the Treatment of Multidrug-Resistant Bacterial Sepsis. Nano Lett. 2022, 22, 7882–7891. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Xu, J.; Gao, C.; Zhang, W.; Fang, X.; Shang, Y. Nanomaterials targeting macrophages in sepsis: A promising approach for sepsis management. Front. Immunol. 2022, 13, 1026173. [Google Scholar] [CrossRef] [PubMed]
| Types | Role | |||||
|---|---|---|---|---|---|---|
| Old | M1 | CD11b, CD11c, CD68, CD86, F4/80 (EMR1 in human), Ly6C, MHCII, iNOS | Pro-inflammation | |||
| M2 | CD11b, CD11c, CD68, CD86, F4/80 (EMR1 in human), Ly6C, CD206, CD163 | Anti-inflammation | ||||
| New | CD11b | CD11c | F4/80 | Other | ||
| 1 | high | high | low | Secreting IL-1β and TNF-α, activating Treg cells | ||
| 2 | high | low | medium | Ly6C | The ability of phagocytosis is higher, but the ability to stimulate T cells is weak | |
| 3 | medium | medium | high | CX3CR1 | Secreting IL-10, activating Th2 cells | |
| 4 | medium | high | low | CD103 | The ability to activate Th1 cells is the strongest. | |
| 5 | low | medium | low | NA | ||
| CD11b | CD11c | F4/80 | CD206 | |||
| 1 | high | high | low | A:+ B:- | The phagocytic ability of A is higher than B. | |
| 2 | high | low | medium | A:+ B:- | The phagocytic ability of A is higher than B. | |
| 3 | medium | medium | high | A:+ B:- | The phagocytic ability of A is higher than B. | |
| 4 | medium | high | low | A:+ B:- | The amount of A is lower than B during acute stage, and gradually decreases with time. | |
| 5 | low | medium | low | A:+ B:- | The amount of A is lower than B during acute stage, and gradually decreases with time. | |
| CD74 + CD81+ | Common markers for mouse, rat, pig, and human renal macrophages | |||||
| Molecule | Introduction | Role |
|---|---|---|
| Mucin 1 | Glycoproteins on the surface of epithelial cells | Blocking the binding of LPS and TLR4 |
| Resveratrol | The natural phenol released by plants when attacked by pathogens | Blocking the binding of LPS and TLR4 |
| Quercetin | A plant flavonol widely found in fruits and vegetables | Blocking the binding of LPS and TLR4 |
| Rhodomeroterpene | New carotenoids isolated from rhododendron | Reducing the inflammatory response of macrophages |
| siRNA-TNF-α | A siRNA that can specifically knock out TNF-α | Reducing the inflammatory response of macrophages |
| Naringin | A flavonoid from citrus fruits, especially grapefruit | Inducing the transformation of macrophages from pro-inflammatory to anti-inflammatory |
| Vitamin C | A natural antioxidant is widely found in fruits and vegetables | Inducing the transformation of macrophages from pro-inflammatory to anti-inflammatory |
| Macrophage nanoparticles | Nanoparticles based on macrophages can absorb substances such as LPS. | Reducing tissue and cell damage |
| Antimicrobial peptides and cathepsin B (nanoparticles) | Nanoparticles formed on the basis of macrophages, having certain bactericidal activity. | Reducing tissue and cell damage |
| Plasmid DNA (nanoparticles) | Nanoparticles formed on the basis of macrophages, carrying antibacterial genes | Reducing tissue and cell damage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Zhao, S.; Duan, M. The Response of Macrophages in Sepsis-Induced Acute Kidney Injury. J. Clin. Med. 2023, 12, 1101. https://doi.org/10.3390/jcm12031101
He J, Zhao S, Duan M. The Response of Macrophages in Sepsis-Induced Acute Kidney Injury. Journal of Clinical Medicine. 2023; 12(3):1101. https://doi.org/10.3390/jcm12031101
Chicago/Turabian StyleHe, Jiawei, Shen Zhao, and Meili Duan. 2023. "The Response of Macrophages in Sepsis-Induced Acute Kidney Injury" Journal of Clinical Medicine 12, no. 3: 1101. https://doi.org/10.3390/jcm12031101
APA StyleHe, J., Zhao, S., & Duan, M. (2023). The Response of Macrophages in Sepsis-Induced Acute Kidney Injury. Journal of Clinical Medicine, 12(3), 1101. https://doi.org/10.3390/jcm12031101






