Arterial Blood-Flow Acceleration Time on Doppler Ultrasound Waveforms: What Are We Talking About?
Abstract
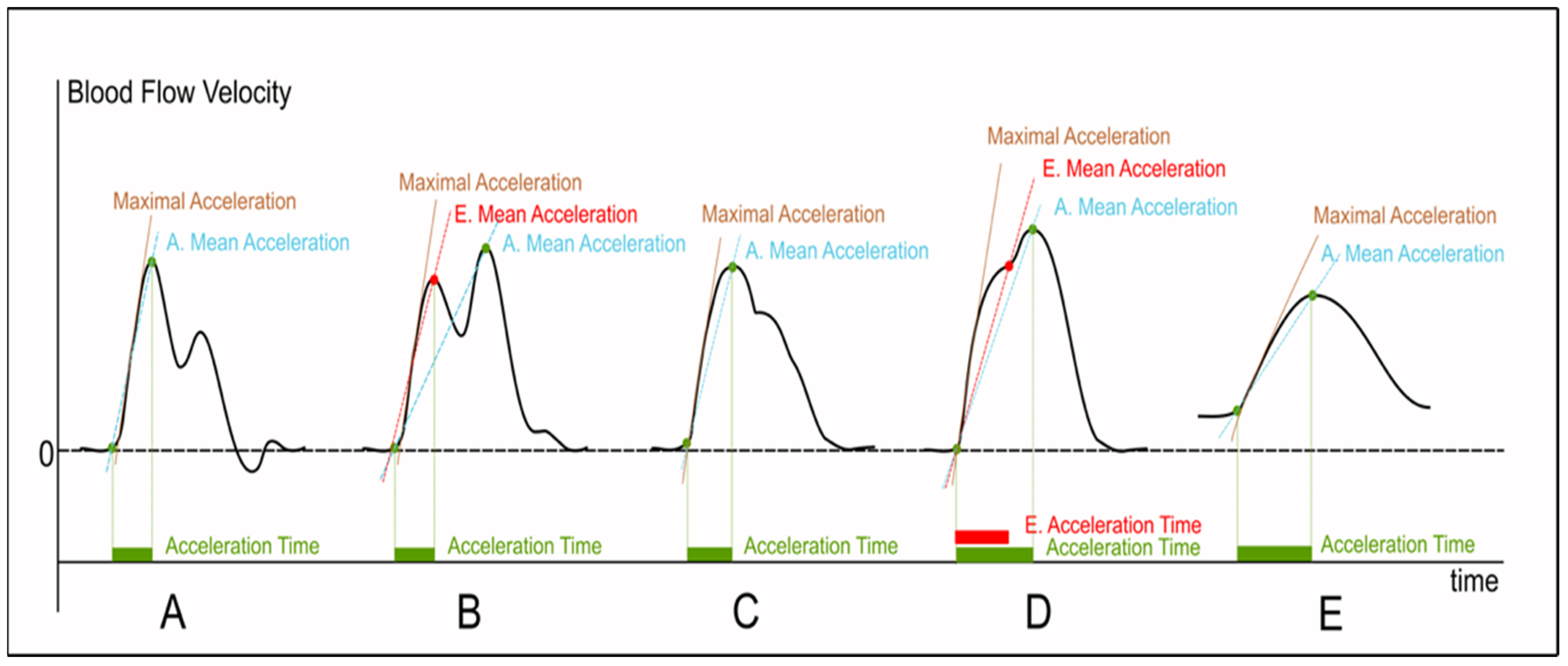
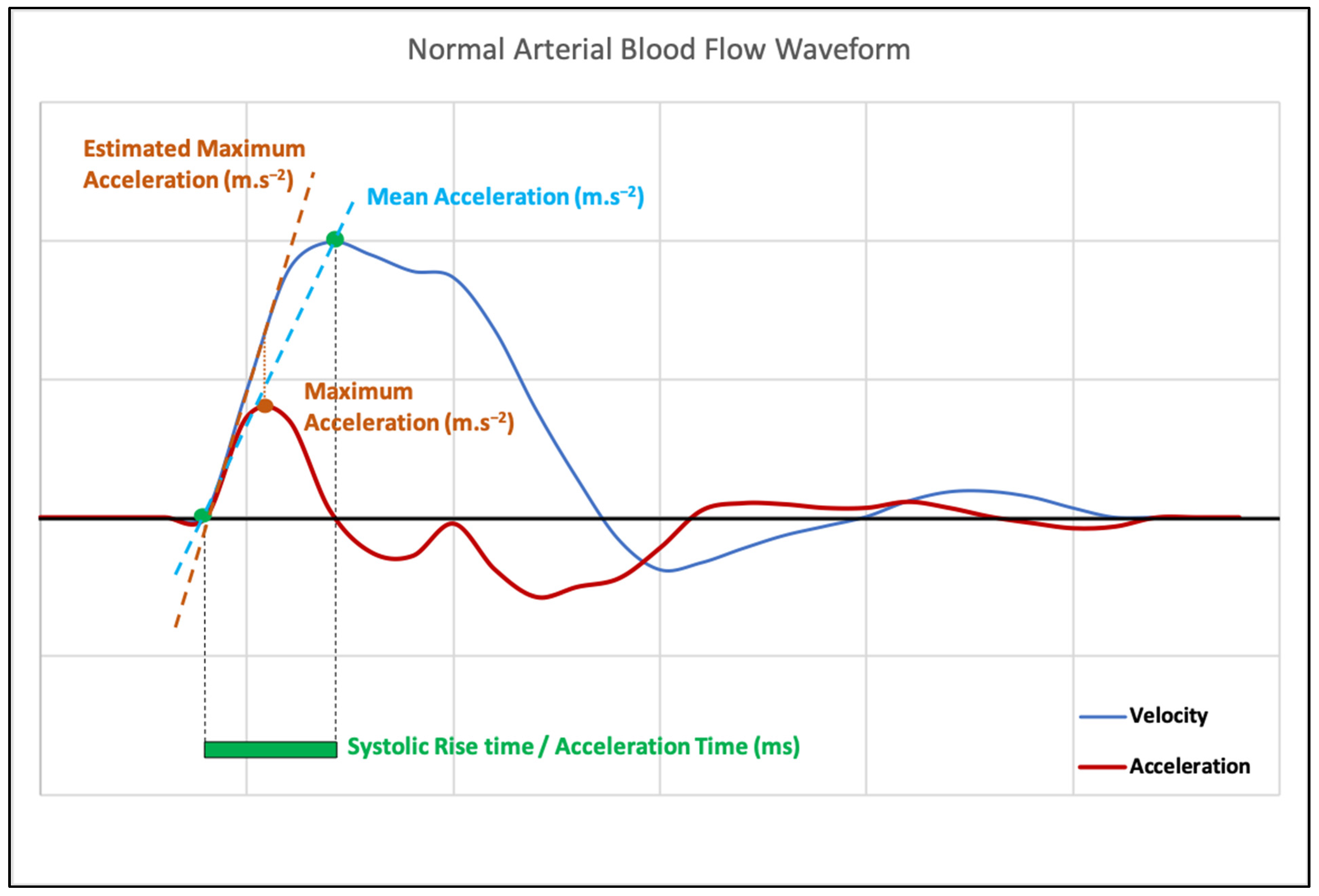
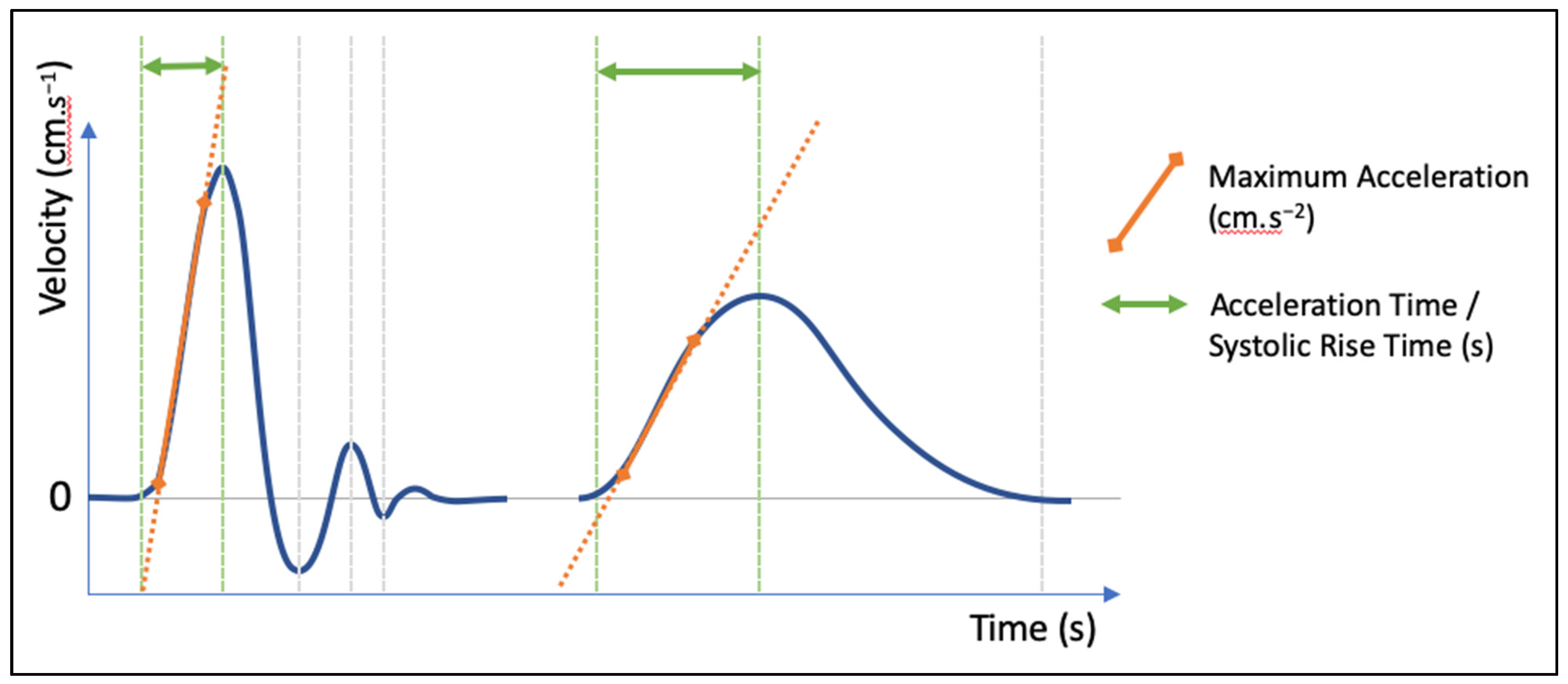
1. Rationale and Semantics
2. Measurement Methods
3. Proposal and Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nichols, W.W.; O’Rourke, M.F.; Vlachopoulos, C. Contours of pressure and flow waves in arteries. In McDonald’s Blood Flow in Arteries—Theoretical, Experimental, and Clinical Principles, 6th ed.; Hodder Arnold: London, UK, 2011; pp. 225–254. [Google Scholar]
- Humphries, K.N.; Hames, T.K.; Smith, S.W.J.; Cannon, V.A.; Chant, A.D.B. Quantitative assessment of the common femoral to popliteal arterial segment using continuous wave Doppler ultrasound. Ultrasound Med. Biol. 1980, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Takahara, S.; Ihara, H.; Ichikawa, Y.; Ishibashi, M.; Sagawa, S.; Nagano, S.; Takaha, M.; Sonoda, T. Predictability of renal allograft prognosis during rejection crisis by ultrasonic Doppler flow technique. Urology 1982, 19, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kitslaar, P.J.; Jörning, P.J.; Köhlen, J.P. Assessment of aortoiliac stenosis by femoral artery pressure measurement and Doppler waveform analysis. Eur. J. Vasc. Surg. 1988, 2, 35–40. [Google Scholar] [CrossRef]
- Mynard, J.P.; Kondiboyina, A.; Kowalski, R.; Cheung, M.M.H.; Smolich, J.J. Measurement, Analysis and Interpretation of Pressure/Flow Waves in Blood Vessels. Front. Physiol. 2020, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Vlachopoulos, C.V.; Alexopoulos, N.A.; Dima, I.; Pietri, P.G.; Protogerou, A.D.; Vyssoulis, G.; Stefanadis, C. The effect of heart rate on wave reflections may be determined by the level of aortic stiffness: Clinical and technical implications. Am. J. Hypertens. 2008, 21, 334–340. [Google Scholar] [CrossRef]
- Cunha, P.G.; Olsen, M.H. Chapter 24—Vascular Aging and Cardiovascular Disease. In Early Vascular Agin (EVA)—New Directions in Cardiovascular Protection; Nilsson, P.M., Olsen, M.H., Laurent, S., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 261–271. ISBN 9780128013878. [Google Scholar]
- Husmann, M.; Jacomella, V.; Thalhammer, C.; Amann-Vesti, B.R. Markers of arterial stiffness in peripheral arterial disease. VASA Z. Gefasskrankh. 2015, 44, 341–348. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. Recent Advances in Arterial Stiffness and Wave Reflection in Human Hypertension. Hypertension 2007, 49, 1202–1206. [Google Scholar] [CrossRef]
- Sommerset, J.; Karmy-Jones, R.; Dally, M.; Feliciano, B.; Vea, Y.; Teso, D. Plantar Acceleration Time: A Novel Technique to Evaluate Arterial Flow to the Foot. Ann. Vasc. Surg. 2019, 60, 308–314. [Google Scholar] [CrossRef]
- Sommerset, J.; Teso, D.; Feliciano, B.; Vea, Y.; Sentman, M.; Zimmerman, N.; Nyholm, C.; Honl, R.; Karmy-Jones, R. Innovative Arterial Duplex Examination: A Guide to Evaluate Flow in the Foot Using Pedal Acceleration Time. J. Vasc. Ultrasound 2019, 43, 11–17. [Google Scholar] [CrossRef]
- Trihan, J.E.; Mahé, G.; Croquette, M.; Coutant, V.; Thollot, C.; Guillaumat, J.; Lanéelle, D. Accuracy of Acceleration Time of Distal Arteries to Diagnose Severe Peripheral Arterial Disease. Front. Cardiovasc. Med. 2022, 8, 744354. [Google Scholar] [CrossRef]
- Yagyu, T.; Funabashi, S.; Yoneda, S.; Noguchi, T.; Yasuda, S. Novel Evaluation Method for Lower Extremity Peripheral Artery Disease with Duplex Ultrasound―Usefulness of Acceleration Time. Circ. J. 2020, 84, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Fukanaga, R.; Ogawa, S.; Matsumoto, M.; Kimura, K.; Kamada, T. A new accurate and non-invasive screening method for renovascular hypertension: The renal artery Doppler technique. J. Hypertens. Suppl. Off. J. Int. Soc. Hypertens. 1988, 6, S458–S460. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Fukunaga, R.; Etani, H.; Yoneda, S.; Kimura, K.; Kamada, T. Efficacy of echo-Doppler examination for the evaluation of renovascular disease. Ultrasound Med. Biol. 1988, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Burdick, L.; Airoldi, F.; Marana, I.; Giussani, M.; Alberti, C.; Cianci, M.; Lovaria, A.; Saccheri, S.; Gazzano, G.; Morganti, A. Superiority of acceleration and acceleration time over pulsatility and resistance indices as screening tests for renal artery stenosis. J. Hypertens. 1996, 14, 1229–1235. [Google Scholar] [CrossRef]
- Strosberg, D.S.; Haurani, M.J.; Satiani, B.; Go, M.R. Common carotid artery end-diastolic velocity and acceleration time can predict degree of internal carotid artery stenosis. J. Vasc. Surg. 2017, 66, 226–231. [Google Scholar] [CrossRef]
- Iizuka, K.; Takekawa, H.; Iwasaki, A.; Igarashi, H.; Suzuki, K.; Kobayashi, S.; Tsukui, D.; Hirata, K. Suitable methods of measuring acceleration time in the diagnosis of internal carotid artery stenosis. J. Med. Ultrason. 2020, 47, 327–333. [Google Scholar] [CrossRef]
- Nishihira, T.; Takekawa, H.; Suzuki, K.; Suzuki, A.; Tsukahara, Y.; Iizuka, K.; Igarashi, H.; Iwasaki, A.; Okamura, M.; Hirata, K. Usefulness of acceleration time ratio in diagnosis of internal carotid artery origin stenosis. J. Med. Ultrason. 2018, 45, 493–500. [Google Scholar] [CrossRef]
- Sharman, J.E.; Davies, J.E.; Jenkins, C.; Marwick, T.H. Augmentation index, left ventricular contractility, and wave reflection. Hypertens. Dallas Tex. 2009, 54, 1099–1105. [Google Scholar] [CrossRef]
- Bardelli, M.; Veglio, F.; Arosio, E.; Cataliotti, A.; Valvo, E.; Morganti, A. New intrarenal echo-Doppler velocimetric indices for the diagnosis of renal artery stenosis. Kidney Int. 2006, 69, 580–587. [Google Scholar] [CrossRef]
- Brouwers, J.J.W.M.; Jiang, J.F.Y.; Feld, R.T.; van Doorn, L.P.; van Wissen, R.C.; van Walderveen, M.A.A.; Hamming, J.F.; Schepers, A. A New Doppler-Derived Parameter to Quantify Internal Carotid Artery Stenosis: Maximal Systolic Acceleration. Ann. Vasc. Surg. 2022, 81, 202–210. [Google Scholar] [CrossRef]
- Brouwers, J.J.W.M.; van Doorn, L.P.; Pronk, L.; van Wissen, R.C.; Putter, H.; Schepers, A.; Hamming, J.F. Doppler Ultrasonography Derived Maximal Systolic Acceleration: Value Determination with Artificially Induced Stenosis. Vasc. Endovascular Surg. 2022, 56, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.J.W.M.; van Doorn, L.P.; van Wissen, R.C.; Putter, H.; Hamming, J.F. Using maximal systolic acceleration to diagnose and assess the severity of peripheral artery disease in a flow model study. J. Vasc. Surg. 2020, 71, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Paglia, A.; Sasso, L.; Pirozzi, F.; Iannuzzi, A.; Carlomagno, A.; Abete, P.; Bonaduce, D. Arterial wave reflections and ventricular-vascular interaction in patients with left ventricular systolic dysfunction. Int. Heart J. 2014, 55, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Jin, Z.; Takei, Y.; Hasegawa, T.; Koshaka, S.; Palmieri, V.; Elkind, M.S.; Homma, S.; Sacco, R.L.; Di Tullio, M.R. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J. Hypertens. 2011, 29, 574–582. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trihan, J.-E.; Mahé, G.; Laroche, J.-P.; Dauzat, M.; Perez-Martin, A.; Croquette, M.; Lanéelle, D. Arterial Blood-Flow Acceleration Time on Doppler Ultrasound Waveforms: What Are We Talking About? J. Clin. Med. 2023, 12, 1097. https://doi.org/10.3390/jcm12031097
Trihan J-E, Mahé G, Laroche J-P, Dauzat M, Perez-Martin A, Croquette M, Lanéelle D. Arterial Blood-Flow Acceleration Time on Doppler Ultrasound Waveforms: What Are We Talking About? Journal of Clinical Medicine. 2023; 12(3):1097. https://doi.org/10.3390/jcm12031097
Chicago/Turabian StyleTrihan, Jean-Eudes, Guillaume Mahé, Jean-Pierre Laroche, Michel Dauzat, Antonia Perez-Martin, Magali Croquette, and Damien Lanéelle. 2023. "Arterial Blood-Flow Acceleration Time on Doppler Ultrasound Waveforms: What Are We Talking About?" Journal of Clinical Medicine 12, no. 3: 1097. https://doi.org/10.3390/jcm12031097
APA StyleTrihan, J.-E., Mahé, G., Laroche, J.-P., Dauzat, M., Perez-Martin, A., Croquette, M., & Lanéelle, D. (2023). Arterial Blood-Flow Acceleration Time on Doppler Ultrasound Waveforms: What Are We Talking About? Journal of Clinical Medicine, 12(3), 1097. https://doi.org/10.3390/jcm12031097




