Humoral and T-Cell Mediated Response after the Third Dose of mRNA Vaccines in Patients with Systemic Lupus Erythematosus on Belimumab
Abstract
:1. Introduction
- 1.
- What is already known on this topic
- B-cell targeted therapies, in particular anti-CD20 monoclonal antibody, impair humoral response to mRNA vaccines against SARS-CoV-2, but few data are available for anti-BAFF antibody, which is the only licensed biologic treatment for SLE.
- 2.
- What this study adds
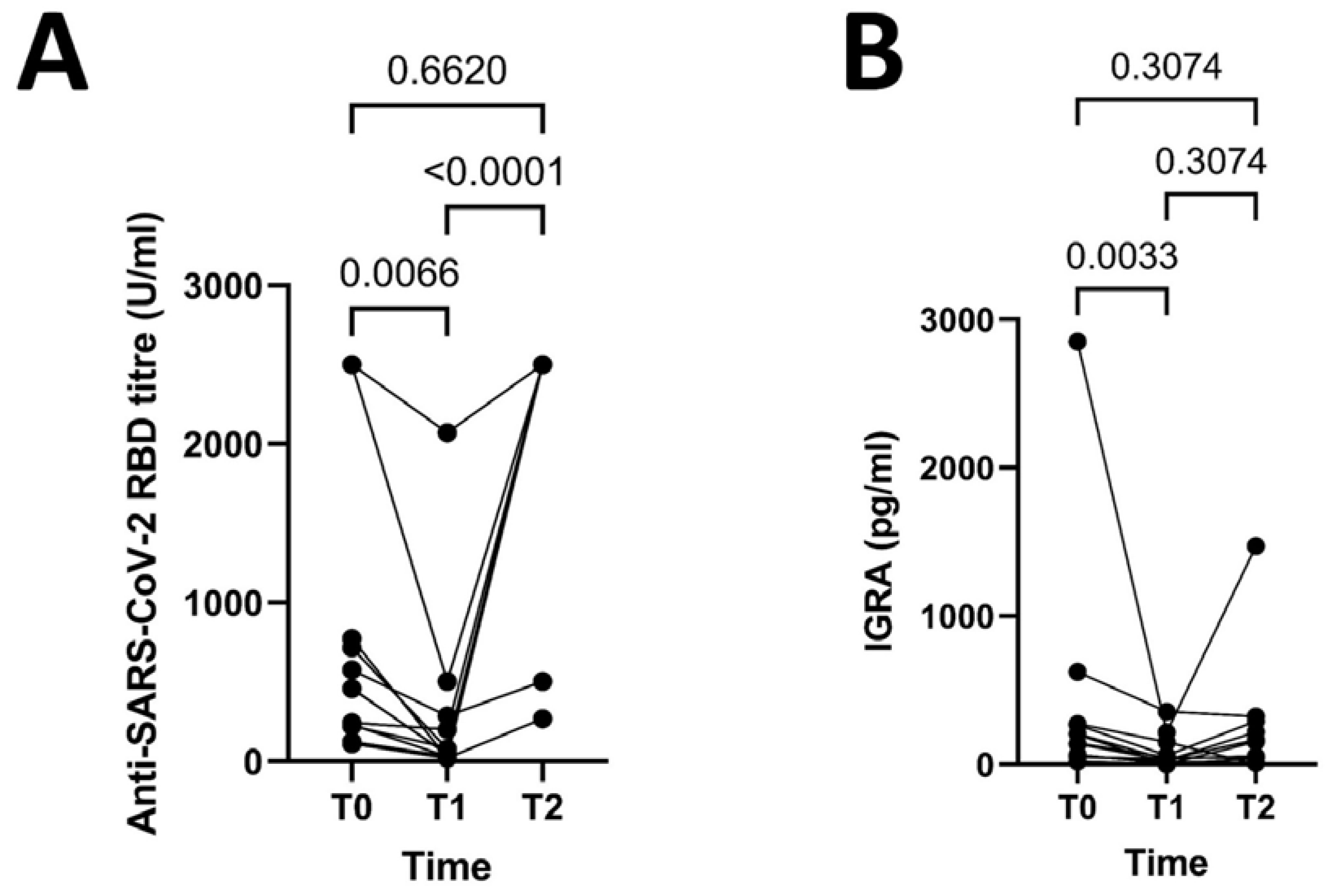
- In patients exposed to Belimumab humoral response declines during the following 6 months after the second dose of mRNA vaccines, and some patients lose cellular-mediated response to mRNA vaccines against SARS-CoV-2 over time under Belimumab. After the third dose of mRNA vaccines both humoral and cellular-mediated response are restored under Belimumab.
- 3.
- How this study might affect research, practice or policy
- Patients under Belimumab should receive further doses of mRNA vaccines to maintain both humoral and cellular-mediated response over time, and they should be monitored for the persistence of immune response over time.
2. Patients and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prendecki, M.; Clarke, C.; Edwards, H.; McIntyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021, 80, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Moor, M.B.; Suter-Riniker, F.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; Borradori, L.; et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): An investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021, 3, e789–e797. [Google Scholar] [CrossRef] [PubMed]
- Fabris, M.; De Marchi, G.; Domenis, R.; Caponnetto, F.; Guella, S.; Secco, C.D.; Cabas, N.; De Vita, S.; Beltrami, A.P.; Curcio, F.; et al. High T-cell response rate after COVID-19 vaccination in belimumab and rituximab recipients. J. Autoimmun. 2022, 129, 102827. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.A.; Connolly, C.M.; Boyarsky, B.J.; Werbel, W.A.; Christopher-Stine, L.; Garonzik-Wang, J.; Segev, D.L.; Paik, J.J. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021, 80, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Ribeiro, A.C.; Aikawa, N.E.; Saad, C.G.S.; Yuki, E.F.N.; Pedrosa, T.; Fusco, S.R.G.; Rojo, P.T.; Pereira, R.M.R.; Shinjo, S.K.; Andrade, D.C.O.; et al. Immunogenicity and safety of the CoronaVac inactivated vaccine in patients with autoimmune rheumatic diseases: A phase 4 trial. Nat. Med. 2021, 27, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Sidler, D.; Born, A.; Schietzel, S.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; et al. Trajectories of humoral and cellular immunity and responses to a third dose of mRNA vaccines against SARS-CoV-2 in patients with a history of anti-CD20 therapy. RMD Open 2022, 8, e002166. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, N.E.; Kupa, L.V.K.; Silva, C.A.; Saad, C.G.S.; Pasoto, S.G.; Yuki, E.F.N.; Fusco, S.R.G.; Shinjo, S.K.; Andrade, D.C.O.; Sampaio-Barros, P.D.; et al. Strong response after fourth dose of mRNA COVID-19 vaccine in autoimmune rheumatic diseases patients with poor response to inactivated vaccine. Rheumatology 2022, 62, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, I.; Olivetta, E.; Natalucci, F.; Olivieri, G.; Presti, A.L.; Fedele, G.; Stefanelli, P.; Ceccarelli, F.; Conti, F. Evidence of immune response to BNT162b2 COVID-19 vaccine in systemic lupus erythematosus patients treated with Belimumab. Lupus 2023, 9612033221151012, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Izmirly, P.M.; Kim, M.Y.; Samanovic, M.; Fernandez-Ruiz, R.; Ohana, S.; Deonaraine, K.K.; Engel, A.J.; Masson, M.; Xie, X.; Cornelius, A.R.; et al. Evaluation of Immune Response and Disease Status in Systemic Lupus Erythematosus Patients Following SARS-CoV-2 Vaccination. Arthritis Rheumatol. 2021, 74, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Afek, A.; Kreiss, Y.; Rahav, G.; Nemet, I.; Kliker, L.; Indenbaum, V.; Ram, E.; Lavee, J.; Segev, A.; et al. Kinetics of cellular and humoral responses to third BNT162B2 COVID-19 vaccine over six months in heart transplant recipients—Implications for the omicron variant. J. Hear. Lung Transplant. 2022, 41, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Tran, M.; Murthy, V.; Gonzalez, E. Immunogenicity and Safety of SARS-CoV-2 Vaccination in Patients With Rheumatic Diseases: A Systematic Review and Meta-analysis. Am. J. Clin. Oncol. 2022, 28, 381–389. [Google Scholar] [CrossRef] [PubMed]
- De Santis, M.; Motta, F.; Isailovic, N.; Clementi, M.; Criscuolo, E.; Clementi, N.; Tonutti, A.; Rodolfi, S.; Barone, E.; Colapietro, F.; et al. Dose-Dependent Impairment of the Immune Response to the Moderna-1273 mRNA Vaccine by Mycophenolate Mofetil in Patients with Rheumatic and Autoimmune Liver Diseases. Vaccines 2022, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Margioris, A.N. Immunosuppressed non-responders to two doses of mRNA SARS-CoV-2 vaccines achieve an immune response comparable to those of immunocompetent individuals after a third dose. Hormones 2022, 21, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Manothummetha, K.; Chuleerarux, N.; Sanguankeo, A.; Kates, O.S.; Hirankarn, N.; Thongkam, A.; Dioverti-Prono, M.V.; Torvorapanit, P.; Langsiri, N.; Worasilchai, N.; et al. Immunogenicity and Risk Factors Associated with Poor Humoral Immune Response of SARS-CoV-2 Vaccines in Recipients of Solid Organ Transplant: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e226822. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M.; Pawliczak, R. COVID-19 vaccination efficacy in numbers including SARS-CoV-2 variants and age comparison: A meta-analysis of randomized clinical trials. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 32. [Google Scholar] [CrossRef] [PubMed]
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Female | Female | Female | Female | Female | Female | Female | Female | Female | Female | Female | Female |
| Age | 58 | 46 | 59 | 42 | 44 | 61 | 51 | 49 | 40 | 42 | 40 | 40 |
| Disease | SLE | SLE | SLE | SLE | SLE | SLE | SLE | SLE + APS | SLE+ APS | SLE | SLE + APS | SLE |
| Concomitant Immunosuppressant | No | No | MMF 1 g/day | No | MMF 500 mg/day | No | MTX 7.5 mg/week | AZA 100 mg/day | No | No | TAC 3 mg/day | CyA 150 mg/day |
| Hydrxychloroquine | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No |
| Corticosteroids | No | No | PD 2.5 mg, 4 times/w | No | No | No | No | No | No | No | No | No |
| Time beetween II and III dose of vaccine (days) | 177 | 188 | 174 | 136 | 213 | 180 | 210 | 142 | 232 | 231 | 203 | 173 |
| Remission (LLDAS) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Humoral and cellular response after the II dose (T0—2D4W) | ||||||||||||
| Anti-RBD antibody titre, U/mL | 715 | 2500 | 773 | 110 | 458 | 232 | 2500 | 223 | 460 | 243 | 124 | 576 |
| Cellular response (quantitative), pg/mL | 138 | 2851 | 144 | 48 | 58 | 624 | 21 | 207 | 265 | 65 | 202 | 274 |
| Cellular response (qualitative) | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present | Present |
| Humoral and cellular response 6-month after the II dose (T1—2D6M) | ||||||||||||
| Anti-RBD antibody titre, U/mL | 78 | 2072 | 21 | 21 | 45 | 30 | 504 | 86 | 43 | 202 | 33 | NA |
| Cellular response (quantitative), pg/mL | 26 | 216 | 49 | 38 | 18 | 356 | 2 | 33 | 61 | 3 | 2 | 150 |
| Cellular response (qualitative) | Present | Present | Present | Present | Present | Present | Indeterminate | Present | Present | Absent | Absent | Present |
| Humoral and cellular response after the III dose (T2—3D4W) | ||||||||||||
| Anti-RBD antibody titre, U/mL | 2500 | 2500 | 268 | 2500 | 2500 | 2500 | 2500 | 2500 | 2500 | 2500 | 2500 | 504 |
| Cellular response (quantitative), pg/mL | 218 | 1472 | 44 | 55 | 12 | 324 | 42 | 167 | 291 | 8 | 153 | 2 |
| Cellular response (qualitative) | Present | Present | Present | Present | Present | Present | Present | Present | Present | Indeterminate | Present | Indeterminate |
| COVID-19 infection after the thirdvaccine dose | No | No | No | Yes | Yes | No | No | No | Yes | No | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartuccio, L.; De Marchi, G.; Domenis, R.; Cabas, N.; Guella, S.; Paradiso, A.; Fabro, C.; Beltrami, A.P.; De Vita, S.; Curcio, F. Humoral and T-Cell Mediated Response after the Third Dose of mRNA Vaccines in Patients with Systemic Lupus Erythematosus on Belimumab. J. Clin. Med. 2023, 12, 1083. https://doi.org/10.3390/jcm12031083
Quartuccio L, De Marchi G, Domenis R, Cabas N, Guella S, Paradiso A, Fabro C, Beltrami AP, De Vita S, Curcio F. Humoral and T-Cell Mediated Response after the Third Dose of mRNA Vaccines in Patients with Systemic Lupus Erythematosus on Belimumab. Journal of Clinical Medicine. 2023; 12(3):1083. https://doi.org/10.3390/jcm12031083
Chicago/Turabian StyleQuartuccio, Luca, Ginevra De Marchi, Rossana Domenis, Nicola Cabas, Silvia Guella, Antonella Paradiso, Cinzia Fabro, Antonio Paolo Beltrami, Salvatore De Vita, and Francesco Curcio. 2023. "Humoral and T-Cell Mediated Response after the Third Dose of mRNA Vaccines in Patients with Systemic Lupus Erythematosus on Belimumab" Journal of Clinical Medicine 12, no. 3: 1083. https://doi.org/10.3390/jcm12031083
APA StyleQuartuccio, L., De Marchi, G., Domenis, R., Cabas, N., Guella, S., Paradiso, A., Fabro, C., Beltrami, A. P., De Vita, S., & Curcio, F. (2023). Humoral and T-Cell Mediated Response after the Third Dose of mRNA Vaccines in Patients with Systemic Lupus Erythematosus on Belimumab. Journal of Clinical Medicine, 12(3), 1083. https://doi.org/10.3390/jcm12031083







