Development of a Machine-Learning Model for Prediction of Extubation Failure in Patients with Difficult Airways after General Anesthesia of Head, Neck, and Maxillofacial Surgeries
Abstract
1. Introduction
2. Materials and Methods
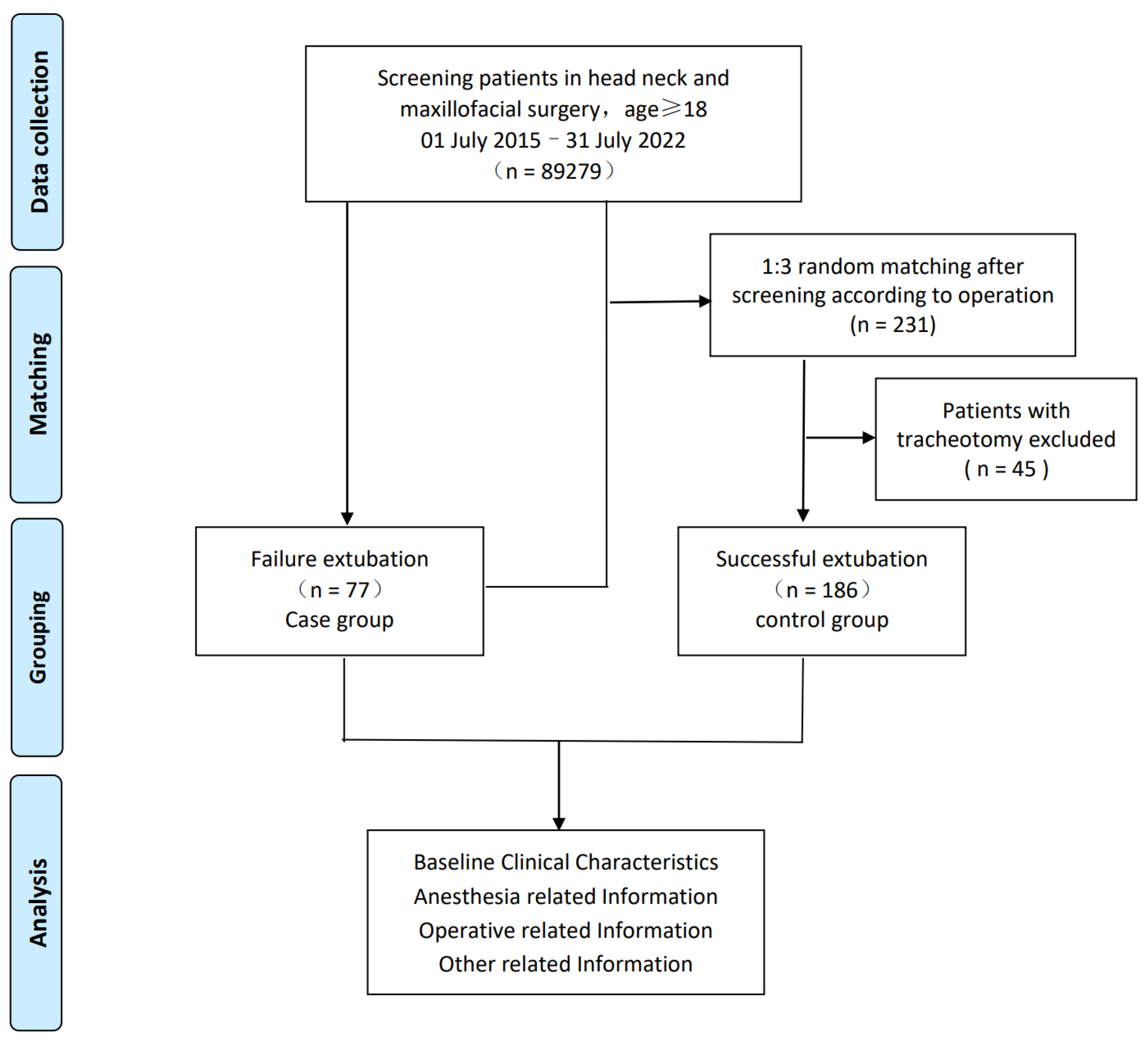
2.1. Study Population and Design
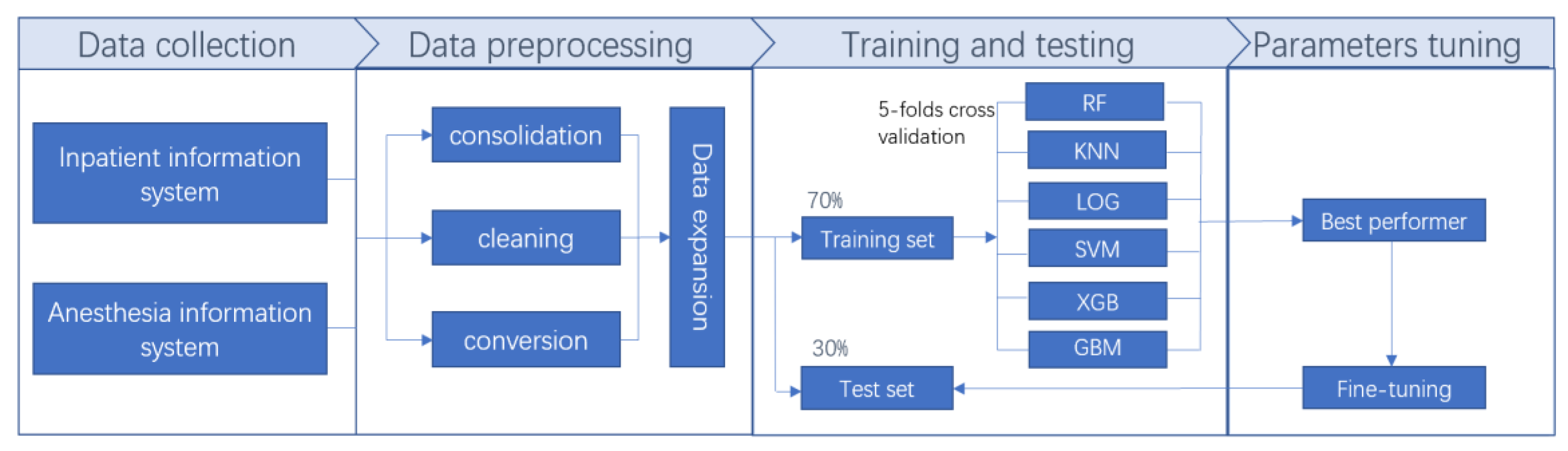
2.2. Data Collection and Data Expansion
2.3. Model Selection
2.4. Statistical Analysis
3. Results
3.1. Baseline Variable Details of the Prediction Model
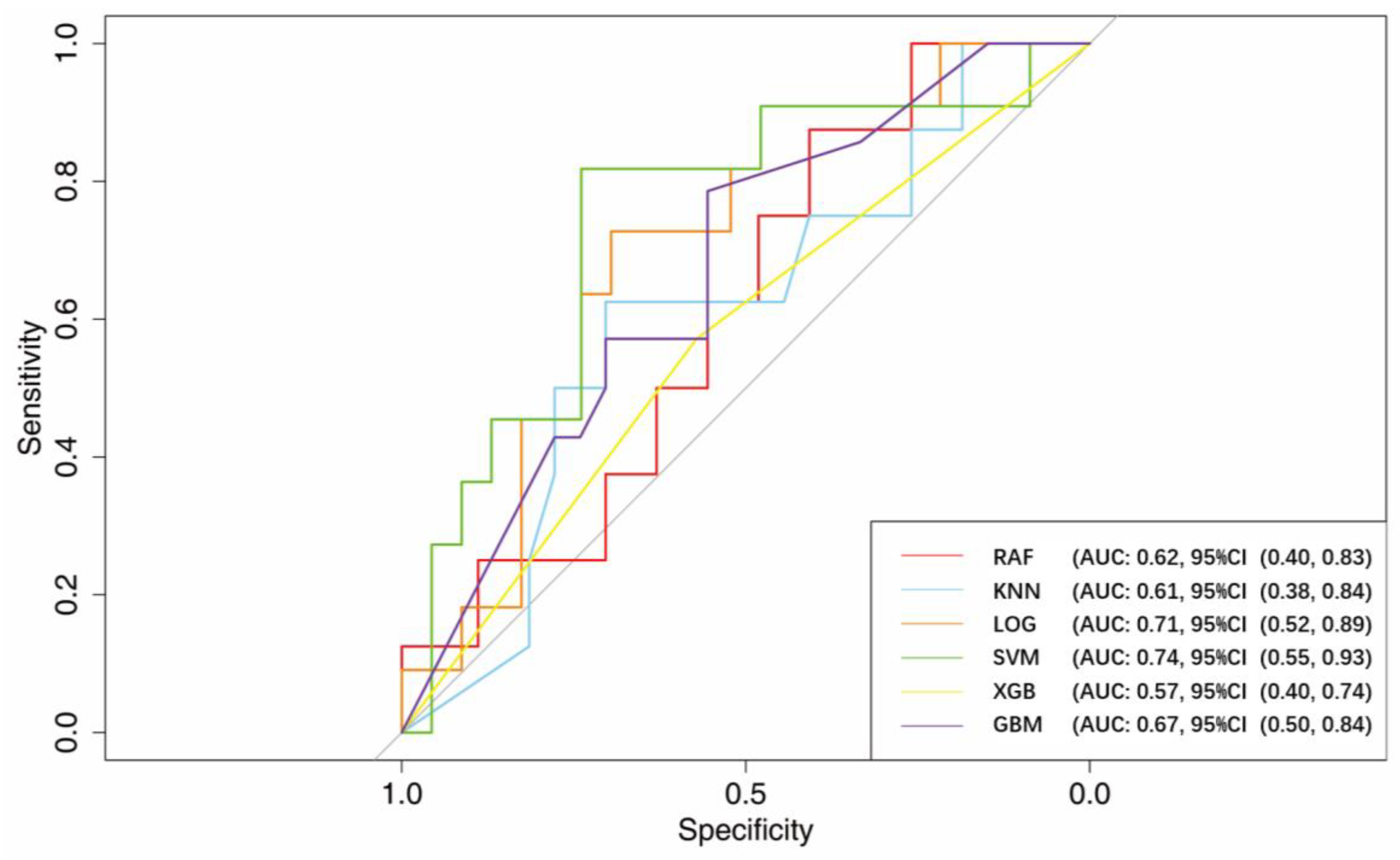
3.2. AUC
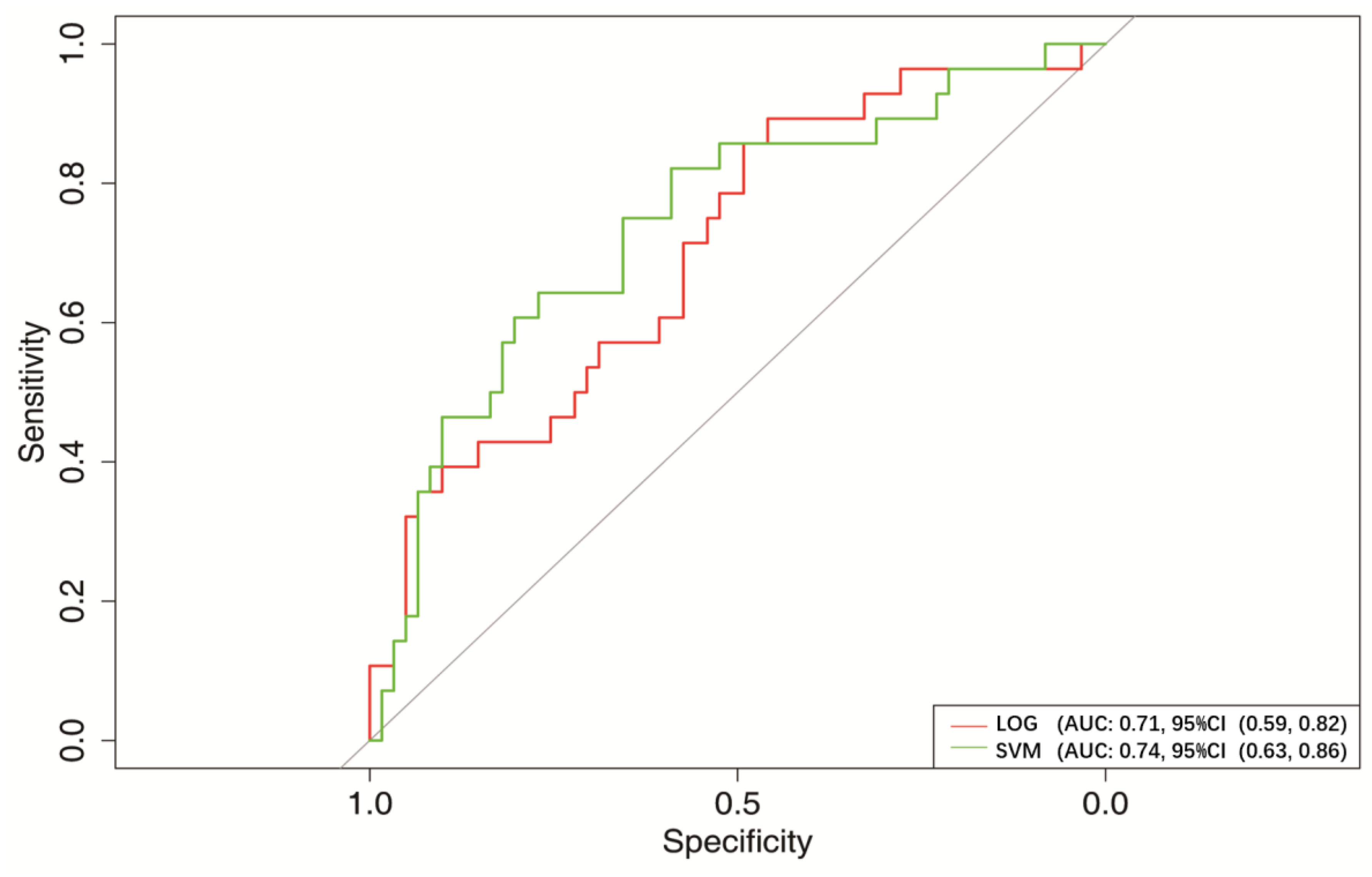
3.3. Optimal Model of Machine-Learning
3.4. Prediction of the Risk of Extubation Failure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, T.M.; Woodall, N.; Frerk, C.; Fourth National Audit, P. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br. J. Anaesth 2011, 106, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Cavallone, L.F.; Vannucci, A. Review article: Extubation of the difficult airway and extubation failure. Anesth Analg. 2013, 116, 368–383. [Google Scholar] [CrossRef] [PubMed]
- Frova, G.; Sorbello, M. Algorithms for difficult airway management: A review. Minerva Anestesiol. 2009, 75, 201–209. [Google Scholar] [PubMed]
- Evans, S.W.; McCahon, R.A. Management of the airway in maxillofacial surgery: Part 1. Br. J. Oral Maxillofac. Surg. 2018, 56, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Difficult Airway Society Extubation Guidelines Group; Popat, M.; Mitchell, V.; Dravid, R.; Patel, A.; Swampillai, C.; Higgs, A. Difficult Airway Society Guidelines for the management of tracheal extubation. Anaesthesia 2012, 67, 318–340. [Google Scholar] [CrossRef]
- Cook, T.M.; Woodall, N.; Harper, J.; Benger, J.; Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: Intensive care and emergency departments. Br. J. Anaesth 2011, 106, 632–642. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Hagberg, C.A.; Connis, R.T.; Abdelmalak, B.B.; Agarkar, M.; Dutton, R.P.; Fiadjoe, J.E.; Greif, R.; Klock, P.A.; Mercier, D.; et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology 2022, 136, 31–81. [Google Scholar] [CrossRef] [PubMed]
- Edelman, D.A.; Perkins, E.J.; Brewster, D.J. Difficult airway management algorithms: A directed review. Anaesthesia 2019, 74, 1175–1185. [Google Scholar] [CrossRef]
- Mort, T.C.; Braffett, B.H. Conventional Versus Video Laryngoscopy for Tracheal Tube Exchange: Glottic Visualization, Success Rates, Complications, and Rescue Alternatives in the High-Risk Difficult Airway Patient. Anesth Analg. 2015, 121, 440–448. [Google Scholar] [CrossRef]
- Vivier, E.; Muller, M.; Putegnat, J.B.; Steyer, J.; Barrau, S.; Boissier, F.; Bourdin, G.; Mekontso-Dessap, A.; Levrat, A.; Pommier, C.; et al. Inability of Diaphragm Ultrasound to Predict Extubation Failure: A Multicenter Study. Chest 2019, 155, 1131–1139. [Google Scholar] [CrossRef]
- Biro, P.; Priebe, H.J. Staged extubation strategy: Is an airway exchange catheter the answer? Anesth Analg. 2007, 105, 1182–1185. [Google Scholar] [CrossRef] [PubMed]
- Mort, T.C. Continuous airway access for the difficult extubation: The efficacy of the airway exchange catheter. Anesth Analg. 2007, 105, 1357–1362, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Apfelbaum, J.L.; Hagberg, C.A.; Caplan, R.A.; Blitt, C.D.; Connis, R.T.; Nickinovich, D.G.; Hagberg, C.A.; Caplan, R.A.; Benumof, J.L.; Berry, F.A.; et al. Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013, 118, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2003, 98, 1269–1277. [CrossRef] [PubMed]
- Connor, C.W. Artificial Intelligence and Machine Learning in Anesthesiology. Anesthesiology 2019, 131, 1346–1359. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.A.; Witkowski, E.; Gao, L.; Meireles, O.; Rosman, G. Artificial Intelligence in Anesthesiology: Current Techniques, Clinical Applications, and Limitations. Anesthesiology 2020, 132, 379–394. [Google Scholar] [CrossRef]
- Reiniš, J.; Petrenko, O.; Simbrunner, B.; Hofer, B.S.; Schepis, F.; Scoppettuolo, M.; Saltini, D.; Indulti, F.; Guasconi, T.; Albillos, A.; et al. Assessment of portal hypertension severity using machine learning models in patients with compensated cirrhosis. J. Hepatol. 2022, 78, 390–400. [Google Scholar] [CrossRef]
- Wesselink, E.M.; Wagemakers, S.H.; van Waes, J.A.R.; Wanderer, J.P.; van Klei, W.A.; Kappen, T.H. Associations between intraoperative hypotension, duration of surgery and postoperative myocardial injury after noncardiac surgery: A retrospective single-centre cohort study. Br. J. Anaesth 2022, 129, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. J. Clin. Epidemiol. 2015, 68, 134–143. [Google Scholar] [CrossRef]
- Parotto, M.; Cooper, R.M.; Behringer, E.C. Extubation of the Challenging or Difficult Airway. Curr. Anesthesiol. Rep. 2020, 10, 334–340. [Google Scholar] [CrossRef]
- Ferraro, F.; Gambardella, C.; Testa, D.; Santini, L.; Marfella, R.; Fusco, P.; Lombardi, C.P.; Polistena, A.; Sanguinetti, A.; Avenia, N.; et al. Nasotracheal prolonged safe extubation in acute respiratory failure post-thyroidectomy: An efficacious technique to avoid tracheotomy? A retrospective analysis of a large case series. Int. J. Surg. (Lond. Engl.) 2017, 41 (Suppl. 1), S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Tapia, B.; Garrido, E.; Cebrian, J.L.; Del Castillo, J.L.; Gonzalez, J.; Losantos, I.; Gilsanz, F. Impact of Goal Directed Therapy in Head and Neck Oncological Surgery with Microsurgical Reconstruction: Free Flap Viability and Complications. Cancers 2021, 13, 1545. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Smielewski, P.; Czosnyka, M.; Ercole, A. Cerebrovascular Signal Complexity Six Hours after Intensive Care Unit Admission Correlates with Outcome after Severe Traumatic Brain Injury. J. Neurotrauma 2016, 33, 2011–2018. [Google Scholar] [CrossRef]
- Kong, V.; Somakhamixay, O.; Cho, W.; Kang, G.; Won, H.; Rah, H.; Bang, H.J.P. Recurrence risk prediction of acute coronary syndrome per patient as a personalized ACS recurrence risk: A retrospective study. PeerJ 2022, 10, e14348. [Google Scholar] [CrossRef]
- Chou, F.S.; Ghimire, L.V. Machine Learning for Mortality Prediction in Pediatric Myocarditis. Front. Pediatr. 2021, 9, 644922. [Google Scholar] [CrossRef]
- Suarez-Ibarrola, R.; Hein, S.; Reis, G.; Gratzke, C.; Miernik, A. Current and future applications of machine and deep learning in urology: A review of the literature on urolithiasis, renal cell carcinoma, and bladder and prostate cancer. World J. Urol. 2020, 38, 2329–2347. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Haimovich, J.; Hurley, N.C.; McNamara, R.; Spertus, J.A.; Desai, N.; Rumsfeld, J.S.; Masoudi, F.A.; Huang, C.; Normand, S.L.; et al. Use of Machine Learning Models to Predict Death After Acute Myocardial Infarction. JAMA Cardiol. 2021, 6, 633–641. [Google Scholar] [CrossRef] [PubMed]
- van Driel, M.E.C.; van Dijk, J.F.M.; Baart, S.J.; Meissner, W.; Huygen, F.; Rijsdijk, M. Development and validation of a multivariable prediction model for early prediction of chronic postsurgical pain in adults: A prospective cohort study. Br. J. Anaesth 2022, 129, 407–415. [Google Scholar] [CrossRef]
- Demirjian, S.; Bashour, C.A.; Shaw, A.; Schold, J.D.; Simon, J.; Anthony, D.; Soltesz, E.; Gadegbeku, C.A. Predictive Accuracy of a Perioperative Laboratory Test-Based Prediction Model for Moderate to Severe Acute Kidney Injury After Cardiac Surgery. Jama 2022, 327, 956–964. [Google Scholar] [CrossRef]
- Kendale, S.; Kulkarni, P.; Rosenberg, A.D.; Wang, J. Supervised Machine-learning Predictive Analytics for Prediction of Postinduction Hypotension. Anesthesiology 2018, 129, 675–688. [Google Scholar] [CrossRef]
- Hatib, F.; Jian, Z.; Buddi, S.; Lee, C.; Settels, J.; Sibert, K.; Rinehart, J.; Cannesson, M. Machine-learning Algorithm to Predict Hypotension Based on High-fidelity Arterial Pressure Waveform Analysis. Anesthesiology 2018, 129, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.; Ensor, J.; Snell, K.; Harrell, F.; Martin, G.; Reitsma, J.; Moons, K.; Collins, G.; van Smeden, M.J.B. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef] [PubMed]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Kloesel, B.; Todd, M.M.; Cole, D.J.; Prielipp, R.C. The Evolution, Current Value, and Future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology 2021, 135, 904–919. [Google Scholar] [CrossRef] [PubMed]
- Sharma, I.; Dong, W.; Vu, C.N.; Zheng, G. The impact of head and neck radiation on airway management: A retrospective data review. Can. J. Anaesth 2022, 69, 1562–1564. [Google Scholar] [CrossRef]
- Soydan, S.S.; Bayram, B.; Akdeniz, B.S.; Kayhan, Z.; Uckan, S. Changes in difficult airway predictors following mandibular setback surgery. Int. J. Oral Maxillofac. Surg. 2015, 44, 1351–1354. [Google Scholar] [CrossRef]
- Asmar, A.; Mohandas, R.; Wingo, C.S. A physiologic-based approach to the treatment of a patient with hypokalemia. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2012, 60, 492–497. [Google Scholar] [CrossRef]

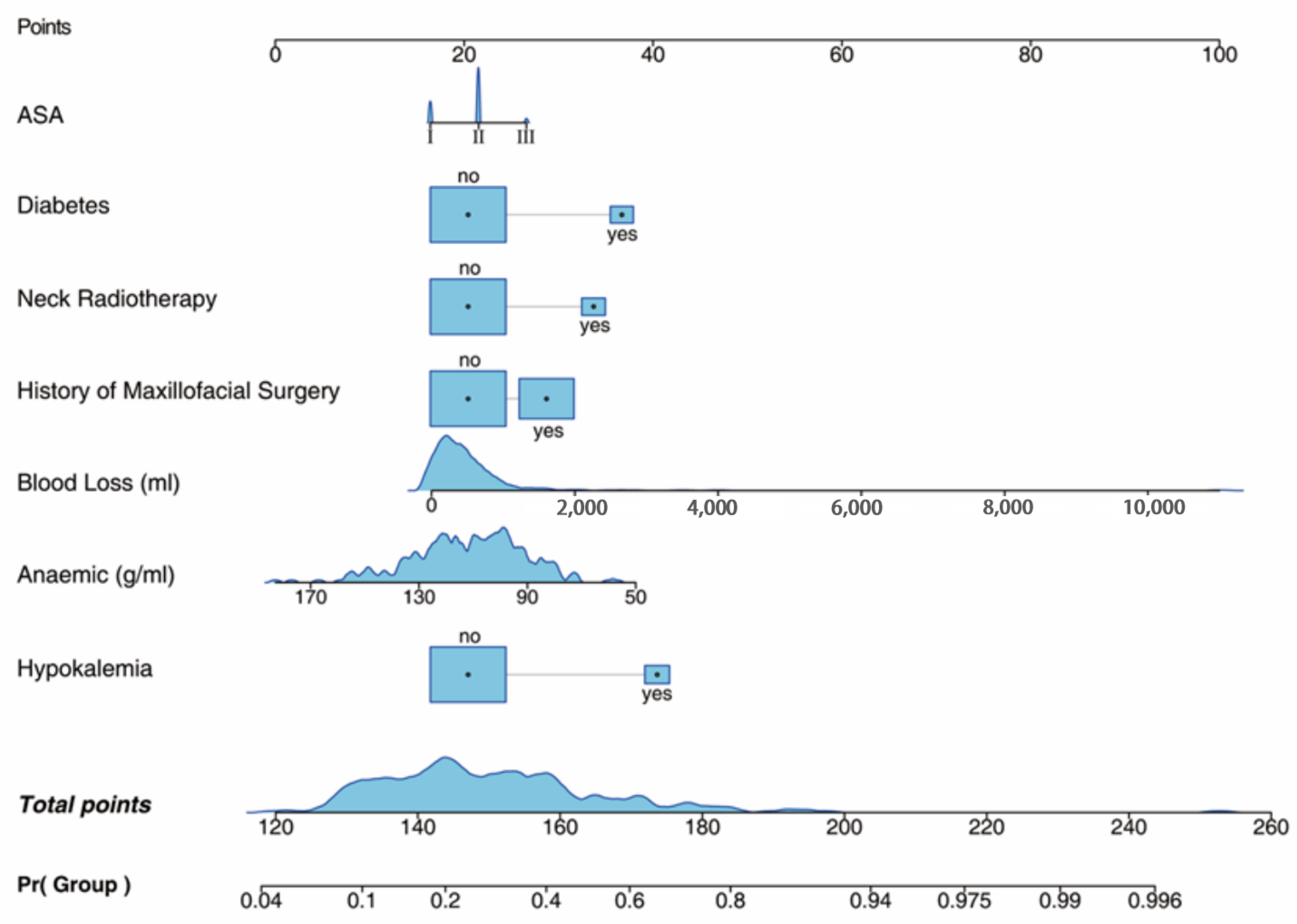
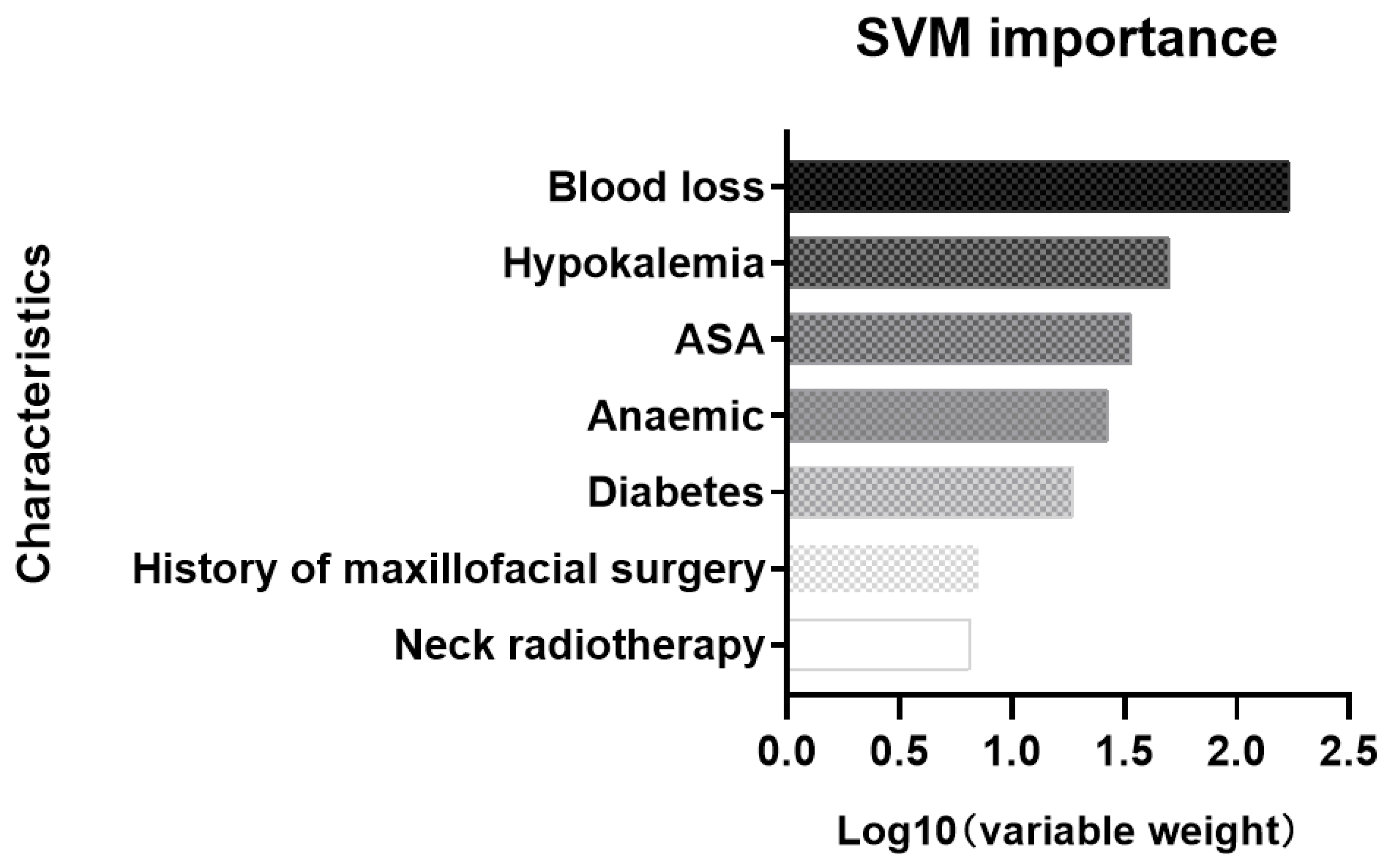
| Characteristic | Case | Control | p |
|---|---|---|---|
| n = 77 | n = 186 | ||
| Baseline Clinical Characteristics | |||
| Sex | |||
| Male (%) | 53 (68.8) | 118 (63.4) | 0.478 |
| Female (%) | 24 (31.2) | 68 (36.6) | |
| Age (year) | 60 (48, 71) | 59 (41, 68) | 0.539 |
| Surgical complexity | |||
| I (%) | 1 (1.3) | 0 (0.0) | 0.061 |
| II (%) | 4 (5.2) | 19 (10.2) | |
| III (%) | 17 (22.1) | 58 (31.2) | |
| IV (%) | 55 (71.4) | 109 (58.6) | |
| ASA grade | |||
| I (%) | 10 (13.2) | 61 (32.8) | 0.003 |
| II (%) | 61 (80.3) | 116 (62.4) | |
| III (%) | 5 (6.6) | 9 (4.8) | |
| COPD | |||
| No (%) | 74 (96.1) | 179 (96.2) | 1 |
| Yes (%) | 3 (3.9) | 7 (3.8) | |
| Hypertension | |||
| No (%) | 57 (74.0) | 144 (77.4) | 0.632 |
| Yes (%) | 20 (26.0) | 42 (22.6) | |
| Diabetes | |||
| No (%) | 66 (85.7) | 173 (93.0) | 0.097 |
| Yes (%) | 11 (14.3) | 13 (7.0) | |
| OSAS | |||
| No (%) | 75 (98.7) | 180 (96.8) | 0.677 |
| Yes (%) | 1 (1.3) | 6 (3.2) | |
| Coronary heart disease | |||
| No (%) | 72 (93.5) | 180 (96.8) | 0.308 |
| Yes (%) | 5 (6.5) | 6 (3.2) | |
| Anesthesia-related Information | |||
| Induction | |||
| Anesthetized (%) | 58 (76.3) | 157 (84.4) | 0.155 |
| Awake (%) | 18 (23.7) | 29 (15.6) | |
| Mouth opening (cm) | 4.00 (3.00, 4.00) | 4.00 (3.50, 4.00) | 0.697 |
| History of neck radiotherapy | |||
| No (%) | 63 (82.9) | 176 (94.6) | 0.007 |
| Yes (%) | 13 (17.1) | 10 (5.4) | |
| History of maxillofacial surgery | |||
| No (%) | 38 (50.0) | 138 (74.2) | <0.001 |
| Yes (%) | 38 (50.0) | 48 (25.8) | |
| Operation-related Information | |||
| Tumor size (cm) | 2.80 (0.00, 4.80) | 2.00 (0.35, 3.12) | 0.103 |
| Operation time (h) | 5.58 (3.75, 8.50) | 4.75 (2.75, 6.75) | 0.003 |
| End time (24 h) | 16 (14, 19) | 16 (14, 18) | 0.444 |
| Blood loss (mL) | 400 (300, 763) | 300 (100, 500) | <0.001 |
| Blood infusion (mL) | 0 (0, 500) | 0 (0, 0) | 0.032 |
| Fluid infusion (mL) | 3400 (2000, 4000) | 2500 (1500, 3500) | 0.002 |
| Surgical site | |||
| Intraoral | 17 (22.1) | 43 (23.1) | 0.995 |
| Neck (%) | 12 (15.6) | 29 (15.6) | |
| Skull base (%) | 20 (26.0) | 45 (24.2) | |
| ≥2 sites (%) | 28 (36.4) | 69 (37.1) | |
| Flap reconstruction | |||
| No (%) | 44 (57.9) | 133 (71.5) | 0.042 |
| Yes (%) | 32 (42.1) | 53 (28.5) | |
| Other Related Information | |||
| Extubation time (h) | 14.50 (6.00, 37.00) | 15.75 (1.00, 39.69) | 0.403 |
| Delirium | |||
| No (%) | 73 (94.8) | 170 (91.4) | 0.448 |
| Yes (%) | 4 (5.2) | 16 (8.6) | |
| Anemia (g/L) | 104 (95, 115) | 116 (100, 130) | <0.001 |
| Hypokalemia | |||
| No (%) | 62 (80.5) | 164 (94.8) | <0.001 |
| Yes (%) | 15 (19.5) | 9 (5.2) | |
| Variables | AUC (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|
| RF | 0.62 (0.40–0.83) | 0.48 (0.19–1.00) | 1.00 (0.38–1.00) | 0.57 (0.37–0.86) |
| KNN | 0.61 (0.38–0.84) | 0.70 (0.15–0.89) | 0.75 (0.38–1.00) | 0.71 (0.34–0.86) |
| LOG | 0.71 (0.52–0.89) | 0.65 (0.30–0.91) | 0.82 (0.45–1.00) | 0.71 (0.53–0.85) |
| SVM | 0.74 (0.55–0.93) | 0.74 (0.52–0.96) | 0.82 (0.55–1.00) | 0.76 (0.62–0.91) |
| XGB | 0.57 (0.40–0.74) | 0.57 (0.00–1.00) | 0.57 (0.00–1.00) | 0.60 (0.40–0.74) |
| GBM | 0.67 (0.50- 0.84) | 0.63 (0.19–0.89) | 0.79 (0.43–1.00) | 0.68 (0.46–0.80) |
| Variables | AUC (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|
| LOG | 0.71 (0.59, 0.82) | 0.56 (0.36, 0.97) | 0.86 (0.36, 1.00) | 0.66 (0.54, 0.82) |
| SVM | 0.74 (0.63, 0.86) | 0.75 (0.49, 0.95) | 0.75 (0.46, 0.96) | 0.74 (0.61, 0.84) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Wang, J.; Zhu, Y.; Liu, J.; Zhang, L.; Shi, W.; Hu, W.; Ding, Y.; Zhou, R.; Jiang, H. Development of a Machine-Learning Model for Prediction of Extubation Failure in Patients with Difficult Airways after General Anesthesia of Head, Neck, and Maxillofacial Surgeries. J. Clin. Med. 2023, 12, 1066. https://doi.org/10.3390/jcm12031066
Huang H, Wang J, Zhu Y, Liu J, Zhang L, Shi W, Hu W, Ding Y, Zhou R, Jiang H. Development of a Machine-Learning Model for Prediction of Extubation Failure in Patients with Difficult Airways after General Anesthesia of Head, Neck, and Maxillofacial Surgeries. Journal of Clinical Medicine. 2023; 12(3):1066. https://doi.org/10.3390/jcm12031066
Chicago/Turabian StyleHuang, Huimin, Jiayi Wang, Ying Zhu, Jinxing Liu, Ling Zhang, Wei Shi, Wenyue Hu, Yi Ding, Ren Zhou, and Hong Jiang. 2023. "Development of a Machine-Learning Model for Prediction of Extubation Failure in Patients with Difficult Airways after General Anesthesia of Head, Neck, and Maxillofacial Surgeries" Journal of Clinical Medicine 12, no. 3: 1066. https://doi.org/10.3390/jcm12031066
APA StyleHuang, H., Wang, J., Zhu, Y., Liu, J., Zhang, L., Shi, W., Hu, W., Ding, Y., Zhou, R., & Jiang, H. (2023). Development of a Machine-Learning Model for Prediction of Extubation Failure in Patients with Difficult Airways after General Anesthesia of Head, Neck, and Maxillofacial Surgeries. Journal of Clinical Medicine, 12(3), 1066. https://doi.org/10.3390/jcm12031066





