Abstract
Dermoscopic features of actinic keratosis (AK) have been widely studied, but there is still little evidence for their diagnostic accuracy. Our study investigates whether established dermoscopic criteria are reliable predictors in differentiating non-pigmented actinic keratosis (NPAK) from pigmented actinic keratosis (PAK). For this purpose, dermoscopic images of 83 clinically diagnosed AK (45 NPAK, 38PAK) were examined, and the sensitivity (Se), specificity (Sp), positive predictive value (PPV), and negative predictive value (NPV) were assessed. Features with statistical significance were the red pseudo-network (p = 0.02) for NPAK and the pigmented pseudo-network (p < 0.001) with a pigment intensity value even less than 10% for PAK (p = 0.001). Pigmented pseudo-network (Se: 89%, Sp: 77%, PPV: 77%, NPV: 89%) with a pigment intensity value of more than 10% (Se: 90%, Sp: 86%, PPV: 79%, NPV: 93%) had excellent diagnostic accuracy for PAK. Scale and widened follicular openings with yellowish dots surrounded by white circles were equally represented in both variants of AK. Linear wavy vessels and shiny streaks were more prominently observed in NPAK, as were rosettes in PAK, but these results failed to meet statistical significance. The red starburst pattern was near statistical significance for PAK. Therefore, pigmentation is the strongest dermoscopic predictor for the differentiation between NPAK and PAK.
1. Introduction
Actinic keratosis (AK) is a premalignant lesion within the cutaneous squamous cell carcinoma (cSCC) spectrum, with a prevalence ranging from 11% to 60% in Caucasians above the age of 40 years and a potential for malignant transformation into invasive cSCCs ranging between 0.1–20% [1,2,3,4,5,6]. It commonly occurs in elderly, fair-skinned male individuals, in areas of sun-damaged skin [1,2,5]. Non-pigmented actinic keratosis (NPAK) and pigmented actinic keratosis (PAK) consist of the main clinical subtypes of AK defined by the absence or presence of clinically obvious pigmentation, respectively [2].
Dermoscopy is a non-invasive examination that improves the clinical diagnosis and early and efficient management decisions for skin lesions [7]. In addition, a dermoscopic examination contributes to its treatment through the preprocedural assessment of lesion surface boundaries, the monitoring of the effects of local therapies, and the long-term monitoring of patients [6,8].
Dermoscopically NPAK facial lesions are characterized by the so-called “strawberry pattern” consisting of a red pseudo-network created by the background erythema with targetoid ostia of hair follicles (white circles corresponding to the follicular openings, surrounding a yellowish keratotic plug) [2,9,10]. On the other hand, PAK lesions of the face demonstrate a pigmented pseudo-network consisting of a pigmented background between targetoid follicular openings, at times associated with a red pseudo-network [11,12]. Additional dermoscopic features include a white-to-yellow surface scale, fine wavy vessels surrounding the hair follicle, and structures only visible in polarized-light dermoscopy. These include a rosette sign (four white, dotted clods forming a four-leaf clover-shaped structure, due to alternating hyperkeratotic and parakeratotic corneal layers in the follicular infundibula with peri-follicular fibrosis) and shiny streaks (white, perpendicular, few-millimeters-long lines caused by the polarization of thickened hyaline fibrous bundles) [9,12,13,14]. The progression to malignancy of the AK is highlighted by the “red starburst pattern”, radially arranged red lines, or hairpin vessels surrounding a yellow-to-white scaly center [2,15].
Our study aims to improve the clinical differentiation between NPAK and PAK of the face and scalp through an evaluation of the diagnostic accuracy of the dermoscopic criteria of AK, as PAK shares clinical and dermoscopic features with Lentigo Malignant (LM). The differential diagnosis of PAK from LM sometimes represents a challenge even for expert dermatologists, and a histopathological examination is important before starting treatment of this variant of AK. Thus, it is highly significant to describe all the aspects of the dermoscopic morphology of PAK.
2. Methods
2.1. Study Population
We included 40 patients with 83 clinically diagnosed naive AK from the Dermato-Oncology Unit of the 1st Department of Dermatology & Venereology of “A. SYGROS” University Hospital in Athens, Greece. The study was approved by the Ethics Committee of the institution (approval code 2530/5,10.2018). All participants were informed about the aims of the study and provided written informed consent. All lesions included in our study had typical clinical and dermoscopic features of AK, and the evaluation of their criteria met an agreement between two independent investigators (DS and AK). In case of differential diagnostic doubt, a skin biopsy was performed and lesions that did not have a definite AK histopathologic diagnosis were excluded from the study. AKs arising on extra-facial anatomic sites were not included in the study. Another exclusion criterion was actinic lesions that were previously treated for AK irrelevant of the therapeutic method.
2.2. Clinical and Dermoscopic Examinations
Initially, the demographic characteristics of the patients were recorded, followed by a clinical examination. AK lesions were clinically divided into two categories for the purposes of the study: either NPAK defined as lesions without clinically obvious pigmentation, or PAK that showed even light, clinically observed brown–black coloration. Moreover, AK lesions were clinically classified according to the Olsen’s system. Lesions better identified by palpation than by inspection were defined as Olsen grade I, lesions easily identified by inspection and palpation were Olsen grade II, while hyperkeratotic lesions were classified as Olsen grade III [16]. Dermoscopic images were obtained using polarized-light, contact dermoscopy, with Dermlite DL 200 Hybrid dermoscope coupled to a Nikon J1 camera. Their evaluation was performed for the predetermined dermoscopic criteria of AK (red pseudo-network, pigmented pseudo-network, scales, yellowish dots within widened follicular openings, white circles around yellowish dots, linear wavy vessels, short shiny streaks, and red starburst pattern).
In addition, the intensity of the pigmentation was measured as the percentage of the surface of the lesion covered by the pigment. According to the intensity of the pigmentation, lesions were classified into 4 categories: lesions without pigmentation, lesions with pigment intensity less than 10%, lesions with pigment intensity of 10–50%, and lesions with pigment intensity greater than 50%.
2.3. Statistical Analysis
The statistical analysis was performed with STAT/IC version 15.1. Continuous variables were checked for normality of distribution with the Shapiro–Wilk test and were presented as mean ± standard deviation. Contingency tables were used for categorical variables. For the detection of statistically significant differences in the presence of dermoscopic criteria between NPAK and PAK, as well as for the rest of the categorical variables, Pearson’s chi-squared and Fisher’s exact tests were used. For continuous variables, Mann–Whitney and Kruskal–Wallis tests were used. Additionally, for the statistically significantly different variables, univariate and multivariate logistic regression analyses were conducted. Finally, in the multivariate logistic regression model, an evaluation of the goodness of fit was performed according to the Hosmer–Lemeshow criterium. Statistically significant differences were considered in cases of p-value < 0.05.
3. Results
3.1. Demographic and Clinical Features of the Study’s Population
In total, 45 NPAK and 38 PAK were diagnosed in 40 patients. Of them, 18 patients presented with a solitary AK lesion, 10 patients with 2 lesions, 6 patients with 3 lesions, 4 patients with 4 lesions, 1 patient with 5 lesions, and 1 with 6 AKs. No major differences were noted regarding the age and sex of our study participants in both types of lesions with AK. Patients with NPAK and PAK tended to be older, with a predilection for the male sex [NPAK: 38/45 (84.4%), PAK: 31/38 (81.6%)] (Table 1).

Table 1.
Demographic and clinical characteristics of the study’s population.
Concerning the Fitzpatrick skin-type classification, the most frequent phototypes were II and III for NPAK and PAK (II: ⅓ in both, III: ¼ of NPAK, IV: ½ of PAK patients) (Table 1).
Regarding the anatomic site of the lesions, common sites of presentation were in NPAK lesions, the scalp in 18/45 (40%), and the lateral face in 9/45 (20%). PAK lesions were approximately equally present on the scalp, temples, and lateral face (26.3%, 26.3%, and 28.9%, respectively) (Table 1).
Furthermore, the macroscopic examination of the lesions revealed that the majority of NPAK and PAK lesions were OLSEN’s stage II (18/45 (40%) and 18/38 (47.4%), respectively, followed by stage I (16/45 (35.6%) and 13/38 (34.2%), respectively (Table 1).
3.2. Dermoscopic Features of NPAK and PAK Lesions
The dermoscopic features of NPAK and PAK are presented in Table 2. With regard to color, the red network was more frequently found in NPAK lesions [38/45 (84.4%), p = 0.01], but also in 60.5% (23/38) of PAK lesions. A pigmented network was present in the vast majority of PAK lesions [10/45 of NPAK (22.2%), 34/38 of PAK (89.5%), p < 0.001].

Table 2.
Dermoscopic features of NPAK and PAK.
Concerning the pigment intensity, even less than 10% was significantly associated with PAK (p = 0.001). Scales were equally observed in NPAK and PAK lesions [38/45 (84.4%), 32/38 (84.2%), respectively]. The structures that were equally recorded in both groups of lesions were widened follicular openings, yellowish dots, and white circles (77.9% NPAK vs. 76.3% PAK, 62.2% NPAK vs. 63.2% PAK, 62.2% NPAK VS. 63.2% PAK, respectively).
Consequently, linear wavy vessels and shiny streaks were more frequently revealed in NPAK lesions (66.7% and 37.8% in NPAK, 57.9% and 29% in PAK, respectively). On the contrary, rosettes and a red starburst pattern were more commonly observed in PAK lesions (42.1% and 21% vs. 31.1% and 8.9%, respectively) (Figure 1).
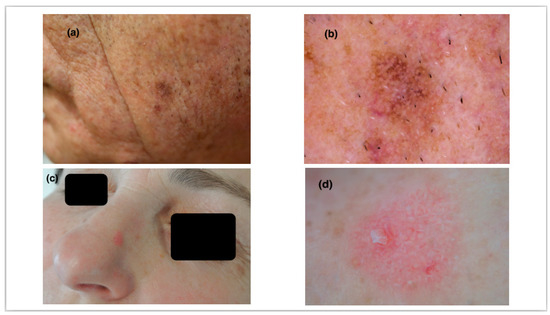
Figure 1.
(a) A 67-year-old male patient with a pigmented scaly plaque on the left lateral side of the face. (b) Pigmented pseudo-network and widened follicular openings along with fine scaling demonstrate a typical pigmented actinic keratosis of the face. (c) A 62-year-old female patient with a pink, slightly elevated plaque on the nose. (d) A red pseudo-network surrounding widened follicular ostia and yellowish dots are features of a non-pigmented actinic keratosis. Linear wavy vessels can also be observed.
3.3. Diagnostic Significance of Dermoscopic Structures
For statistically significant dermoscopic features with p < 0.05 of NPAK and PAK, Se, Sp, PPV, and NPV were calculated (Table 3). Among the dermoscopic results of NPAK, the red network had high Se (84%) but low Sp (39%) values, while the pigmented network and pigment intensity less than 10% had high Se (89% and 83%, respectively), Sp (77% for both), and NPV values (89% and 93%, respectively). When the pigment intensity was greater than 10%, Se, Sp, PPV, and NPV values were high (90%, 86%, 79%, and 93%, respectively).

Table 3.
Se, Sp, PPV, and NPV of significantly present dermoscopic criteria for NPAK and PAK.
Finally, the results of the logistic regression analysis are presented in Table 4. The odds ratio for the red pseudo-network is 0.28, 29.75 for pigmented pseudo-network, 17.22 for pigment intensity less than 10%, and 58.9 for pigment intensity greater than 10%.

Table 4.
Logistic regression analysis of statistically significant dermoscopic criteria.
4. Discussion
In this study, we examined the demographic, clinical, and dermoscopic characteristics aiming to detect special observations that could accurately differentiate NPAK from PAK.
Concerning age, gender, and skin type according to the Fitzpatrick classification of the participant, no significant differences were observed between NPAK and PAK lesions. Patients of both groups tended to be of an older age, with a preponderance of the male sex, and observations in agreement with previous epidemiological studies [5,17]. In the NPAK group, skin phototype II was predominant, while in the PAK group, phototype III was more common. Phototype I was rarely observed in PAK compared to NPAK, a result consistent with other reports, suggestive of the tendency of PAK to present darker phototypes [1].
Moreover, results regarding the macroscopic examination of lesions revealed no statistical significance. The majority (approximately one-half) of NPAK were located in the scalp, and less than one-quarter in the lateral face, while PAK was equally distributed in the lateral face, scalp, and temples. Our observation was in line with the previous data [17]. In addition, according to Olsen’s clinical grading, NPAK and PAK displayed similar frequencies in stages I to III, as mentioned in the existing literature [18].
The dermoscopic examination could confidently differentiate NPAK from PAK through color. A pigmented pseudo-network is a potent predictor of PAK (22.2% NPAK vs. 89.5% PAK) demonstrating high Se and NPV values, moderate Sp and PPV values, and an odds ratio of 29.75 for pigmented actinic lesions in accordance with the previous reports [19]. Pigment intensity of more than 10% had discriminating efficiency for PAK versus NPAK with high Se and NPV, moderate Sp and PPV, and an odds ratio of 58.9. Pigment intensity of less than 10% was also statistically significant with high NPP, moderate Se and Sp, low PPV, and an odds ratio of 17.22. The presence of a red pseudo-network was significantly predominant in NPAK lesions (84.4% NPAK vs. 60.5% PAK) but without discriminative potential (moderate Se, low PPV, and NPV, very-low Sp, odds ratio of 0.28), in concordance with the previous dermoscopic data [15]. The detection of a red pseudo-network in PAK could be correlated with the presence of significant histological inflammation, which is a characteristic of these pre-malignant lesions [20].
Finally, the presence of scale, targetoid follicular openings, rosettes, linear wavy vessels, and shiny streaks did not meet statistical importance for the differentiation between NPAK and PAK, since these features were observed at almost similar frequencies in both types of lesions. Interestingly, the red starburst pattern, a sign of more aggressive lesions, was close to statistical significance for PAK (8.9% NPAK vs. 21% PAK, p = 0.13).
The limitations of our study were the relatively small number of examined lesions, the non-blinded selection of patients and lesions, and the fact that the investigators were aware of the diagnosis. In addition, the accuracy of the dermoscopic criteria referred only to the differentiation of NPAK from PAK, and therefore could not be generalized. Therefore, further studies should be conducted, including non-pigmented and pigmented skin lesions with similar macroscopic presentations, in order to observe special AK dermoscopic features.
5. Conclusions
Our study determined that pigmentation is the main variable that could clinically and dermoscopically differentiate NPAK from PAK. Regarding other well-established AK dermoscopic features (i.e., scale, targetoid follicular openings, rosettes, linear wavy vessels, and shiny streaks), these are evenly observed among NPAK and PAK failing to show statistical significance. The presence of a pigmented pseudo-network with a pigment intensity of more than 10% is an accurate, statistically important dermoscopic clue for the differential diagnosis between NPAK and PAK.
Author Contributions
Conceptualization, D.S. and A.C.K.; Methodology, D.S., A.L., Z.A. and A.C.K.; Software, T.Z.; Formal analysis, A.L., Z.A., A.S., D.R. and A.C.K.; Investigation, D.S., Z.A., A.Z., K.L., G.P., E.P., F.K. and A.P.; Data curation, T.Z., A.S. and D.R.; Writing—original draft, D.S. and M.T.; Writing—review & editing, D.S., M.T., A.L. and A.C.K.; Supervision, D.S., A.P., A.S., D.R. and A.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Ethics Committee of the institution (approval code 2530/5,10.2018).
Informed Consent Statement
All participants were informed about the aims of the study and provided written informed consent.
Conflicts of Interest
None to declare for the purposes of this study.
References
- Reinehr, C.P.H.; Bakos, R.M. Actinic keratoses: Review of clinical, dermoscopic, and therapeutic aspects. An. Bras. Dermatol. 2019, 94, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Casari, A.; Chester, J.; Pellacani, G. Actinic Keratosis and Non-Invasive Diagnostic Techniques: An Update. Biomedicines 2018, 6, 8. [Google Scholar] [CrossRef]
- Glogau, R.G. The risk of progression to invasive disease. J. Am. Acad. Dermatol. 2000, 42, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Rennie, G.; Selwood, T.S. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet 1988, 1, 795–797. [Google Scholar] [CrossRef]
- Sgouros, D.; Milia-Argyti, A.; Arvanitis, D.K.; Polychronaki, E.; Kousta, F.; Panagiotopoulos, A.; Theotokoglou, S.; Syrmali, A.; Theodoropoulos, K.; Stratigos, A.; et al. Actinic Keratoses (AK): An Exploratory Questionnaire-Based Study of Patients’ Illness Perceptions. Curr. Oncol. 2022, 29, 5150–5163. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, D.; Theofili, M.; Damaskou, V.; Theotokoglou, S.; Theodoropoulos, K.; Stratigos, A.; Theofilis, P.; Panayiotides, I.; Rigopoulos, D.; Katoulis, A. Dermoscopy as a Tool in Differentiating Cutaneous Squamous Cell Carcinoma from Its Variants. Dermatol. Pract. Concept. 2021, 11, e2021050. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.; Piccolo, V.; Lallas, A.; Argenziano, G. Recent advances in dermoscopy. F1000Research 2016, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Argenziano, G.; Zendri, E.; Moscarella, E.; Longo, C.; Grenzi, L.; Pellacani, G.; Zalaudek, I. Update on non-melanoma skin cancer and the value of dermoscopy in its diagnosis and treatment monitoring. Expert Rev. Anticancer. Ther. 2013, 13, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Giacomel, J.; Argenziano, G.; Hofmann-Wellenhof, R.; Micantonio, T.; Di Stefani, A.; Oliviero, M.; Rabinovitz, H.; Soyer, H.P.; Peris, K. Dermoscopy of facial nonpigmented actinic keratosis. Br. J. Dermatol. 2006, 155, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Argenziano, G. Dermoscopy of actinic keratosis, intraepidermal carcinoma and squamous cell carcinoma. Curr. Probl. Dermatol. 2015, 46, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Ferrara, G.; Leinweber, B.; Mercogliano, A.; D’Ambrosio, A.; Argenziano, G. Pitfalls in the clinical and dermoscopic diagnosis of pigmented actinic keratosis. J. Am. Acad. Dermatol. 2005, 53, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Akay, B.N.; Kocyigit, P.; Heper, A.O.; Erdem, C. Dermatoscopy of flat pigmented facial lesions: Diagnostic challenge between pigmented actinic keratosis and lentigo maligna. Br. J. Dermatol. 2010, 163, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Ertop Dogan, P.; Akay, B.N.; Okcu Heper, A.; Rosendahl, C.; Erdem, C. Dermatoscopic findings and dermatopathological correlates in clinical variants of actinic keratosis, Bowen’s disease, keratoacanthoma, and squamous cell carcinoma. Dermatol. Ther. 2021, 34, e14877. [Google Scholar] [CrossRef] [PubMed]
- Balagula, Y.; Braun, R.P.; Rabinovitz, H.S.; Dusza, S.W.; Scope, A.; Liebman, T.N.; Mordente, I.; Siamas, K.; Marghoob, A.A. The significance of crystalline/chrysalis structures in the diagnosis of melanocytic and nonmelanocytic lesions. J. Am. Acad. Dermatol. 2012, 67, 194.E1–194.E8. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Giacomel, J.; Schmid, K.; Bondino, S.; Rosendahl, C.; Cavicchini, S.; Tourlaki, A.; Gasparini, S.; Bourne, P.; Keir, J.; et al. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: A progression model. J. Am. Acad. Dermatol. 2012, 66, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Abernethy, M.L.; Kulp-Shorten, C.; Callen, J.P.; Glazer, S.D.; Huntley, A.; McCray, M.; Monroe, A.B.; Tschen, E.; Wolf, J.E., Jr. A double-blind, vehicle-controlled study evaluating masoprocol cream in the treatment of actinic keratoses on the head and neck. J. Am. Acad. Dermatol. 1991, 24, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C. Epidemiology of actinic keratoses. Curr. Probl. Dermatol. 2015, 46, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; McGuigan, K.L.; Osley, K.L.; Zendell, K.; Lee, J.B. Pigmented solar (actinic) keratosis: An underrecognized collision lesion. J. Am. Acad. Dermatol. 2013, 68, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Kelati, A.; Baybay, H.; Moscarella, E.; Argenziano, G.; Gallouj, S.; Mernissi, F.Z. Dermoscopy of Pigmented Actinic Keratosis of the Face: A Study of 232 Cases. Actas Dermosifiliogr. 2017, 108, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Labadie, J.G.; Compres, E.; Sunshine, J.C.; Alam, M.; Gerami, P.; Harikumar, V.; Poon, E.; Arndt, K.A.; Dover, J.S. Actinic Keratosis Color and Its Associations: A Retrospective Photographic, Dermoscopic, and Histologic Evaluation. Dermatol. Surg. 2022, 48, 57–60. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).