Long Pentraxin 3 as a New Biomarker for Diagnosis of Hip and Knee Periprosthetic Joint Infections
Abstract
:1. Introduction
2. Materials and Methods
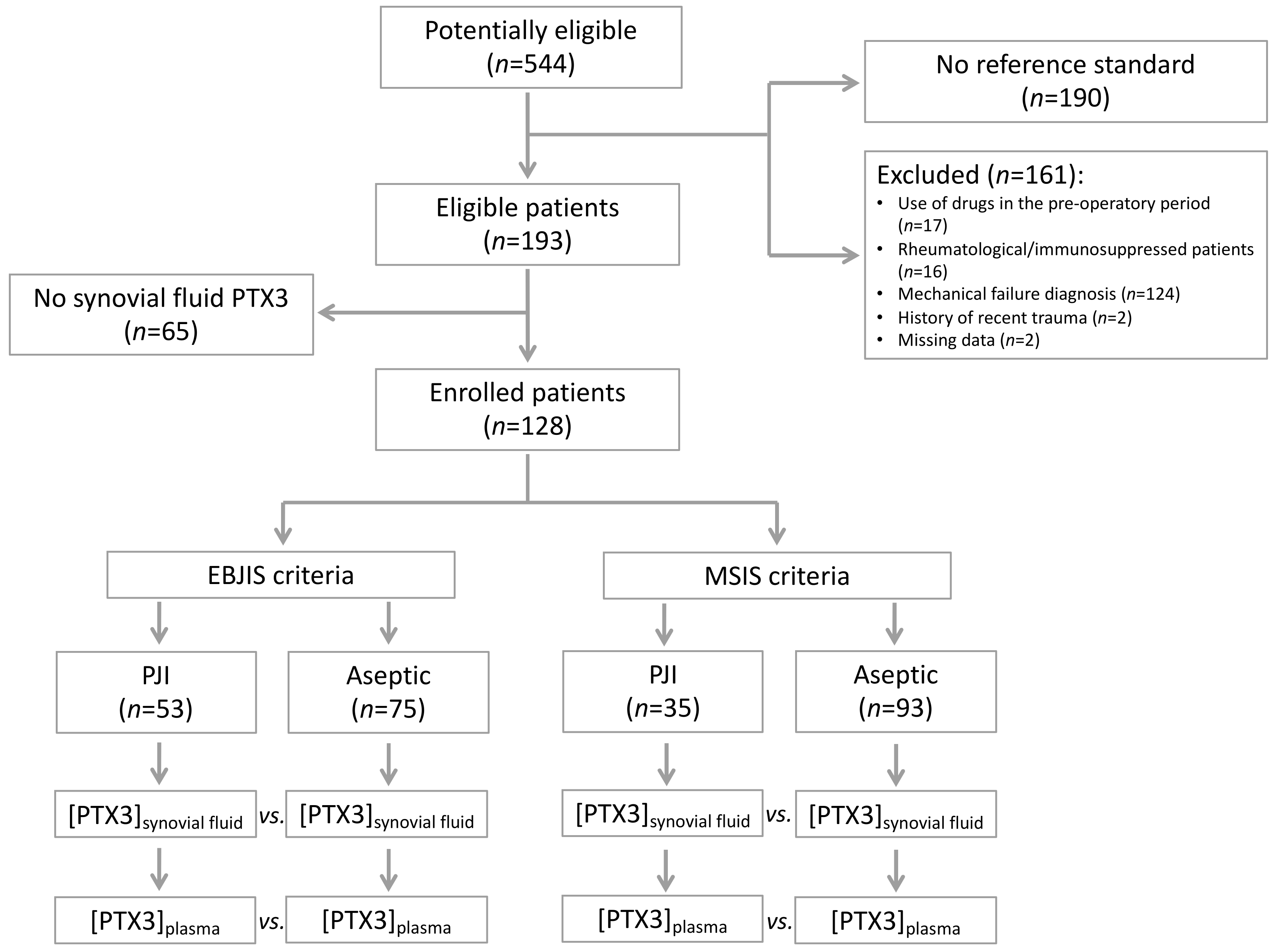
2.1. Study Design and Sample Size
2.2. Study Population
2.3. Description of Treatment
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Microbiological Analysis
3.3. Laboratory Analysis
3.4. Clinical Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertsson, O.; Lidgren, L.; Sundberg, M.; W-Dahl, A. The Swedish Knee Arthroplasty Register—Annual Report 2019; The Swedish Knee Arthroplasty Register: Lund, Sweden, 2019; ISBN 978-91-88017-29-1. [Google Scholar]
- Kärrholm, J.; Rogmark, C.; Nauclér, E.; Nåtman, J.; Vinblad, J.; Mohaddes, M.; Rolfson, O. Swedish Hip Arthroplasty Register—Annual Report 2019; Sahlgrenska University Hospital: Göteborg, Swenden, 2019; ISBN 978-91-986612-0-0. ISSN 1654-5982. [Google Scholar]
- The Workgroup of the Musculoskeletal Infection Society. New Definition for Periprosthetic Joint Infection. J. Arthroplast. 2011, 26, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Gehrke, T.; International Consensus Group on Periprosthetic Joint Infection. Definition of Periprosthetic Joint Infection. J. Arthroplast. 2014, 29, 1331. [Google Scholar] [CrossRef] [Green Version]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corvec, S.; Portillo, M.E.; Pasticci, B.M.; Borens, O.; Trampuz, A. Epidemiology and New Developments in the Diagnosis of Prosthetic Joint Infection. Int. J. Artif. Organs 2012, 35, 923–934. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis. 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Renz, N.; Yermak, K.; Perka, C.; Trampuz, A. Alpha Defensin Lateral Flow Test for Diagnosis of Periprosthetic Joint Infection: Not a Screening but a Confirmatory Test. J. Bone Joint Surg. Am. 2018, 100, 742–750. [Google Scholar] [CrossRef]
- Bellova, P.; Knop-Hammad, V.; Königshausen, M.; Mempel, E.; Frieler, S.; Gessmann, J.; Schildhauer, T.A.; Baecker, H. Sonication of Retrieved Implants Improves Sensitivity in the Diagnosis of Periprosthetic Joint Infection. BMC Musculoskelet. Disord. 2019, 20, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebše, R. The EBJIS Definition of Periprosthetic Joint Infection. Bone Joint J. 2021, 103-B, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Parvizi, J. The Role of Biomarkers in the Diagnosis of Periprosthetic Joint Infection. EFORT Open Rev. 2016, 1, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Okroj, K.T.; Calkins, T.E.; Kayupov, E.; Kheir, M.M.; Bingham, J.S.; Beauchamp, C.P.; Parvizi, J.; Della Valle, C.J. The Alpha-Defensin Test for Diagnosing Periprosthetic Joint Infection in the Setting of an Adverse Local Tissue Reaction Secondary to a Failed Metal-on-Metal Bearing or Corrosion at the Head-Neck Junction. J. Arthroplast. 2018, 33, 1896–1898. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.C.; Beswick, A.D.; Kunutsor, S.K.; Wilson, M.J.; Whitehouse, M.R.; Blom, A.W. The Alpha-Defensin Immunoassay and Leukocyte Esterase Colorimetric Strip Test for the Diagnosis of Periprosthetic Infection: A Systematic Review and Meta-Analysis. J. Bone Jt. Surg. Am. 2016, 98, 992–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inforzato, A.; Baldock, C.; Jowitt, T.A.; Holmes, D.F.; Lindstedt, R.; Marcellini, M.; Rivieccio, V.; Briggs, D.C.; Kadler, K.E.; Verdoliva, A.; et al. The Angiogenic Inhibitor Long Pentraxin PTX3 Forms an Asymmetric Octamer with Two Binding Sites for FGF2. J. Biol. Chem. 2010, 285, 17681–17692. [Google Scholar] [CrossRef] [Green Version]
- Noone, D.P.; Dijkstra, D.J.; van der Klugt, T.T.; van Veelen, P.A.; de Ru, A.H.; Hensbergen, P.J.; Trouw, L.A.; Sharp, T.H. PTX3 Structure Determination Using a Hybrid Cryoelectron Microscopy and AlphaFold Approach Offers Insights into Ligand Binding and Complement Activation. Proc. Natl. Acad. Sci. USA 2022, 119, e2208144119. [Google Scholar] [CrossRef]
- Abderrahim-Ferkoune, A.; Bezy, O.; Chiellini, C.; Maffei, M.; Grimaldi, P.; Bonino, F.; Moustaid-Moussa, N.; Pasqualini, F.; Mantovani, A.; Ailhaud, G.; et al. Characterization of the Long Pentraxin PTX3 as a TNFalpha-Induced Secreted Protein of Adipose Cells. J. Lipid Res. 2003, 44, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Alles, V.V.; Bottazzi, B.; Peri, G.; Golay, J.; Introna, M.; Mantovani, A. Inducible Expression of PTX3, a New Member of the Pentraxin Family, in Human Mononuclear Phagocytes. Blood 1994, 84, 3483–3493. [Google Scholar] [CrossRef] [Green Version]
- Doni, A.; Peri, G.; Chieppa, M.; Allavena, P.; Pasqualini, F.; Vago, L.; Romani, L.; Garlanda, C.; Mantovani, A. Production of the Soluble Pattern Recognition Receptor PTX3 by Myeloid, but Not Plasmacytoid, Dendritic Cells. Eur. J. Immunol. 2003, 33, 2886–2893. [Google Scholar] [CrossRef]
- Goodman, A.R.; Levy, D.E.; Reis, L.F.; Vilcek, J. Differential Regulation of TSG-14 Expression in Murine Fibroblasts and Peritoneal Macrophages. J. Leukoc. Biol. 2000, 67, 387–395. [Google Scholar] [CrossRef]
- Klouche, M.; Peri, G.; Knabbe, C.; Eckstein, H.-H.; Schmid, F.-X.; Schmitz, G.; Mantovani, A. Modified Atherogenic Lipoproteins Induce Expression of Pentraxin-3 by Human Vascular Smooth Muscle Cells. Atherosclerosis 2004, 175, 221–228. [Google Scholar] [CrossRef]
- Breviario, F.; d’Aniello, E.M.; Golay, J.; Peri, G.; Bottazzi, B.; Bairoch, A.; Saccone, S.; Marzella, R.; Predazzi, V.; Rocchi, M. Interleukin-1-Inducible Genes in Endothelial Cells. Cloning of a New Gene Related to C-Reactive Protein and Serum Amyloid P Component. J. Biol. Chem. 1992, 267, 22190–22197. [Google Scholar] [CrossRef]
- Vouret-Craviari, V.; Matteucci, C.; Peri, G.; Poli, G.; Introna, M.; Mantovani, A. Expression of a Long Pentraxin, PTX3, by Monocytes Exposed to the Mycobacterial Cell Wall Component Lipoarabinomannan. Infect. Immun. 1997, 65, 1345–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doni, A.; Musso, T.; Morone, D.; Bastone, A.; Zambelli, V.; Sironi, M.; Castagnoli, C.; Cambieri, I.; Stravalaci, M.; Pasqualini, F.; et al. An Acidic Microenvironment Sets the Humoral Pattern Recognition Molecule PTX3 in a Tissue Repair Mode. J. Exp. Med. 2015, 212, 905–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaillon, S.; Peri, G.; Delneste, Y.; Frémaux, I.; Doni, A.; Moalli, F.; Garlanda, C.; Romani, L.; Gascan, H.; Bellocchio, S.; et al. The Humoral Pattern Recognition Receptor PTX3 Is Stored in Neutrophil Granules and Localizes in Extracellular Traps. J. Exp. Med. 2007, 204, 793–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diniz, S.N.; Nomizo, R.; Cisalpino, P.S.; Teixeira, M.M.; Brown, G.D.; Mantovani, A.; Gordon, S.; Reis, L.F.L.; Dias, A.A.M. PTX3 Function as an Opsonin for the Dectin-1-Dependent Internalization of Zymosan by Macrophages. J. Leukoc. Biol. 2004, 75, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Parente, R.; Doni, A.; Bottazzi, B.; Garlanda, C.; Inforzato, A. The Complement System in Aspergillus Fumigatus Infections and Its Crosstalk with Pentraxins. FEBS Lett. 2020, 594, 2480–2501. [Google Scholar] [CrossRef] [Green Version]
- Grčević, D.; Sironi, M.; Valentino, S.; Deban, L.; Cvija, H.; Inforzato, A.; Kovačić, N.; Katavić, V.; Kelava, T.; Kalajzić, I.; et al. The Long Pentraxin 3 Plays a Role in Bone Turnover and Repair. Front. Immunol. 2018, 9, 417. [Google Scholar] [CrossRef] [Green Version]
- Parente, R.; Sobacchi, C.; Bottazzi, B.; Mantovani, A.; Grčevic, D.; Inforzato, A. The Long Pentraxin PTX3 in Bone Homeostasis and Pathology. Front. Immunol. 2019, 10, 2628. [Google Scholar] [CrossRef] [Green Version]
- Brunetta, E.; Folci, M.; Bottazzi, B.; De Santis, M.; Gritti, G.; Protti, A.; Mapelli, S.N.; Bonovas, S.; Piovani, D.; Leone, R.; et al. Macrophage Expression and Prognostic Significance of the Long Pentraxin PTX3 in COVID-19. Nat. Immunol. 2021, 22, 19–24. [Google Scholar] [CrossRef]
- Caironi, P.; Masson, S.; Mauri, T.; Bottazzi, B.; Leone, R.; Magnoli, M.; Barlera, S.; Mamprin, F.; Fedele, A.; Mantovani, A.; et al. Pentraxin 3 in Patients with Severe Sepsis or Shock: The ALBIOS Trial. Eur. J. Clin. Investig. 2017, 47, 73–83. [Google Scholar] [CrossRef]
- Jenny, N.S.; Arnold, A.M.; Kuller, L.H.; Tracy, R.P.; Psaty, B.M. Associations of Pentraxin 3 with Cardiovascular Disease and All-Cause Death: The Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Latini, R.; Maggioni, A.P.; Peri, G.; Gonzini, L.; Lucci, D.; Mocarelli, P.; Vago, L.; Pasqualini, F.; Signorini, S.; Soldateschi, D.; et al. Prognostic Significance of the Long Pentraxin PTX3 in Acute Myocardial Infarction. Circulation 2004, 110, 2349–2354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doni, A.; Stravalaci, M.; Inforzato, A.; Magrini, E.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Link Between Innate Immunity, Tissue Remodeling, and Cancer. Front. Immunol. 2019, 10, 712. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Li, T.; Yan, S.; Liu, M.; Liu, K.; Zhang, H.; Gao, M.; Xiao, X. Pentraxin-3 Is a Strong Biomarker of Sepsis Severity Identification and Predictor of 90-Day Mortality in Intensive Care Units via Sepsis 3.0 Definitions. Diagnostics 2021, 11, 1906. [Google Scholar] [CrossRef]
- Davoudian, S.; Piovani, D.; Desai, A.; Mapelli, S.N.; Leone, R.; Sironi, M.; Valentino, S.; Silva-Gomes, R.; Stravalaci, M.; Asgari, F.; et al. A Cytokine/PTX3 Prognostic Index as a Predictor of Mortality in Sepsis. Front. Immunol. 2022, 13, 979232. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Puchner, S.E.; Windhager, R. Serum Inflammatory Biomarkers in the Diagnosis of Periprosthetic Joint Infections. Biomedicines 2021, 9, 1128. [Google Scholar] [CrossRef]
- Huard, M.; Detrembleur, C.; Poilvache, H.; Pastor, Y.; Geels, I.; Van Cauter, M.; Driesen, R.; Yombi, J.-C.; Neyt, J.; Cornu, O. Alpha Defensin: A Diagnostic Accuracy Depending on the Infection Definition Used. J. Arthroplast. 2020, 35, 1355–1360. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Perino, G.; Kienapfel, H.; Ascherl, R.; Hassenpflug, G.J.; Thomsen, M.; Thomas, P.; Huber, M.; Kendoff, D.; et al. Revised Histopathological Consensus Classification of Joint Implant Related Pathology. Pathol. Res. Pract. 2014, 210, 779–786. [Google Scholar] [CrossRef]
- Portillo, M.E.; Salvadó, M.; Trampuz, A.; Plasencia, V.; Rodriguez-Villasante, M.; Sorli, L.; Puig, L.; Horcajada, J.P. Sonication versus Vortexing of Implants for Diagnosis of Prosthetic Joint Infection. J. Clin. Microbiol. 2013, 51, 591–594. [Google Scholar] [CrossRef]
- Knoflach, M.; Kiechl, S.; Mantovani, A.; Cuccovillo, I.; Bottazzi, B.; Xu, Q.; Xiao, Q.; Gasperi, A.; Mayr, A.; Kehrer, M.; et al. Pentraxin-3 as a Marker of Advanced Atherosclerosis Results from the Bruneck, ARMY and ARFY Studies. PLoS ONE 2012, 7, e31474. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Coppadoro, A.; Bombino, M.; Bellani, G.; Zambelli, V.; Fornari, C.; Berra, L.; Bittner, E.A.; Schmidt, U.; Sironi, M.; et al. Alveolar Pentraxin 3 as an Early Marker of Microbiologically Confirmed Pneumonia: A Threshold-Finding Prospective Observational Study. Crit. Care 2014, 18, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuiper, J.W.P.; Verberne, S.J.; Vos, S.J.; van Egmond, P.W. Does the Alpha Defensin ELISA Test Perform Better Than the Alpha Defensin Lateral Flow Test for PJI Diagnosis? A Systematic Review and Meta-Analysis of Prospective Studies. Clin. Orthop. Relat. Res. 2020, 478, 1333–1344. [Google Scholar] [CrossRef] [PubMed]

| MSIS (1/2 Major Criteria or 3/5 Minor Criteria) | EBJIS (1/4 Criteria) |
|---|---|
| Major criteria: | Purulence around the prosthesis or sinus tract |
| Two positive periprosthetic cultures | Increased synovial fluid leukocyte count |
| Sinus tract communicating with the prosthesis | Positive histopathology |
| Minor criteria: | Confirmatory microbial growth in synovial fluid, periprosthetic tissue, or sonication culture |
| Elevated CRP and ESR | |
| Elevated synovial fluid leukocyte count or positive leukocyte esterase strip test (++ or +++) | |
| Elevated synovial fluid percentage of granulocytes | |
| A single positive culture | |
| Positive histological analysis of periprosthetic tissue |
| EBJIS | MSIS | ||||||
|---|---|---|---|---|---|---|---|
| All patients (n = 128) | Infected (n = 53) | Non-infected (n = 75) | p | Infected (n = 35) | Non-infected (n = 93) | p | |
| Sex (M:F) | 51:77 | 26:27 | 25:50 | 0.074 b | 17:18 | 34:59 | 0.216 b |
| BMI | 28.37 a (24.54–30.94) | 29.14 (24.23–32.04) | 27.13 (24.60–29.66) | 0.163 c | 29.14 (24.08–33.38) | 27.27 (24.65–29.76) | 0.144 c |
| Charlson score | 0: 11 (8%) 1: 16 (13%) 2: 31 (24%) 3: 42 (33%) 4: 17 (13%) 5: 9 (7%) 6: 2 (2%) | 0: 6 (11%) 1: 9 (17%) 2: 15 (28%) 3: 12 (23%) 4: 6 (11%) 5: 4 (8%) 6: 1 (2%) | 0: 5 (7%) 1: 7 (9%) 2: 16 (21%) 3: 30 (40%) 4: 11 (15%) 5: 5 (7%) 6: 1 (1%) | 0.416 b | 0: 5 (14%) 1: 8 (23%) 2: 5 (14%) 3: 10 (29%) 4: 4 (11%) 5: 3 (9%) 6: 0 | 0: 6 (6%) 1: 8 (9%) 2: 26 (28%) 3: 32 (35%) 4: 13 (14%) 5: 6 (6%) 6: 2 (2%) | 0.162 b |
| ASA score | 1: 22 (17%) 2: 72 (56%) 3: 34 (27%) | 1: 9 (17%) 2: 30 (57%) 3: 14 (26%) | 1: 13 (17%) 2: 42 (56%) 3: 20 (27%) | 0.998 b | 1: 6 (17%) 2: 19 (54%) 3: 10 (29%) | 1: 16 (17%) 2: 53 (57%) 3: 24 (26%) | 0.948 b |
|
Age at surgery | 70 (61–76) a | 69 (56–73) | 70 (62–78) | 0.242 c | 70 (52–74) | 70 (62–77) | 0.165 c |
| Pathogens | PJI (EBJIS) n (Hip:Knee) | PJI (MSIS) n (Hip:Knee) |
|---|---|---|
| Coagulase Negative Staphylococci | 15 (14:1) | 13 (12:1) |
| Staphylococcus Aureus | 7 (6:1) | 7 (6:1) |
| Streptococcus Species | 5 (4:1) | 4 (3:1) |
| Pseudomonas Aeruginosa | 2 (2:0) | 2 (2:0) |
| Enterococcus Faecalis | 2 (1:1) | 1 (1:0) |
| Enterococcus Faecium | 2 (1:1) | 1 (1:0) |
| Corynebacterium Sp. | 1 (0:1) | - |
| Microbacterium Sp. | 1 (1:0) | - |
| Propionibacterium Acnes | 1 (1:0) | - |
| Ralstonia Pickettii | 1 (1:0) | - |
| Aspergillus Fumigatus | 1 (1:0) | - |
| Brevibacterium Casei | 1 (0:1) | - |
| Brevibacterium Laterosporus | 1 (0:1) | - |
| Enterobacter Cloacae | 1 (0:1) | - |
| Escherichia Coli | 1 (0:1) | - |
| Not identified | 16 (12:4) | 9 (7:2) |
| EBJIS | MSIS | ||||||
|---|---|---|---|---|---|---|---|
| All Patients (n = 128) | Infected (n = 53) | Non-Infected (n = 75) | p b | Infected (n = 35) | Non-Infected (n = 93) | p | |
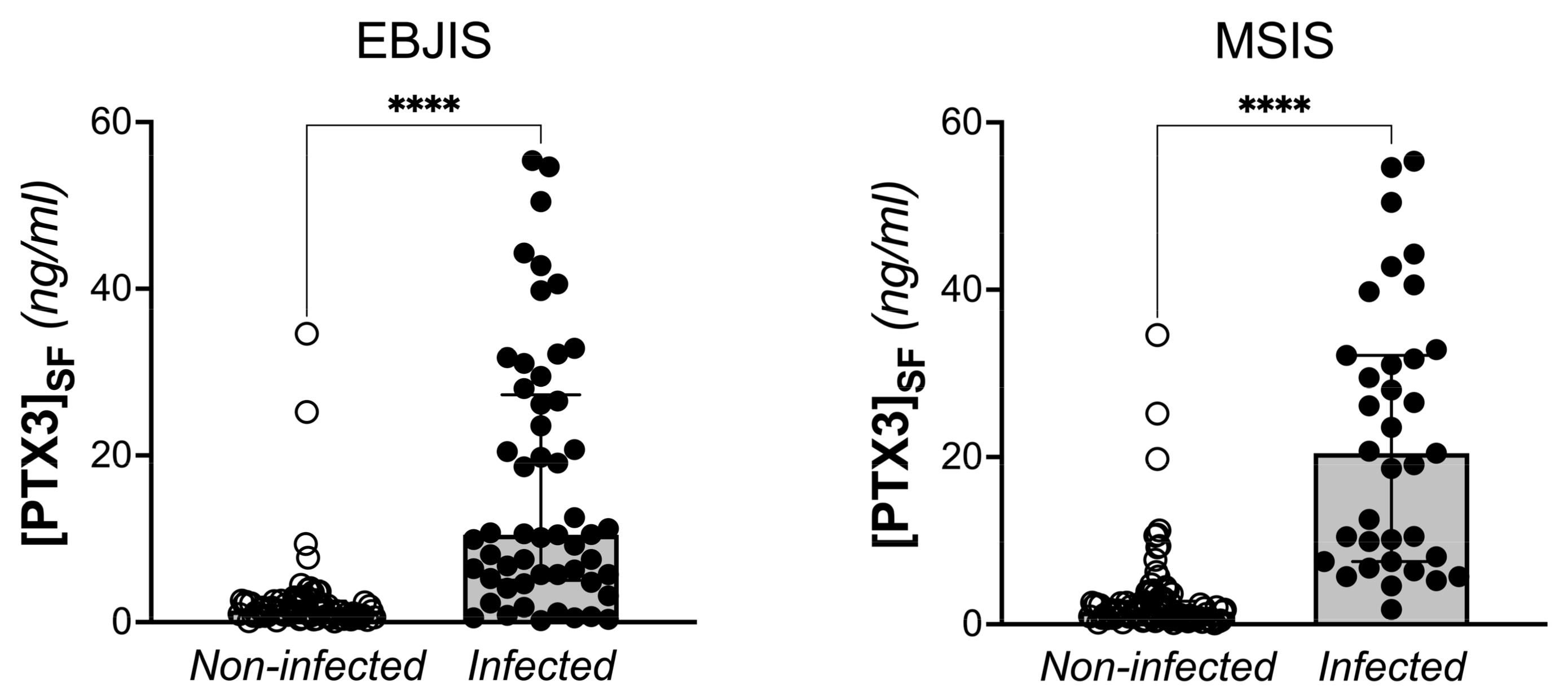
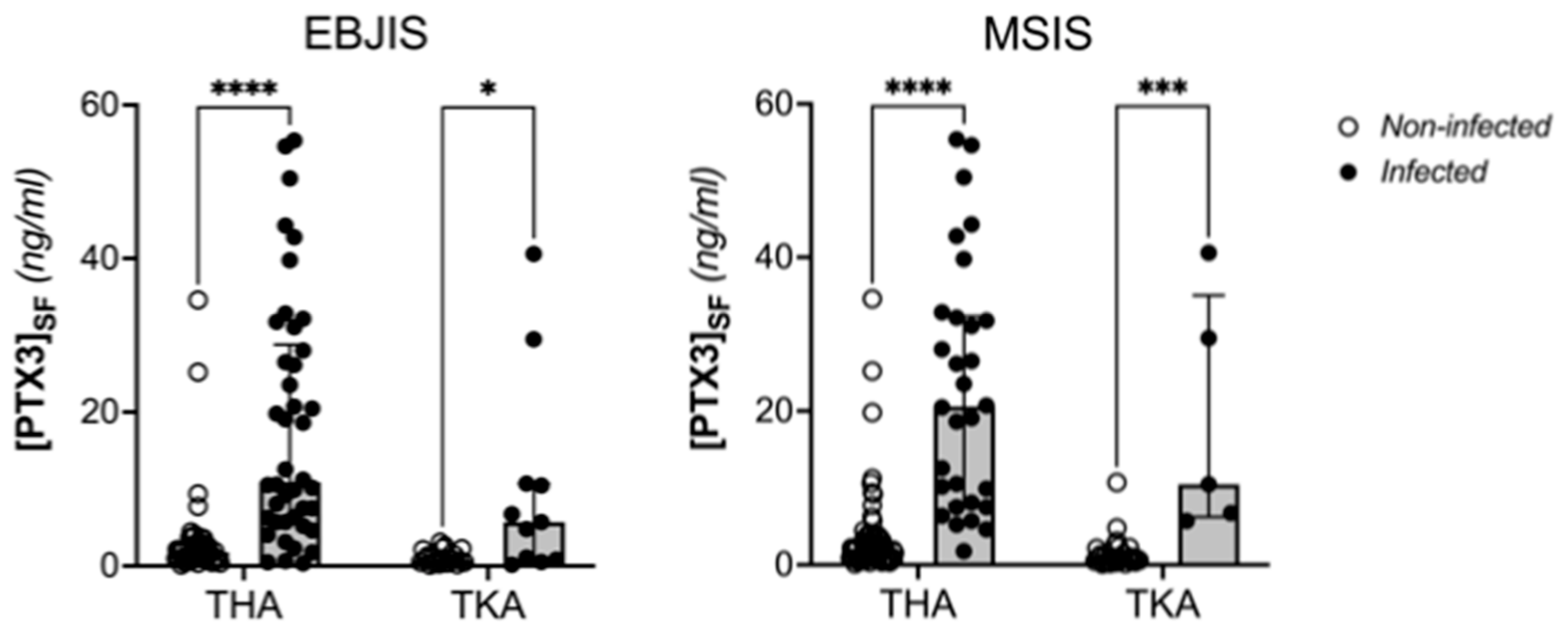
| Synovial PTX3 (ng/mL) | 2.41 (0.91–9.33) a | 10.48 (5.02–27.27) | 1.40 (0.76–2.52) | <0.0001 | 20.46 (7.51–32.15) | 1.51 (0.74–2.79) | <0.0001 |
| Plasmatic PTX3 (ng/mL) | 4.89 (3.59–6.43) | 4.66 (3.28–6.34) | 5.25 (3.76–6.46) | 0.347 | 4.66 (3.36–6.21) | 5.23 (3.61–6.46) | 0.567 |
| Plasmatic CRP (ng/mL) | 0.65 (0.22–1.61) | 1.33 (0.76–3.34) | 0.27 (0.16–0.90) | <0.0001 | 2.06 (0.88–4.26) | 0.40 (0.17–0.94) | <0.0001 |
|
ESR (mm/h) | 20.00 (10.00–38.75) | 20.00 (10.25–55.75) | 20.00 (9.25–34.00) | 0.200 | 22.00 (11.50–61.00) | 20.00 (9.00–34.00) | 0.105 |
|
D-Dimer (ng/mL) | 325 (249–449) | 318 (247–503) | 342 (226–440) | 0.936 | 347 (241–610) | 325 (252–413) | 0.433 |
| EBJS | Obs a | Threshold b | Sensitivity | Specificity | Accuracy | SE c | LR+ d | LR- e | AUC | p |
|---|---|---|---|---|---|---|---|---|---|---|
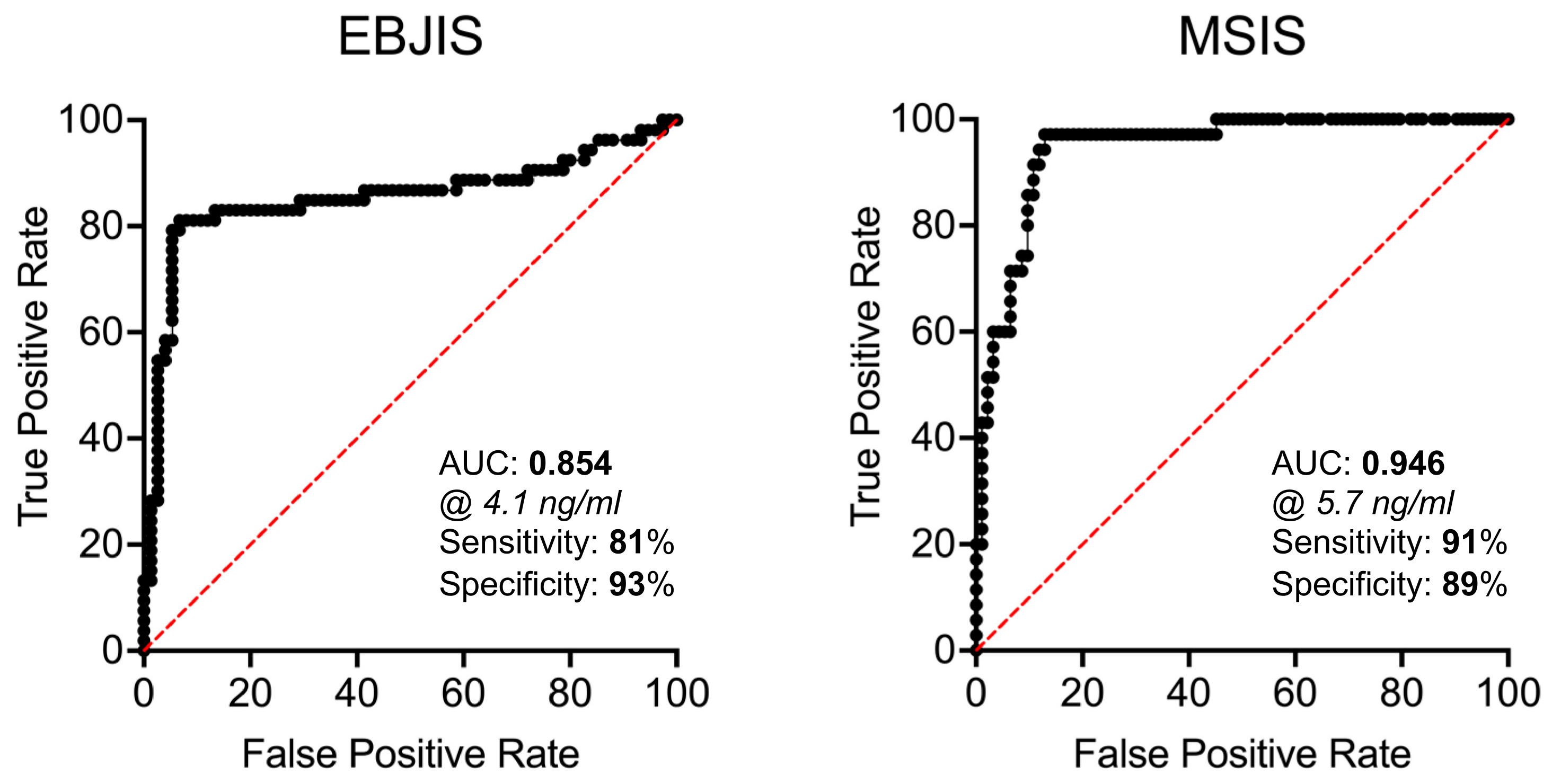
| Synovial PTX3 (ng/mL) | 128 | 4.1 | 81.13% | 93.33% | 87.5% | 0.04 | 11.89 | 0.22 | 0.85 (0.78–0.93) f | <0.0001 |
| Plasmatic PTX3 (ng/mL) | 124 | 4.7 | 54.00% | 59.46% | 57.45% | 0.05 | 1.28 | 0.72 | 0.55 (0.45–0.66) | 0.345 |
| Plasmatic CRP (mg/dL) | 128 | 0.94 | 62.26% | 80.00% | 72.66% | 0.04 | 3.23 | 0.49 | 0.80 (0.72–0.88) | <0.0001 |
| ESR (mm/h) | 96 | 40 | 36.11% | 86.67% | 67.71% | 0.06 | 2.71 | 0.74 | 0.58 (0.45–0.70) | 0.198 |
| D-Dimer (ng/mL) | 33 | 516 | 25.00% | 82.24% | 57.58% | 0.10 | 2.13 | 0.85 | 0.51 (0.31–0.71) | 0.928 |
| MSIS | Obs | Threshold | Sensitivity | Specificity | Accuracy | SE | LR+ | LR- | AUC | p |
| Synovial PTX3 (ng/mL) | 128 | 5.7 | 91.43% | 89.25% | 89.84% | 0.02 | 8.50 | 0.10 | 0.95 (0.91–0.98) | <0.001 |
| Plasmatic PTX3 (ng/mL) | 124 | 4.8 | 54.44% | 55.88% | 50.81% | 0.06 | 0.77 | 1.17 | 0.53 (0.42–0.65) | 0.564 |
| Plasmatic CRP (mg/dL) | 128 | 0.94 | 71.43% | 75.27% | 74.22% | 0.04 | 2.90 | 0.41 | 0.81 (0.72–0.89) | <0.0001 |
| ESR (mm/h) | 96 | 40 | 42.86% | 84.00% | 75.00% | 0.07 | 2.68 | 0.68 | 0.62 (0.47–0.76) | 0.104 |
| D-Dimer (ng/mL) | 33 | 516 | 40.00% | 91.30% | 75.76% | 0.11 | 4.60 | 0.66 | 0.59 (0.36–0.81) | 0.422 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loppini, M.; Di Maio, M.; Avigni, R.; Leone, R.; Inforzato, A.; Grappiolo, G.; Mantovani, A.; Bottazzi, B. Long Pentraxin 3 as a New Biomarker for Diagnosis of Hip and Knee Periprosthetic Joint Infections. J. Clin. Med. 2023, 12, 1055. https://doi.org/10.3390/jcm12031055
Loppini M, Di Maio M, Avigni R, Leone R, Inforzato A, Grappiolo G, Mantovani A, Bottazzi B. Long Pentraxin 3 as a New Biomarker for Diagnosis of Hip and Knee Periprosthetic Joint Infections. Journal of Clinical Medicine. 2023; 12(3):1055. https://doi.org/10.3390/jcm12031055
Chicago/Turabian StyleLoppini, Mattia, Marco Di Maio, Roberta Avigni, Roberto Leone, Antonio Inforzato, Guido Grappiolo, Alberto Mantovani, and Barbara Bottazzi. 2023. "Long Pentraxin 3 as a New Biomarker for Diagnosis of Hip and Knee Periprosthetic Joint Infections" Journal of Clinical Medicine 12, no. 3: 1055. https://doi.org/10.3390/jcm12031055
APA StyleLoppini, M., Di Maio, M., Avigni, R., Leone, R., Inforzato, A., Grappiolo, G., Mantovani, A., & Bottazzi, B. (2023). Long Pentraxin 3 as a New Biomarker for Diagnosis of Hip and Knee Periprosthetic Joint Infections. Journal of Clinical Medicine, 12(3), 1055. https://doi.org/10.3390/jcm12031055






