Abstract
Background: Preoperative diagnosis of periprosthetic joint infections (PJIs) poses an unmet clinical challenge. The long pentraxin PTX3 is a component of the innate immune system involved in infection immunity. This study evaluated the potential of synovial and plasmatic PTX3 in the diagnosis of hip and knee PJIs. Methods: Consecutive total hip and knee arthroplasty (THA/TKA) revisions were prospectively included and classified as septic or aseptic according to the European Bone and Joint Infection Society (EBJIS) and Musculoskeletal Infection Society (MSIS) criteria. The concentration of PTX3 in plasma and synovial fluid samples was measured with ELISA. The AUC, threshold value, sensitivity, specificity, and positive and negative likelihood ratios were calculated using the ROC (receiver operating characteristic) curve method. Results: The study population included 128 patients (94 THAs; 34 TKAs). The AUC of the synovial PTX3 based on EBJIS criteria was 0.85 (p < 0.0001), with a sensitivity of 81.13% and a specificity of 93.33%. The AUC based on MSIS criteria was 0.95 (p < 0.001), with a sensitivity of 91.43% and a specificity of 89.25%. Plasmatic PTX3 failed to discriminate infected from non-infected patients. Conclusions: Synovial PTX3 demonstrated an excellent diagnostic potential in hip and knee PJIs, with a very high specificity irrespective of the diagnostic criteria for PJI.
1. Introduction
Periprosthetic joint infections (PJIs) are the most common cause of total hip and knee arthroplasty (THA and TKA, respectively) revision within two years of surgery, accounting for 62% of revisions in the biennium 2017–2018 [1,2]. Moreover, PJIs are the second cause of THA and TKA revision, after aseptic loosening, regardless of time since surgery. Given that the reoperation rate is 1.6% to 2.4% [1,2], the incidence of PJIs lies in the 1–1.5% range. According to the Swedish Hip and Knee Replacement Registries, 30,404 TKAs and 36,791 THAs have been performed in the biennium 2017–2018 [1,2]. Therefore, a significant number of patients develop septic complications after replacement surgery every year. Preoperative diagnosis of PJI is still challenging. Over time, several classification systems have been developed to formulate a proper diagnosis of PJI, such as the Musculoskeletal Infection Society (MSIS) [3,4], International Consensus Meeting (ICM) [5], Infectious Diseases Society of America (IDSA) [6], and European Bone and Joint Infection Society (EBJIS) [7,8,9,10,11] criteria. Confirmation of diagnosis requires isolation of the pathogen from explanted prosthesis and/or biopsies and its identification through microbiological techniques. On the other hand, preoperative diagnosis is highly desirable to inform and orient the clinical management of PJI patients prior to revision surgery. Preoperative diagnostic criteria integrate varying levels of information that are contributed by a number of clinical and biochemical parameters and have been consistently upgraded over recent years.
In this regard, several biomarkers have been assessed for diagnosis of PJIs, in particular those present in the synovial fluid and serum [12]. A major research focus has been on the relationship between infection and soluble mediators of the innate immune system [13,14]. At a systemic level, C-reactive protein (CRP), a paradigmatic component of the acute phase reaction, has been extensively studied as a serum biomarker of PJI, in conjunction with characteristics of haemostasis, such as erythrocyte sedimentation rate (ESR) and plasmatic concentration of D-dimer [5]. In addition to circulating biomarkers of PJI, molecular and cellular components of the synovial fluid, including α-defensin and leukocyte esterase (LE), which act as local “signs” of pathology, have received great attention [14]. Moreover, evidence is being accumulated that additional players of the innate immune system are involved in the pathogenesis of PJIs [12], which makes them suitable candidates for the development of novel diagnostic tools.
The long pentraxin 3 (PTX3) is a member of the pentraxin superfamily of proteins that includes CRP and Serum Amyloid P component (SAP, an acute phase protein in rodents), and is characterized by the presence of an N-terminal domain in addition to a C-terminal pentraxin-like domain (distinctive of the family) [15]. Folded into a glycosylated homo-octamer with a unique quaternary structure [16,17], this protein is made and released by different cell types such as mononuclear phagocytes, dendritic cells, smooth muscle cells, adipocytes, and fibroblasts [18,19,20,21,22]. The expression of PTX3 is induced by pro-inflammatory signals, such as IL-1β, TNF-α, and Toll-like receptors (TLR) agonists [15,23,24], via both MyD88- and TRIF-dependent pathways [25]. Gene transcription and protein synthesis are also elicited through PI3K/Akt and JNK pathways, or downstream of FUS/CHOP translocation [15]. Moreover, the protein is stored in the lactoferrin+ granules of neutrophils and is rapidly mobilized and secreted upon stimulation with micro-organisms, TLR agonists, and pro-inflammatory cytokines [26]. Neutrophils are promptly recruited to sites of infection and inflammation, and the rapid release of neutrophil-stored PTX3 is taken as an early immediate mechanism of innate defence in that this protein acts as an “opsonin” that facilitates pathogen recognition, internalization, and killing by professional phagocytes (including neutrophils themselves) [27,28]. Importantly, this long pentraxin is an intrinsic component of the bone microenvironment, where it is expressed by osteoprogenitor cells and is involved both in bone homeostasis and fracture healing [29,30].
Similarly to the close relative CRP, the plasmatic levels of PTX3 rapidly increase in several pathological conditions with inflammatory and/or infectious aetiology, including acute myocardial infarction, sepsis, and SARS-CoV-2 infections, and have been associated with severity of the disease and risk of mortality [31,32,33,34,35]. In most cases, PTX3 correlates with CRP; however, while CRP is mostly synthesized by hepatocytes and is extensively used as a systemic, though generic, marker of inflammation, PTX3 is locally induced at sites of infection and inflammation by proinflammatory mediators and is predicted to be an earlier and local marker of disease [36]. The circulating levels of PTX3 also correlate with other markers of bacterial infections, such as procalcitonin (PCT) and D-dimer, as recently reported in septic patients [37,38]. However, both PCT and D-dimer had limited accuracy as PJI markers [39], and their diagnostic potential in this pathology requires further investigation. Therefore, PTX3, at the interface between infection immunity and bone biology, stands out as an ideal candidate for investigations into new biomarkers of PJI.
Based on this rationale, the primary aim of this study was to assess the diagnostic potential of synovial and plasmatic PTX3 in PJI patients undergoing THA or TKA revision. In this regard, previous studies have shown that the diagnostic accuracy of an established PJI biomarker, i.e., alpha-defensin, depends on the clinical definition of infection [40]. To account for this, in our investigations, two internationally recognized and clinically validated criteria were adopted to define the infection status of the study population, based on the indications of MSIS [4] and EBJIS [7,8].
2. Materials and Methods
2.1. Study Design and Sample Size
This study complied with the provisions of the Declaration of Helsinki and was approved by the Institutional Review Board of IRCCS Humanitas Research Hospital (registration number: 165/2017). Confidentiality of patient data was preserved, and no patient identifiers were used in the dataset. Patients were enrolled only after obtaining a signature of written informed consent. The present prospective diagnostic study was performed at our institution from October 2016 to December 2019, following the International Standards for Reporting Diagnostic Accuracy (STARD) guidelines. The minimum follow-up was 24 months.
The primary aim of this study was to assess the performance of PTX3 (i.e., the concentration of the protein in the synovial fluid and plasma) in the diagnosis of PJI in patients undergoing total THA or TKA revision. As secondary aims, the diagnostic power of PTX3 (either in the synovial fluid or plasma) was compared with that of plasmatic CRP, and correlations were assessed between the concentration of PTX3 and that of other clinically established inflammatory markers.
A total of 128 patients were enrolled in this study, of which 53/35 were infected and 75/93 were non-infected (based on EBJIS/MSIS criteria). In this regard, it is worth noting here that in a pilot investigation on 40 patients receiving THA or TKA revision at IRCCS Humanitas Research Hospital, the concentration of PTX3 in the synovial fluid was able to detect PJI with an AUC of 0.93 (95% confidence interval—CI, 0.84–1.0). Given the primary aim of the study and assuming an AUC of 0.95 and a marginal error of 0.05 with 95% CI, the required number of patients to achieve a statistical power of 80% was estimated as 50 in both infected and non-infected groups.
2.2. Study Population
Patients eligible for THA and TKA revision surgery were enrolled prospectively and consecutively. To be included in the study, patients had to meet the following inclusion criteria: painful THA or TKA for at least 3 months, and sufficient clinical and laboratory data to define the presence or absence of PJI. Exclusion criteria included: use of antibiotics, glucocorticoids, and anti-histaminic therapy in the 2 weeks prior to surgery; rheumatoid arthritis and other rheumatic disorders; revision surgery for spacer removal and reimplantation; metallosis, prosthetic dislocation, periprosthetic fracture, limb length discrepancy, prosthetic rupture, and polyethylene wear.
Revisions were classified as septic or aseptic according to the MSIS [4] and EBJIS criteria [7,8] (Table 1).

Table 1.
Definition of periprosthetic joint infections.
For the MSIS criteria, elevated CRP was defined as >10 mg/L or >100 mg/L in chronic or acute infections, respectively; elevated synovial fluid leukocyte count was defined as >3000 leukocytes/mL or >10,000 leukocytes/mL in chronic or acute infections, respectively; elevated synovial fluid percentage of granulocytes was defined as >80% or >90% in chronic or acute infections, respectively; and positive histological analysis of periprosthetic tissue was defined as >5 neutrophils per high-power field (HPF) in 5 HPFs observed on periprosthetic tissue at ×400 magnification. For the EBJIS criteria, increased synovial fluid leukocyte count was defined as a leukocyte count of >2000/mL or >70% granulocytes; positive histopathology was defined as a mean of >23 granulocytes per 10 HPFs [41]; confirmatory microbial growth in synovial fluid and periprosthetic tissue culture was considered positive if ≥1 specimen was positive for highly virulent organisms (e.g., Staphylococcus aureus) or ≥2 specimens were positive for low virulent pathogens, and sonication culture was considered positive if >50 colony-forming units (CFU)/mL of sonicated fluid were counted [42].
2.3. Description of Treatment
Preoperative assessment of patients was based on physical examination, laboratory tests, including CRP and ESR, and plain radiographs including anterior–posterior (AP) views of the pelvis and axial views of the hip for THA, AP and lateral views of the knee, axial views of patella, and full-length weight-bearing views of bilateral lower extremities for TKA. Bone scintigraphy, CT scan, or MRI were performed according to the surgeon’s indications. Preoperative joint aspiration of synovial fluid for leukocyte counting and microbiology culturing was performed in case of high levels of CRP and/or ESR, or in the presence of high clinical suspicion for PJI based on multiple surgery, history of surgical site infection in the index joint or prior PJI.
All procedures were performed using the posterolateral approach with the patient in lateral decubitus for the hip surgery, and through the standard medial parapatellar approach for the knee surgery. The antibiotic prophylaxis with cefazolin or clindamycin was administered in all patients before surgery. Blood samples were withdrawn into EDTA Vacutainer immediately before surgery, from which plasma was isolated (see the Section 2.4 below) and used to measure the plasmatic concentration of the PTX3 protein. Synovial fluid was collected during surgery before capsulotomy to prevent blood contamination. Synovial fluid samples were used for microbiological and laboratory analyses. During surgery, five to seven intra-operative periprosthetic tissue samples were obtained for microbiological and histological analysis. Presence and identity of aerobic and anaerobic bacteria and fungi was assessed through microbiological cultures (14 to 21 days) of tissue extracts in selective media. The removed prosthesis was sonicated.
The enrolled patients underwent either one-stage or two-stage revision according to presence/absence of a suspicion of PJI (based on preoperative workout). The measured concentrations of synovial and plasmatic PTX3 were not provided to the surgeon at the time of surgery; therefore, they did not affect diagnosis of PJI and the clinical post-surgery management of patients.
After surgery, all patients received an empiric antibiotic treatment with vancomycin and piperacillin-tazobactam, vancomycin and ciprofloxacin or levofloxacin, or cefazolin until microbiological results were available. The antibiotic regimen was chosen on the basis of known patient risk factors and intolerance to penicillin. The empiric antibiotic treatment was interrupted after 7 days in patients without evidence of PJI. In patients with PJI, the antibiotic treatment was aetiologic in culture-positive cases and empiric in culture-negative cases. The antibiotic treatment was performed until reimplantation in patients who underwent two-stage revision. In patients having one-stage revision, the duration of the antibiotic therapy was three months.
2.4. Laboratory Analysis
All plasma and synovial fluid samples were sent to the laboratory on the day of surgery. Venous blood samples were collected in the presence of EDTA and centrifuged within 2 h at 2000 rpm for 10 min at room temperature. Plasma and synovial fluids were stored at −80 °C until use. Synovial and plasmatic PTX3 were measured by Sandwich ELISA using a home-made colorimetric assay, as previously described [43]. In this assay, a signal was obtained from the chromogenic substrate 3–3′ 5–5′ tetramethylbenzidine (TMB, 1-step ultra TMB-ELISA; Thermo Scientific, Waltham, MA, US), and colour development was stopped by addition of sulfuric acid. Absorbance was measured at 450 nm using an automated microplate reader (Versamax, Molecular Device Corporation, San Jose, CA, US). The concentration of PTX3 was calculated based on a standard curve of the recombinant protein (linear range: 75 pg/mL to 2.4 ng/mL) using the SoftMax PRO software (version 5.3; Molecular Device Corporation, San Jose, CA, US). Plasmatic CRP levels were quantified by immunoturbidimetry on a Beckman Coulter instrument (Beckman Coulter, Milan, Italy), while D-dimer was measured according to routine protocols using the ACL-TOP 750 LAS (Werfen, Milan, Italy) coagulation analyser. ESR and synovial fluid leukocyte count were measured by standard procedures in use in the Institutional Clinical Laboratory.
2.5. Statistical Analysis
Descriptive statistics and statistical testing were performed using Prism version 9.4.1 (GraphPad, Boston, MA, USA). In an initial round of analysis, normality of values measured for the synovial and plasmatic markers in the study (including PTX3; continuous variables) was assessed by means of the Shapiro–Wilk and Kolomogorov–Smirnov tests. The distribution of these observations across groups of patients (i.e., infected vs. non-infected) was evaluated with the Mann–Whitney test. In all cases, values were represented as median ± interquartile range (IQR). Categorical variables (e.g., sex, Charlson and ASA scores) were expressed as frequencies and analysed using contingency tables by employing the Pearson chi-square test to assess differences between groups. Correlations between continuous variables (i.e., concentration of PTX3 and leukocyte count in the synovial fluid) were assessed using the Spearman’s rank correlation coefficient method.
In a second round of analysis, a logistic regression model was fitted to the data using the concentration values of synovial and plasmatic markers as explanatory variables and the infection status (defined based on either MSIS or EBJIS criteria) as the response variable. ROC (receiver operating characteristic) curves were generated to assess the overall quality of fitting. For each synovial and plasmatic marker, threshold value, sensitivity, specificity, and positive and negative likelihood ratios were calculated using the corresponding ROC curves according to the Youden index (J). The area under the ROC curve (AUC) was then calculated and reported as a synthetic index of diagnostic performance of the selected markers.
3. Results
3.1. Study Population
From October 2016 to December 2019, a total of 544 patients who were referred to our institute for THA and TKA revision were classified as potentially eligible. Of these, 190 patients could not enter the study because they did not fulfil the MSIS and EBJIS criteria, and 161 were excluded according to the exclusion criteria. Of the remaining 193 eligible patients, 65 were excluded because of lack of synovial fluid to perform the index tests (Figure 1).
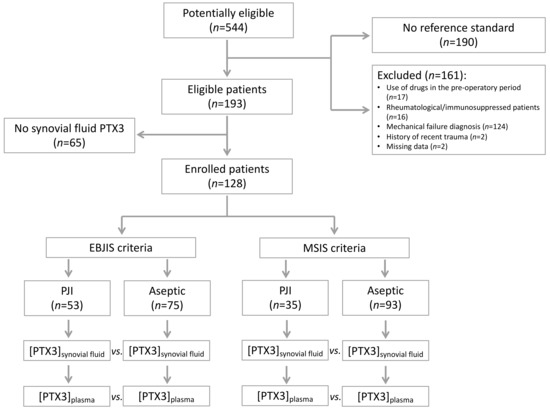
Figure 1.
Flow-chart of the patients included in the study.
The study population consisted of 128 patients, including both THA (n = 94) and TKA (n = 34) revisions. A total of 53 patients had PJI according to the EBJIS criteria. Of these, 35 were deemed infected according to the MSIS criteria too. No patient with a diagnosis of PJI according to the MSIS criteria was deemed non-infected according to the EBJS criteria. All patients were comparable in age, gender, BMI, Charlson score, and ASA scores (Table 2).

Table 2.
Demographics of the study population.
3.2. Microbiological Analysis
The most frequent pathogens isolated in the infected patients (Table 3) were the coagulase-negative Staphylococci (15 and 13 patients in the EBJIS- and MSIS-positive groups, respectively), followed by Staphylococcus Aureus (7 in both groups) and Streptococci (5 and 4). The percentage of poly-microbial infections was 11% and 9% in the EBJIS- and MSIS-positive groups, respectively.

Table 3.
Pathogens isolated at the microbiological analysis after total hip or knee arthroplasty revision.
3.3. Laboratory Analysis
Synovial PTX3 and plasmatic CRP levels were significantly higher in the infected patients compared to the non-infected ones when either EBJIS or MSIS criteria were considered for diagnosis of PJI (p < 0.0001). No differences were found for plasmatic PTX3, ESR, and D-dimer (Table 4, Figure 2, and Supplementary Figure S1).

Table 4.
Concentration of synovial and plasmatic biomarkers.
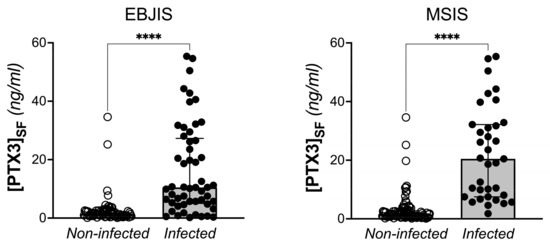
Figure 2.
Concentration of PTX3 in the synovial fluid of THA and TKA patients with and without PJI, based on EBJIS and MSIS criteria (Mann-Withney test, **** p < 0.0001).
When patients were stratified based on the explanted implant (THA or TKA), the synovial levels of PTX3 remained significantly higher in the infected individuals compared to the ones who had aseptic revision, regardless of the criteria used for diagnosis of PJI (Figure 3).
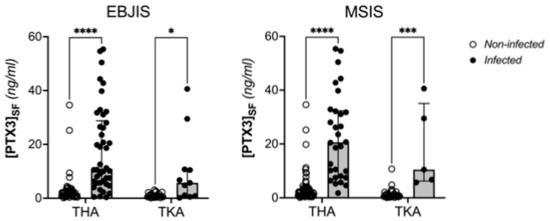
Figure 3.
Distribution of the concentration of PTX3 in the synovial fluid of aseptic and infected patients according to surgery (TKA or THA) and EBJIS/MSIS criteria (Two Way ANOVA, **** p < 0.0001, *** p < 0.0005, * p < 0.05).
To better assess the diagnostic power of PTX3, a logistic regression analysis was run on the datasets generated in this study, which allowed calculation of the AUC and other testing parameters. In this regard, the AUC of synovial PTX3 was 0.85 (95%IC, 0.78–0.93, p < 0.0001), with a sensitivity of 81.13% and a specificity 93.33% when using EBJIS criteria for diagnosis of PJI. When the MSIS criteria were considered, the diagnostic power of synovial PTX3 was even higher, with an AUC of 0.95 (95%IC, 0.91–0.98, p < 0.001), a sensitivity of 91.43%, and a specificity of 89.25% (Table 5). As a comparison, the AUC of plasmatic CRP, a clinically established biomarker of PJI, was 0.80 (95%IC, 0.72–0.88, p < 0.0001), with a sensitivity of 62.26% and a specificity of 80% (according with EBJIS), or 0.81 (95%IC, 0.72–0.89, p < 0.0001), with a sensitivity of 71.43% and a specificity of 75.27% (according with MSIS) (Table 5 and Figure S2). Furthermore, synovial PTX3 retained elevated diagnostic performance regardless of sites of revision surgery (either TKA or THA; Figures S3 and S4, and Table S1).

Table 5.
ROC curve analysis of synovial and plasmatic biomarkers.
Plasmatic PTX3, ESR, and D-dimer proved poor diagnostic markers and failed to discriminate infected from non-infected patients (regardless of the criteria used for diagnosis of PJI) (Table 5).
ROC curves of synovial and plasmatic PTX3 are shown in Figure 4 and Figure S5. For synovial PTX3, the best concentration values to discriminate between infected and aseptic patients was 4.1 ng/mL and 5.7 ng/mL for EBJIS and MSIS criteria, respectively.
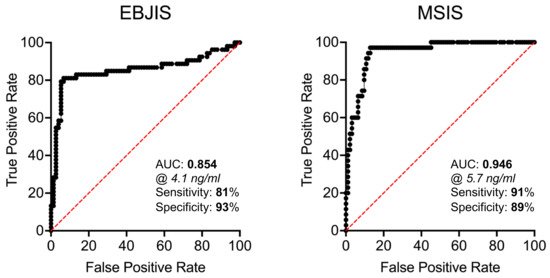
Figure 4.
ROC curves of the concentration of PTX3 in the synovial fluid of THA and TKA patients with and without PJI (based on EBJIS and MSIS criteria).
The leukocyte count in the synovial fluid was significantly higher in the infected revisions compared with aseptic revisions (p < 0.0001), according with the MSIS criteria. Moreover, the concentration of PTX3 was significantly correlated with leukocyte count in the synovial fluid.
3.4. Clinical Outcome
Among the 53 patients with a diagnosis of PJI according to the EBJIS criteria, 29 underwent two-stage revision, 12 total one-stage revision, and 12 partial one-stage revision. The treatment failed in five patients: three individuals (two two-stage and one one-stage revision) underwent long-term antibiotic suppression treatment, one patient underwent surgical debridement (DAIR) for peri-operative infection after the two-stage revision, and one patient underwent further revision for septic acetabular loosening after the one-stage partial revision. Among the 75 aseptic patients, 3 underwent two-stage revision, 46 total one-stage revision, and 26 partial one-stage revision. No patients reported the failure of treatment.
4. Discussion
Periprosthetic joint infections (PJIs) represent one of the most common causes of joint replacement failure [1,2]. Although several classification systems have been developed to formulate a proper diagnosis, the diagnosis of PJIs is still challenging, particularly in septic cases associated with low-virulence and biofilm-forming pathogens. Accurate preoperative diagnosis is needed to choose proper antibiotic therapies and surgical strategies. Indeed, undetected and/or mistreated PJIs at the time of revision surgery can result in persistence of the infection, failure of the revised implant, longer hospital stays, multiple surgeries, prolonged immobilization and rehabilitation, together with increased overall costs. To support and orient diagnosis of PJI prior to surgery it is therefore timely to identify and validate novel biomarkers.
The long pentraxin PTX3 is an established humoral component of the innate immune system and an emerging player in bone biology [29,30]. This protein is synthesized and released at sites of infection/inflammation by a number of immune and non-immune cells, including macrophages, dendritic cells, neutrophils, and cells of the osteoblastogenic lineage, after stimulation with primary proinflammatory cytokines (e.g., TNF-α and IL-1β) and microbial components (e.g., LPS) [22,23,24,26]. The locally made protein binds a broad spectrum of microorganisms, including selected fungi, viruses, and bacteria, and exerts a number of host-protective functions [27,35]. Previous studies demonstrated that high levels of plasmatic PTX3 are associated with risk and severity of several inflammatory and infective diseases [31,32,33,34,35]. Therefore, the present study was designed to assess the potential of synovial and plasmatic PTX3 as diagnostic biomarker of PJIs in patients undergoing THA or TKA revision. The main finding of our investigation was that the concentration of PTX3 in the synovial fluid was elevated in THA/TKA patients with PJI (compared to those who had aseptic prosthesis revision) and able to predict the infection with high accuracy (i.e., AUC values of 0.85 and 0.95 for EBJIS and MSIS criteria, respectively). Importantly, the specificity of synovial PTX3 was consistently high across the applied diagnostic classifications (93% and 89% for EBJIS and MSIS, respectively), which suggests that measuring the levels of this long pentraxin in the synovial fluid might provide clinically useful information to confirm diagnosis of PJI rather than support screening investigations. As opposed to the synovial protein, the plasmatic concentration of PTX3 had poor diagnostic value, likely due to this long pentraxin being mainly synthesized in loco at sites of infection, unlike the short pentraxin CRP that is systemically produced by the liver in response to IL-6. In a previous study, Mauri et al. [44] demonstrated that a PTX3 level ≥ 1 ng/mL in the bronchoalveolar lavage fluid was discriminative of microbiologically confirmed pneumonia in mechanically ventilated patients. On the other hand, the plasmatic PTX3 was not effective for the diagnosis. Consistent with this view, a correlation was found in our study between leukocyte count and concentration of PTX3 in the synovial fluid, which suggests that the latter is likely contributed by white blood cells (mostly neutrophils) infiltrating the inflamed synovium during the infection, where these cells are known to be a source of PTX3 in inflammatory conditions (Figure 5).
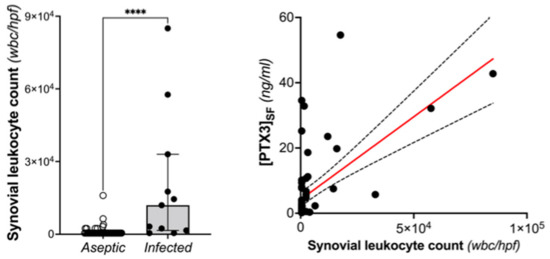
Figure 5.
Leukocyte count in the synovial fluid of THA and TKA patients with and without PJI (based on MSIS criteria; Mann-Withney, **** p < 0.0001) and its correlation with the concentration of PTX3 (Spearman r: 0.498, p < 0.0001).
In a previous meta-analysis, Wyatt et al. [14] reported a pooled diagnostic sensitivity and specificity of alpha-defensin for PJI of 1.00 (95% CI, 0.82 to 1.00) and 0.96 (95% CI, 0.89 to 0.99), respectively. They also observed a pooled diagnostic sensitivity and specificity of leukocyte esterase for PJI of 0.81 (95% CI, 0.49 to 0.95) and 0.97 (95% CI, 0.82 to 0.99), respectively. In a more recent meta-analysis, Kuiper et al. [45] compared the diagnostic potential of laboratory-based (ELISA) and lateral-flow (LF) alpha-defensin in a pooled cohort that included THA and TKA patients. The authors did not observe significant differences between ELISA and LF alpha-defensin in terms of sensitivity (90% versus 86%) and specificity (97% versus 96%). In both studies, the original or modified MSIS criteria were used as the reference standard for the diagnosis of PJI. The diagnostic potential of synovial PTX3, as assessed in the present study, is therefore in line with that of other synovial inflammatory markers; however, a thorough comparison would require further studies in homogenous cohorts of patients.
No serum markers have been shown to be accurate enough to diagnose PJIs in THA/TKA patients (i.e., AUC values of serum markers have been consistently documented to be below 70% [39]). In this regard, the positive likelihood ratio of serum markers has been reported to be below six points, indicating a <35% increase in probability of finding an infection [40]. Therefore, only the synovial fluid could provide useful and reliable information for diagnosis of PJIs [12]. Compared to the previous literature [9,12,13,14,45], the present study further corroborates the notion that serum markers have a limited role in the detection of PJIs and cannot be used on their own to assess septic patients. On the other hand, to support diagnosis of PJI, the synovial fluid needs to be gathered when the infection is suspected.
Due to the large variety of pathogens found in the specimens analysed in this study, we could not make any statistically sound association between the levels of PTX3 and the identity of the pathogens. However, it is licit to speculate that highly virulent pathogens can strongly stimulate the immune system and therefore induce high levels of PTX3. In this respect, both in preclinical models and in patients, PTX3 plasma levels rapidly increase in response to different infectious agents, including bacteria, viruses, and fungi, and correlate with disease severity and unfavourable outcomes [35].
A limitation of the present study is that the reference standards used for diagnosis of PJI (MSIS and EBJIS criteria) did not include synovial alpha-defensin. In fact, our study was authorized and commenced prior to publication of the latest (alpha-defensin including) version of the EBJIS and ICM criteria. Consequently, we could not make any direct comparison between synovial alpha-defensin and PTX3 as for their diagnostic potential in PJI. Another limitation is that patients with clear or suspected auto-inflammatory conditions were excluded based on the reasoning that sterile activation of the immune system could have potentially resulted in elevation of the PTX3 levels in the synovial fluid and/or blood regardless of the presence of PJI. On the same line, patients undergoing prosthetic revision due to metallosis or severe wear of the polyethylene were not eligible. Further studies should be performed to investigate the diagnostic potential of PTX3 in these subgroups of patients.
5. Conclusions
Synovial PTX3 demonstrated an excellent diagnostic potential in hip and knee PJIs, with a very high specificity regardless of the criteria used for diagnosis of PJI. This points to PTX3 being a useful marker to confirm a suspected infection. Further studies are needed to develop and investigate the diagnostic performance for PJI of a new classification system that includes synovial PTX3.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12031055/s1, Figure S1: Concentration of PTX3 in the plasma of THA and TKA patients with and without PJI (based on EBJIS and MSIS criteria); Figure S2: Distribution and ROC curve of the concentration of CRP in the plasma of THA and TKA patients with and without PJI (according to MSIS criteria); Figure S3: ROC curves of the concentration of PTX3 in the plasma of THA and TKA patients with and without PJI (based on EBJIS and MSIS criteria); Figure S4: ROC curves of the concentration of PTX3 in the synovial fluid of patients with and without PJI according to surgery (THA or TKA) and EBJIS criteria; Figure S5: ROC curves of the concentration of PTX3 in the plasma of THA and TKA patients with and without PJI (based on EBJIS and MSIS criteria); Table S1: ROC curve analysis of synovial PTX3 in THA and TKA patients.
Author Contributions
Conceptualization, M.L., G.G., B.B. and A.M.; formal analysis, M.L. and A.I.; investigation, M.D.M., R.A. and R.L.; resources, R.A. and R.L.; data curation, M.L. and M.D.M.; writing—original draft preparation, M.L., M.D.M., B.B. and A.I.; writing—review and editing, M.L, B.B. and A.I.; supervision, M.L. and B.B.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. We gratefully acknowledge the contribution of the European Research Council (ERC n° 669415) to A.M. and Fondazione Beppe & Nuccy Angiolini to A.I. and A.M.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of IRCCS Humanitas Research Hospital (registration number: 165/17; date of approval: 11 April 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data supporting reported results can be found in a repository (Zenodo).
Acknowledgments
The Livio Sciutto Foundation (www.fondazione.it) for Medical Research is gratefully acknowledged. This is a non-profit social organization that recorded in its database the demographic and surgical data of the patients included in the study, with the previous consent of the patients and respecting the current law on the privacy. Finally, all the patients who participated in the study, together with physicians, nurses, and laboratory personnel of the Humanitas Clinical Laboratory, are gratefully acknowledged.
Conflicts of Interest
A.M., B.B. and A.I. are inventors of patents on PTX3. Additionally, A.M. and B.B. obtain royalties on PTX3-related reagents. M.L., G.G., M.D.M., R.A. and R.L. declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Robertsson, O.; Lidgren, L.; Sundberg, M.; W-Dahl, A. The Swedish Knee Arthroplasty Register—Annual Report 2019; The Swedish Knee Arthroplasty Register: Lund, Sweden, 2019; ISBN 978-91-88017-29-1. [Google Scholar]
- Kärrholm, J.; Rogmark, C.; Nauclér, E.; Nåtman, J.; Vinblad, J.; Mohaddes, M.; Rolfson, O. Swedish Hip Arthroplasty Register—Annual Report 2019; Sahlgrenska University Hospital: Göteborg, Swenden, 2019; ISBN 978-91-986612-0-0. ISSN 1654-5982. [Google Scholar]
- The Workgroup of the Musculoskeletal Infection Society. New Definition for Periprosthetic Joint Infection. J. Arthroplast. 2011, 26, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Gehrke, T.; International Consensus Group on Periprosthetic Joint Infection. Definition of Periprosthetic Joint Infection. J. Arthroplast. 2014, 29, 1331. [Google Scholar] [CrossRef]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R.; Infectious Diseases Society of America. Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [PubMed]
- Corvec, S.; Portillo, M.E.; Pasticci, B.M.; Borens, O.; Trampuz, A. Epidemiology and New Developments in the Diagnosis of Prosthetic Joint Infection. Int. J. Artif. Organs 2012, 35, 923–934. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis. 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Renz, N.; Yermak, K.; Perka, C.; Trampuz, A. Alpha Defensin Lateral Flow Test for Diagnosis of Periprosthetic Joint Infection: Not a Screening but a Confirmatory Test. J. Bone Joint Surg. Am. 2018, 100, 742–750. [Google Scholar] [CrossRef]
- Bellova, P.; Knop-Hammad, V.; Königshausen, M.; Mempel, E.; Frieler, S.; Gessmann, J.; Schildhauer, T.A.; Baecker, H. Sonication of Retrieved Implants Improves Sensitivity in the Diagnosis of Periprosthetic Joint Infection. BMC Musculoskelet. Disord. 2019, 20, 623. [Google Scholar] [CrossRef] [PubMed]
- McNally, M.; Sousa, R.; Wouthuyzen-Bakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebše, R. The EBJIS Definition of Periprosthetic Joint Infection. Bone Joint J. 2021, 103-B, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Shahi, A.; Parvizi, J. The Role of Biomarkers in the Diagnosis of Periprosthetic Joint Infection. EFORT Open Rev. 2016, 1, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Okroj, K.T.; Calkins, T.E.; Kayupov, E.; Kheir, M.M.; Bingham, J.S.; Beauchamp, C.P.; Parvizi, J.; Della Valle, C.J. The Alpha-Defensin Test for Diagnosing Periprosthetic Joint Infection in the Setting of an Adverse Local Tissue Reaction Secondary to a Failed Metal-on-Metal Bearing or Corrosion at the Head-Neck Junction. J. Arthroplast. 2018, 33, 1896–1898. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.C.; Beswick, A.D.; Kunutsor, S.K.; Wilson, M.J.; Whitehouse, M.R.; Blom, A.W. The Alpha-Defensin Immunoassay and Leukocyte Esterase Colorimetric Strip Test for the Diagnosis of Periprosthetic Infection: A Systematic Review and Meta-Analysis. J. Bone Jt. Surg. Am. 2016, 98, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Inforzato, A.; Baldock, C.; Jowitt, T.A.; Holmes, D.F.; Lindstedt, R.; Marcellini, M.; Rivieccio, V.; Briggs, D.C.; Kadler, K.E.; Verdoliva, A.; et al. The Angiogenic Inhibitor Long Pentraxin PTX3 Forms an Asymmetric Octamer with Two Binding Sites for FGF2. J. Biol. Chem. 2010, 285, 17681–17692. [Google Scholar] [CrossRef]
- Noone, D.P.; Dijkstra, D.J.; van der Klugt, T.T.; van Veelen, P.A.; de Ru, A.H.; Hensbergen, P.J.; Trouw, L.A.; Sharp, T.H. PTX3 Structure Determination Using a Hybrid Cryoelectron Microscopy and AlphaFold Approach Offers Insights into Ligand Binding and Complement Activation. Proc. Natl. Acad. Sci. USA 2022, 119, e2208144119. [Google Scholar] [CrossRef]
- Abderrahim-Ferkoune, A.; Bezy, O.; Chiellini, C.; Maffei, M.; Grimaldi, P.; Bonino, F.; Moustaid-Moussa, N.; Pasqualini, F.; Mantovani, A.; Ailhaud, G.; et al. Characterization of the Long Pentraxin PTX3 as a TNFalpha-Induced Secreted Protein of Adipose Cells. J. Lipid Res. 2003, 44, 994–1000. [Google Scholar] [CrossRef]
- Alles, V.V.; Bottazzi, B.; Peri, G.; Golay, J.; Introna, M.; Mantovani, A. Inducible Expression of PTX3, a New Member of the Pentraxin Family, in Human Mononuclear Phagocytes. Blood 1994, 84, 3483–3493. [Google Scholar] [CrossRef]
- Doni, A.; Peri, G.; Chieppa, M.; Allavena, P.; Pasqualini, F.; Vago, L.; Romani, L.; Garlanda, C.; Mantovani, A. Production of the Soluble Pattern Recognition Receptor PTX3 by Myeloid, but Not Plasmacytoid, Dendritic Cells. Eur. J. Immunol. 2003, 33, 2886–2893. [Google Scholar] [CrossRef]
- Goodman, A.R.; Levy, D.E.; Reis, L.F.; Vilcek, J. Differential Regulation of TSG-14 Expression in Murine Fibroblasts and Peritoneal Macrophages. J. Leukoc. Biol. 2000, 67, 387–395. [Google Scholar] [CrossRef]
- Klouche, M.; Peri, G.; Knabbe, C.; Eckstein, H.-H.; Schmid, F.-X.; Schmitz, G.; Mantovani, A. Modified Atherogenic Lipoproteins Induce Expression of Pentraxin-3 by Human Vascular Smooth Muscle Cells. Atherosclerosis 2004, 175, 221–228. [Google Scholar] [CrossRef]
- Breviario, F.; d’Aniello, E.M.; Golay, J.; Peri, G.; Bottazzi, B.; Bairoch, A.; Saccone, S.; Marzella, R.; Predazzi, V.; Rocchi, M. Interleukin-1-Inducible Genes in Endothelial Cells. Cloning of a New Gene Related to C-Reactive Protein and Serum Amyloid P Component. J. Biol. Chem. 1992, 267, 22190–22197. [Google Scholar] [CrossRef]
- Vouret-Craviari, V.; Matteucci, C.; Peri, G.; Poli, G.; Introna, M.; Mantovani, A. Expression of a Long Pentraxin, PTX3, by Monocytes Exposed to the Mycobacterial Cell Wall Component Lipoarabinomannan. Infect. Immun. 1997, 65, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Doni, A.; Musso, T.; Morone, D.; Bastone, A.; Zambelli, V.; Sironi, M.; Castagnoli, C.; Cambieri, I.; Stravalaci, M.; Pasqualini, F.; et al. An Acidic Microenvironment Sets the Humoral Pattern Recognition Molecule PTX3 in a Tissue Repair Mode. J. Exp. Med. 2015, 212, 905–925. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Peri, G.; Delneste, Y.; Frémaux, I.; Doni, A.; Moalli, F.; Garlanda, C.; Romani, L.; Gascan, H.; Bellocchio, S.; et al. The Humoral Pattern Recognition Receptor PTX3 Is Stored in Neutrophil Granules and Localizes in Extracellular Traps. J. Exp. Med. 2007, 204, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Diniz, S.N.; Nomizo, R.; Cisalpino, P.S.; Teixeira, M.M.; Brown, G.D.; Mantovani, A.; Gordon, S.; Reis, L.F.L.; Dias, A.A.M. PTX3 Function as an Opsonin for the Dectin-1-Dependent Internalization of Zymosan by Macrophages. J. Leukoc. Biol. 2004, 75, 649–656. [Google Scholar] [CrossRef]
- Parente, R.; Doni, A.; Bottazzi, B.; Garlanda, C.; Inforzato, A. The Complement System in Aspergillus Fumigatus Infections and Its Crosstalk with Pentraxins. FEBS Lett. 2020, 594, 2480–2501. [Google Scholar] [CrossRef]
- Grčević, D.; Sironi, M.; Valentino, S.; Deban, L.; Cvija, H.; Inforzato, A.; Kovačić, N.; Katavić, V.; Kelava, T.; Kalajzić, I.; et al. The Long Pentraxin 3 Plays a Role in Bone Turnover and Repair. Front. Immunol. 2018, 9, 417. [Google Scholar] [CrossRef]
- Parente, R.; Sobacchi, C.; Bottazzi, B.; Mantovani, A.; Grčevic, D.; Inforzato, A. The Long Pentraxin PTX3 in Bone Homeostasis and Pathology. Front. Immunol. 2019, 10, 2628. [Google Scholar] [CrossRef]
- Brunetta, E.; Folci, M.; Bottazzi, B.; De Santis, M.; Gritti, G.; Protti, A.; Mapelli, S.N.; Bonovas, S.; Piovani, D.; Leone, R.; et al. Macrophage Expression and Prognostic Significance of the Long Pentraxin PTX3 in COVID-19. Nat. Immunol. 2021, 22, 19–24. [Google Scholar] [CrossRef]
- Caironi, P.; Masson, S.; Mauri, T.; Bottazzi, B.; Leone, R.; Magnoli, M.; Barlera, S.; Mamprin, F.; Fedele, A.; Mantovani, A.; et al. Pentraxin 3 in Patients with Severe Sepsis or Shock: The ALBIOS Trial. Eur. J. Clin. Investig. 2017, 47, 73–83. [Google Scholar] [CrossRef]
- Jenny, N.S.; Arnold, A.M.; Kuller, L.H.; Tracy, R.P.; Psaty, B.M. Associations of Pentraxin 3 with Cardiovascular Disease and All-Cause Death: The Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Latini, R.; Maggioni, A.P.; Peri, G.; Gonzini, L.; Lucci, D.; Mocarelli, P.; Vago, L.; Pasqualini, F.; Signorini, S.; Soldateschi, D.; et al. Prognostic Significance of the Long Pentraxin PTX3 in Acute Myocardial Infarction. Circulation 2004, 110, 2349–2354. [Google Scholar] [CrossRef] [PubMed]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Doni, A.; Stravalaci, M.; Inforzato, A.; Magrini, E.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Link Between Innate Immunity, Tissue Remodeling, and Cancer. Front. Immunol. 2019, 10, 712. [Google Scholar] [CrossRef]
- Chen, H.; Li, T.; Yan, S.; Liu, M.; Liu, K.; Zhang, H.; Gao, M.; Xiao, X. Pentraxin-3 Is a Strong Biomarker of Sepsis Severity Identification and Predictor of 90-Day Mortality in Intensive Care Units via Sepsis 3.0 Definitions. Diagnostics 2021, 11, 1906. [Google Scholar] [CrossRef]
- Davoudian, S.; Piovani, D.; Desai, A.; Mapelli, S.N.; Leone, R.; Sironi, M.; Valentino, S.; Silva-Gomes, R.; Stravalaci, M.; Asgari, F.; et al. A Cytokine/PTX3 Prognostic Index as a Predictor of Mortality in Sepsis. Front. Immunol. 2022, 13, 979232. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Puchner, S.E.; Windhager, R. Serum Inflammatory Biomarkers in the Diagnosis of Periprosthetic Joint Infections. Biomedicines 2021, 9, 1128. [Google Scholar] [CrossRef]
- Huard, M.; Detrembleur, C.; Poilvache, H.; Pastor, Y.; Geels, I.; Van Cauter, M.; Driesen, R.; Yombi, J.-C.; Neyt, J.; Cornu, O. Alpha Defensin: A Diagnostic Accuracy Depending on the Infection Definition Used. J. Arthroplast. 2020, 35, 1355–1360. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Perino, G.; Kienapfel, H.; Ascherl, R.; Hassenpflug, G.J.; Thomsen, M.; Thomas, P.; Huber, M.; Kendoff, D.; et al. Revised Histopathological Consensus Classification of Joint Implant Related Pathology. Pathol. Res. Pract. 2014, 210, 779–786. [Google Scholar] [CrossRef]
- Portillo, M.E.; Salvadó, M.; Trampuz, A.; Plasencia, V.; Rodriguez-Villasante, M.; Sorli, L.; Puig, L.; Horcajada, J.P. Sonication versus Vortexing of Implants for Diagnosis of Prosthetic Joint Infection. J. Clin. Microbiol. 2013, 51, 591–594. [Google Scholar] [CrossRef]
- Knoflach, M.; Kiechl, S.; Mantovani, A.; Cuccovillo, I.; Bottazzi, B.; Xu, Q.; Xiao, Q.; Gasperi, A.; Mayr, A.; Kehrer, M.; et al. Pentraxin-3 as a Marker of Advanced Atherosclerosis Results from the Bruneck, ARMY and ARFY Studies. PLoS ONE 2012, 7, e31474. [Google Scholar] [CrossRef] [PubMed]
- Mauri, T.; Coppadoro, A.; Bombino, M.; Bellani, G.; Zambelli, V.; Fornari, C.; Berra, L.; Bittner, E.A.; Schmidt, U.; Sironi, M.; et al. Alveolar Pentraxin 3 as an Early Marker of Microbiologically Confirmed Pneumonia: A Threshold-Finding Prospective Observational Study. Crit. Care 2014, 18, 562. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.W.P.; Verberne, S.J.; Vos, S.J.; van Egmond, P.W. Does the Alpha Defensin ELISA Test Perform Better Than the Alpha Defensin Lateral Flow Test for PJI Diagnosis? A Systematic Review and Meta-Analysis of Prospective Studies. Clin. Orthop. Relat. Res. 2020, 478, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).