The Clinical Implications of Serum Carbohydrate Antigen 19-9 Levels in Patients with Nontuberculous Mycobacteria Pulmonary Disease
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Data Collection
2.3. Measurement of CA 19-9 Levels
2.4. NTM Identification and Microbiological Response
2.5. Statistical Analysis
3. Results
3.1. Comparison of the Characteristics of Patients with and without an Elevated CA19-9
3.2. Microbiological Response in Patients Who Received Antibiotic Therapy
3.3. Factors Associated with Microbiological Cure including CA 19-9 Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoefsloot, W.; van Ingen, J.; Andrejak, C.; Angeby, K.; Bauriaud, R.; Bemer, P.; Beylis, N.; Boeree, M.J.; Cacho, J.; Chihota, V.; et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: An NTM-NET collaborative study. Eur. Respir. J. 2013, 42, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Falkinham, J.O., 3rd. Ecology of nontuberculous mycobacteria—Where do human infections come from? Semin. Respir. Crit. Care Med. 2013, 34, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Kartalija, M.; Ovrutsky, A.R.; Bryan, C.L.; Pott, G.B.; Fantuzzi, G.; Thomas, J.; Strand, M.J.; Bai, X.; Ramamoorthy, P.; Rothman, M.S.; et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am. J. Respir. Crit. Care Med. 2013, 187, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lee, E.J.; Kim, S.K.; Chang, J.; Jeong, S.H.; Kang, Y.A. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand. J. Infect. Dis. 2012, 44, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Hamed, K.A.; Tillotson, G. A narrative review of nontuberculous mycobacterial pulmonary disease: Microbiology, epidemiology, diagnosis, and management challenges. Expert. Rev. Respir. Med. 2023, 1–16. [Google Scholar] [CrossRef]
- Hwang, J.A.; Kim, S.; Jo, K.W.; Shim, T.S. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur. Respir. J. 2017, 49, 1600537. [Google Scholar] [CrossRef]
- Kwon, B.S.; Lee, J.H.; Koh, Y.; Kim, W.S.; Song, J.W.; Oh, Y.M.; Lee, S.D.; Lee, S.W.; Lee, J.S.; Lim, C.M.; et al. The natural history of non-cavitary nodular bronchiectatic Mycobacterium avium complex lung disease. Respir. Med. 2019, 150, 45–50. [Google Scholar] [CrossRef]
- Shafran, S.D.; Singer, J.; Zarowny, D.P.; Phillips, P.; Salit, I.; Walmsley, S.L.; Fong, I.W.; Gill, M.J.; Rachlis, A.R.; Lalonde, R.G.; et al. A comparison of two regimens for the treatment of Mycobacterium avium complex bacteremia in AIDS: Rifabutin, ethambutol, and clarithromycin versus rifampin, ethambutol, clofazimine, and ciprofloxacin. Canadian HIV Trials Network Protocol 010 Study Group. N. Engl. J. Med. 1996, 335, 377–383. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown, B.A.; Murphy, D.T.; Girard, W.M.; Couch, L.; Wallace, R.J., Jr. Initial (6-month) results of three-times-weekly azithromycin in treatment regimens for Mycobacterium avium complex lung disease in human immunodeficiency virus-negative patients. J. Infect. Dis. 1998, 178, 121–126. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown, B.A.; Girard, W.M.; Griffith, B.E.; Couch, L.A.; Wallace, R.J., Jr. Azithromycin-containing regimens for treatment of Mycobacterium avium complex lung disease. Clin. Infect. Dis. 2001, 32, 1547–1553. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown-Elliott, B.A.; Langsjoen, B.; Zhang, Y.; Pan, X.; Girard, W.; Nelson, K.; Caccitolo, J.; Alvarez, J.; Shepherd, S.; et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2006, 174, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J., Jr.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72, ii1–ii64. [Google Scholar] [CrossRef]
- Fukushima, K.; Kitada, S.; Abe, Y.; Yamamoto, Y.; Matsuki, T.; Kagawa, H.; Oshitani, Y.; Tsujino, K.; Yoshimura, K.; Miki, M.; et al. Long-term treatment outcome of progressive Mycobacterium avium complex pulmonary disease. J. Clin. Med. 2020, 9, 1315. [Google Scholar] [CrossRef]
- Pasipanodya, J.G.; Ogbonna, D.; Ferro, B.E.; Magombedze, G.; Srivastava, S.; Deshpande, D.; Gumbo, T. Systematic review and meta-analyses of the effect of chemotherapy on pulmonary Mycobacterium abscessus outcomes and disease recurrence. Antimicrob. Agents Chemother. 2017, 61, e01206. [Google Scholar] [CrossRef]
- Pasipanodya, J.G.; Ogbonna, D.; Deshpande, D.; Srivastava, S.; Gumbo, T. Meta-analyses and the evidence base for microbial outcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J. Antimicrob. Chemother. 2017, 72, i3–i19. [Google Scholar] [CrossRef]
- Park, J.; Cho, J.; Lee, C.H.; Han, S.K.; Yim, J.J. Progression and treatment outcomes of lung disease caused by Mycobacterium abscessus and Mycobacterium massiliense. Clin. Infect. Dis. 2017, 64, 301–308. [Google Scholar] [CrossRef]
- Tempero, M.A.; Uchida, E.; Takasaki, H.; Burnett, D.A.; Steplewski, Z.; Pour, P.M. Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res. 1987, 47, 5501–5503. [Google Scholar]
- Mukae, H.; Hirota, M.; Kohno, S.; Komori, K.; Fukushima, K.; Hiratani, K.; Kadota, J.; Hara, K. Elevation of tumor-associated carbohydrate antigens in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 1993, 148, 744–751. [Google Scholar] [CrossRef]
- Chang, B.; Han, S.G.; Kim, W.; Ko, Y.; Song, J.; Hong, G.; Eom, J.S.; Lee, J.H.; Jhun, B.W.; Koh, W.J. Normalization of elevated CA 19-9 level after treatment in a patient with the nodular bronchiectatic form of Mycobacterium abscessus lung disease. Tuberc. Respir. Dis. 2013, 75, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yong, S.H.; Lee, S.H.; Lee, S.H.; Leem, A.Y.; Kim, S.Y.; Chung, K.; Kim, E.Y.; Jung, J.Y.; Park, M.S.; et al. Correlation between serum carbohydrate antigen 19-9 levels and computed tomography severity score in patients with nontuberculous mycobacterial pulmonary disease. Sci. Rep. 2021, 11, 2777. [Google Scholar] [CrossRef]
- Hong, J.Y.; Jang, S.H.; Kim, S.Y.; Chung, K.S.; Song, J.H.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; Kang, Y.A. Elevated serum CA 19-9 levels in patients with pulmonary nontuberculous mycobacterial disease. Braz. J. Infect. Dis. 2016, 20, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Jhun, B.W.; Kim, S.Y.; Moon, S.M.; Jeon, K.; Kwon, O.J.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Development of macrolide resistance and reinfection in refractory Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Jhun, B.W.; Moon, S.M.; Jeon, K.; Kwon, O.J.; Yoo, H.; Carriere, K.C.; Huh, H.J.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Prognostic factors associated with long-term mortality in 1445 patients with nontuberculous mycobacterial pulmonary disease: A 15-year follow-up study. Eur Respir J 2020, 55, 1900798. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwak, N.; Hong, H.; Kang, N.; Im, Y.; Jhun, B.W.; Yim, J.J. BACES score for predicting mortality in nontuberculous mycobacterial pulmonary disease. Am. J. Respir. Crit. Care Med. 2021, 203, 230–236. [Google Scholar] [CrossRef]
- Kim, B.G.; Jhun, B.W.; Kim, H.; Kwon, O.J. Treatment outcomes of Mycobacterium avium complex pulmonary disease according to disease severity. Sci. Rep. 2022, 12, 1970. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, M.J.; Kwon, B.S.; Kim, Y.W.; Lim, S.Y.; Lee, Y.J.; Park, J.S.; Cho, Y.J.; Lee, C.T.; Lee, J.H. Usefulness of the BACES score in nontuberculous mycobacterial pulmonary disease for various clinical outcomes. Sci. Rep. 2023, 13, 7495. [Google Scholar] [CrossRef]
- van Ingen, J.; Aksamit, T.; Andrejak, C.; Böttger, E.C.; Cambau, E.; Daley, C.L.; Griffith, D.E.; Guglielmetti, L.; Holland, S.M.; Huitt, G.A.; et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: An NTM-NET consensus statement. Eur. Respir. J. 2018, 51, 1800170. [Google Scholar] [CrossRef]
- Lee, B.Y.; Kim, S.; Hong, Y.; Lee, S.D.; Kim, W.S.; Kim, D.S.; Shim, T.S.; Jo, K.W. Risk factors for recurrence after successful treatment of Mycobacterium avium complex lung disease. Antimicrob. Agents Chemother. 2015, 59, 2972–2977. [Google Scholar] [CrossRef]
- Yan, M.; Fraser, B.; McArthur, E.; Mehrabi, M.; Brode, S.K.; Marras, T.K. External validation of the BACES Score in Canadian patients with nontuberculous mycobacterial pulmonary disease. Chest 2023, in press. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total (n = 1112) | Elevated CA 19-9 (n = 322) | Normal CA 19-9 (n = 790) | p-Value |
|---|---|---|---|---|
| Sex, female | 677 (61) | 228 (71) | 449 (57) | <0.001 |
| Age, year | 60 (52–68) | 60 (53–70) | 60 (52–67) | 0.190 |
| Body mass index, kg/m2 | 20.7 (19.2–22.5) | 20.3 (18.8–21.9) | 20.9 (19.3–22.9) | 0.001 |
| Smoking status | 0.001 | |||
| Never | 799 (72) | 256 (80) | 543 (69) | |
| Ex | 283 (25) | 62 (19) | 221 (28) | |
| Current | 30 (3) | 4 (1) | 26 (3) | |
| Comorbidity | ||||
| Bronchiectasis | 922 (83) | 299 (93) | 623 (79) | <0.001 |
| Previous pulmonary tuberculosis | 414 (37) | 121 (38) | 293 (37) | 0.878 |
| Other malignancy | 172 (16) | 39 (12) | 133 (17) | 0.048 |
| Obstructive lung disease | 82 (7) | 18 (6) | 64 (8) | 0.146 |
| Chronic pulmonary aspergillosis | 24 (2) | 5 (2) | 19 (2) | 0.375 |
| Idiopathic pulmonary fibrosis | 12 (1) | 5 (2) | 7 (1) | 0.344 |
| Lung cancer | 12 (1) | 3 (1) | 9 (1) | 1.000 |
| Positive sputum AFB smear | 482 (43) | 143 (44) | 339 (43) | 0.647 |
| Radiological form | <0.001 | |||
| Nodular bronchiectatic form | 902 (81) | 297 (92) | 605 (77) | |
| Fibrocavitary form | 154 (14) | 20 (6) | 134 (17) | |
| Non-classifiable form | 56 (5) | 5 (2) | 51 (7) | |
| Cavity | 259 (23) | 64 (20) | 195 (25) | 0.085 |
| Etiology | ||||
| M. avium complex | 797 (72) | 209 (65) | 588 (74) | 0.001 |
| M. massiliense | 109 (10) | 37 (12) | 72 (9) | 0.227 |
| M. abscessus | 103 (9) | 45 (14) | 58 (7) | 0.001 |
| Mixed | 59 (5) | 21 (7) | 38 (5) | 0.248 |
| Others | 44 (4) | 10 (3) | 34 (4) | 0.353 |
| Laboratory test | ||||
| ESR, mm/h (n = 811) | 27 (15–47) | 31 (18–54) | 26 (14–45) | <0.001 |
| CRP, mg/dL (n = 906) | 0.13 (0.05–0.68) | 0.16 (0.06–0.68) | 0.12 (0.05–0.70) | 0.029 |
| Initiation of antibiotic treatment | 688 (62) | 239 (74) | 449 (57) | <0.001 |
| BACES severity score (n = 811) ¶ | 0.842 * | |||
| Mild | 360 (44) | 116 (46) | 244 (44) | |
| Moderate | 372 (46) | 115 (45) | 257 (46) | |
| Severe | 79 (10) | 23 (9) | 56 (10) |
| Variables | Total (n = 688) | Elevated CA 19-9 (n = 239) | Normal CA 19-9 (n = 449) | p-Value |
|---|---|---|---|---|
| Treatment duration, month | 19.4 (15.2–24.5) | 19.0 (15.2–24.5) | 19.6 (15.2–24.6) | 0.949 |
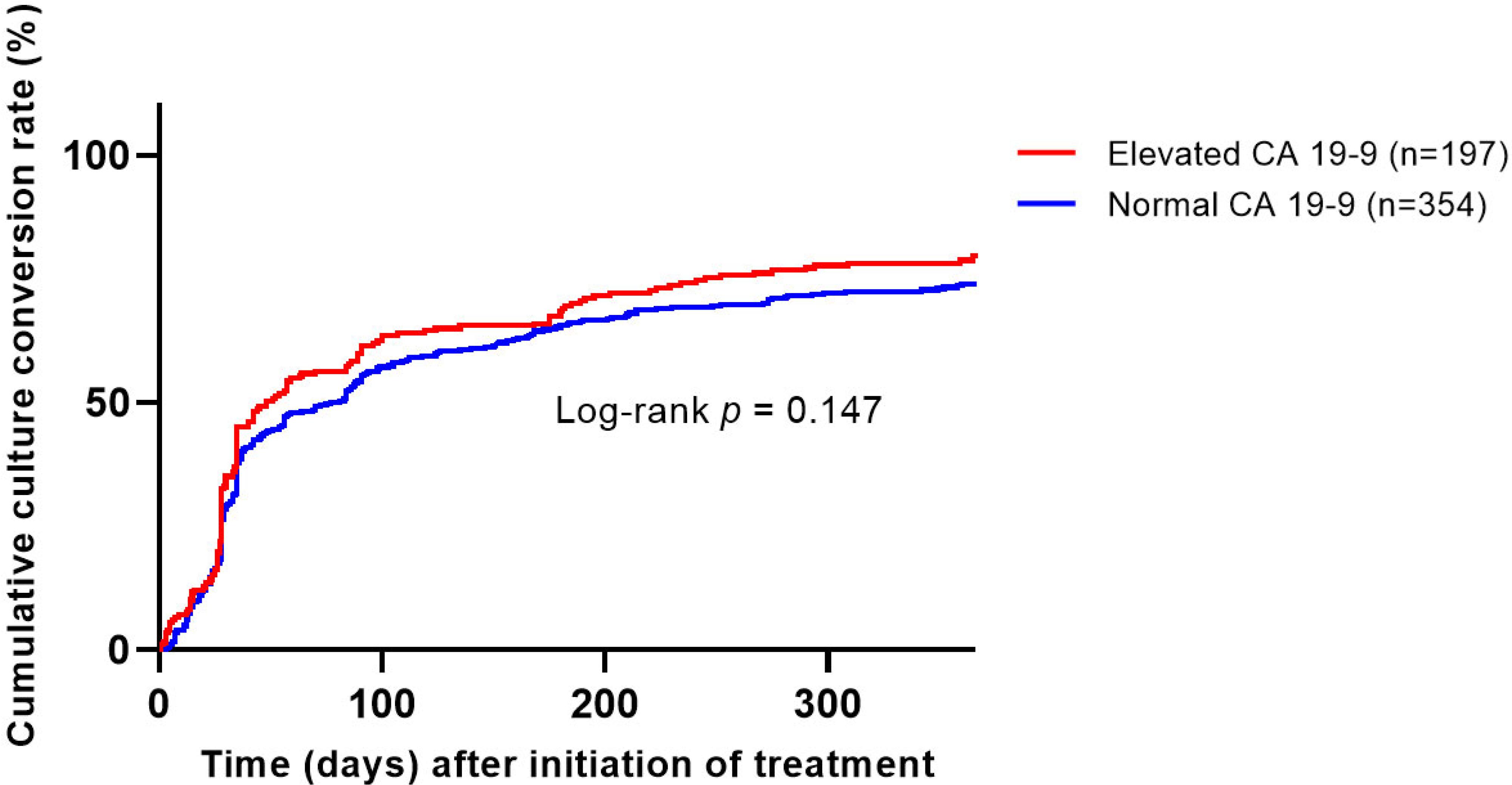
| Culture conversion, within one year | 437/583 (75) | 164/206 (80) | 273/377 (72) | 0.055 |
| Microbiological cure | 466/551 (85) | 175/197 (89) | 291/354 (82) | 0.039 |
| Time to culture conversion, month ¶ | 1.2 (0.9–5.0) | 1.4 (0.9–5.8) | 1.2 (0.9–4.5) | 0.338 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odd Ratio (95% Confidence Interval) | p-Value | Adjusted Odd Ratio (95% Confidence Interval) | p-Value | |
| Sex, female | 1.934 (1.209–3.095) | 0.006 | – | – |
| Age, year | 0.985 (0.964–1.006) | 0.162 | – | – |
| Body mass index, kg/m2 | 1.043 (0.948–1.147) | 0.389 | – | – |
| Smoking status | ||||
| Never | reference | – | reference | – |
| Ex | 0.625 (0.369–1.059) | 0.081 | 0.617 (0.313–1.216) | 0.163 |
| Current | 0.395 (0.120–1.301) | 0.127 | 0.159 (0.037–0.686) | 0.014 |
| Comorbidities | ||||
| Bronchiectasis | 0.748 (0.338–1.439) | 0.384 | 0.358 (0.153–0.837) | 0.018 |
| Obstructive lung disease | 1.250 (0.545–2.867) | 0.598 | – | – |
| Chronic pulmonary aspergillosis | 1.285 (0.287–5.761) | 0.743 | – | – |
| Idiopathic pulmonary fibrosis | 1.281 (0.156–10.547) | 0.818 | – | – |
| Previous pulmonary tuberculosis | 0.603 (0.378–0.960) | 0.033 | – | – |
| Positive sputum AFB smear | 0.370 (0.223–0.615) | <0.001 | 0.289 (0.155–0.541) | <0.001 |
| Cavity | 0.836 (0.514–1.360) | 0.471 | – | – |
| Etiology | ||||
| M. avium complex | 1.364 (0.846–2.199) | 0.203 | – | – |
| M. massiliense | 3.520 (1.249–9.917) | 0.017 | 3.108 (1.055–9.150) | 0.040 |
| M. abscessus | 0.329 (0.172–0.629) | 0.001 | 0.329 (0.147–0.735) | 0.007 |
| Laboratory findings | ||||
| ESR, mm/h | 1.004 (0.995–1.014) | 0.367 | – | – |
| CRP, mg/dL | 0.918 (0.809–1.043) | 0.189 | – | – |
| CA 19-9 (U/mL) | 1.002 (0.999–1.005) | 0.262 | 1.004 (0.999–1.008) | 0.121 |
| BACES severity score ¶ | ||||
| Mild | reference | – | reference | – |
| Moderate | 0.838 (0.492–1.426) | 0.514 | – | – |
| Severe | 0.563 (0.233–1.358) | 0.201 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Jhun, B.W. The Clinical Implications of Serum Carbohydrate Antigen 19-9 Levels in Patients with Nontuberculous Mycobacteria Pulmonary Disease. J. Clin. Med. 2023, 12, 7751. https://doi.org/10.3390/jcm12247751
Lee D, Jhun BW. The Clinical Implications of Serum Carbohydrate Antigen 19-9 Levels in Patients with Nontuberculous Mycobacteria Pulmonary Disease. Journal of Clinical Medicine. 2023; 12(24):7751. https://doi.org/10.3390/jcm12247751
Chicago/Turabian StyleLee, Daegeun, and Byung Woo Jhun. 2023. "The Clinical Implications of Serum Carbohydrate Antigen 19-9 Levels in Patients with Nontuberculous Mycobacteria Pulmonary Disease" Journal of Clinical Medicine 12, no. 24: 7751. https://doi.org/10.3390/jcm12247751
APA StyleLee, D., & Jhun, B. W. (2023). The Clinical Implications of Serum Carbohydrate Antigen 19-9 Levels in Patients with Nontuberculous Mycobacteria Pulmonary Disease. Journal of Clinical Medicine, 12(24), 7751. https://doi.org/10.3390/jcm12247751






