Predictive Performance of Neuron-Specific Enolase (NSE) for Survival after Resuscitation from Cardiac Arrest: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
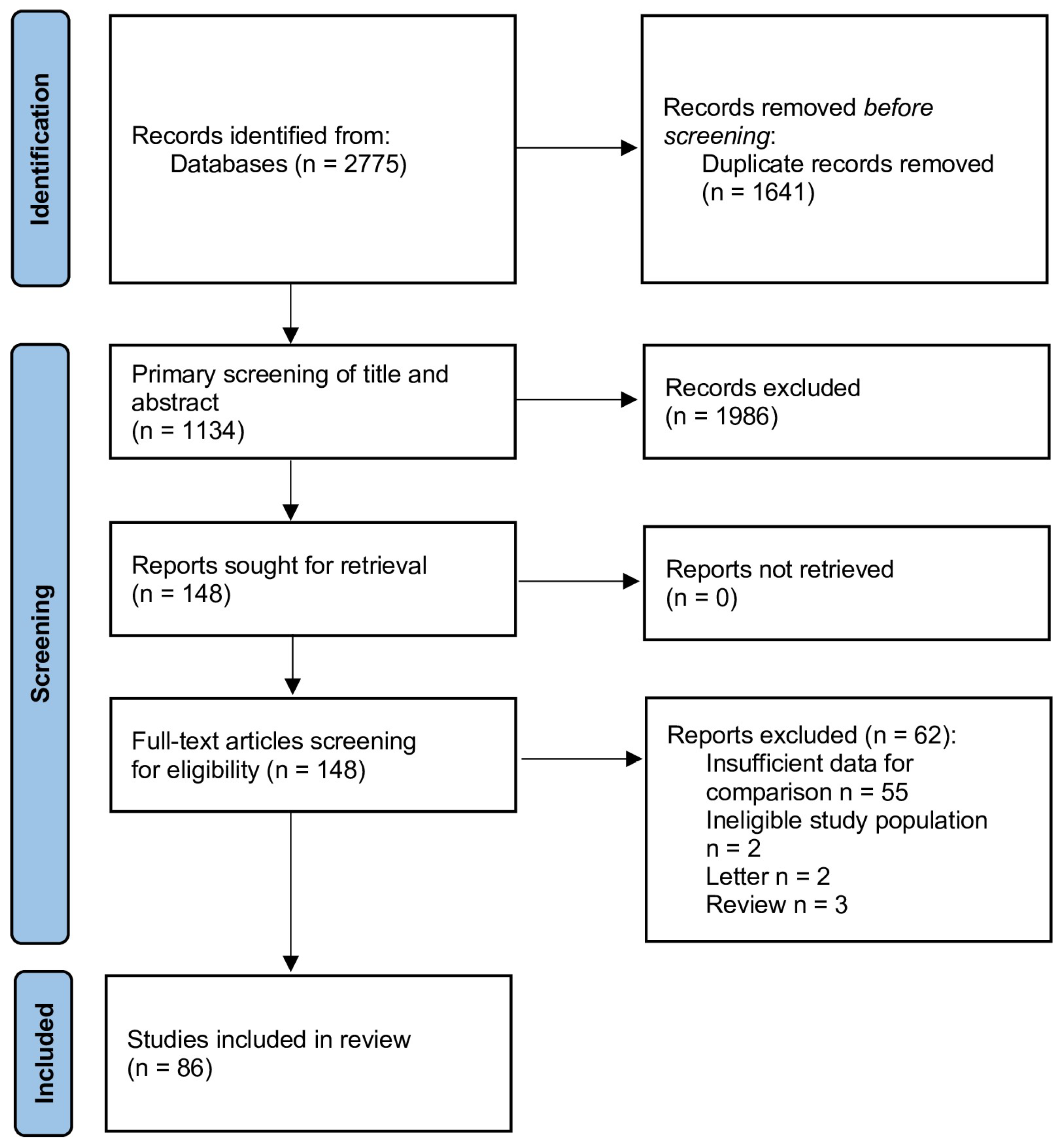
2.1. Literature Search and Selection
2.2. Data Extraction and Quality Assessment
2.3. Data Synthesis and Meta-Analysis
3. Results
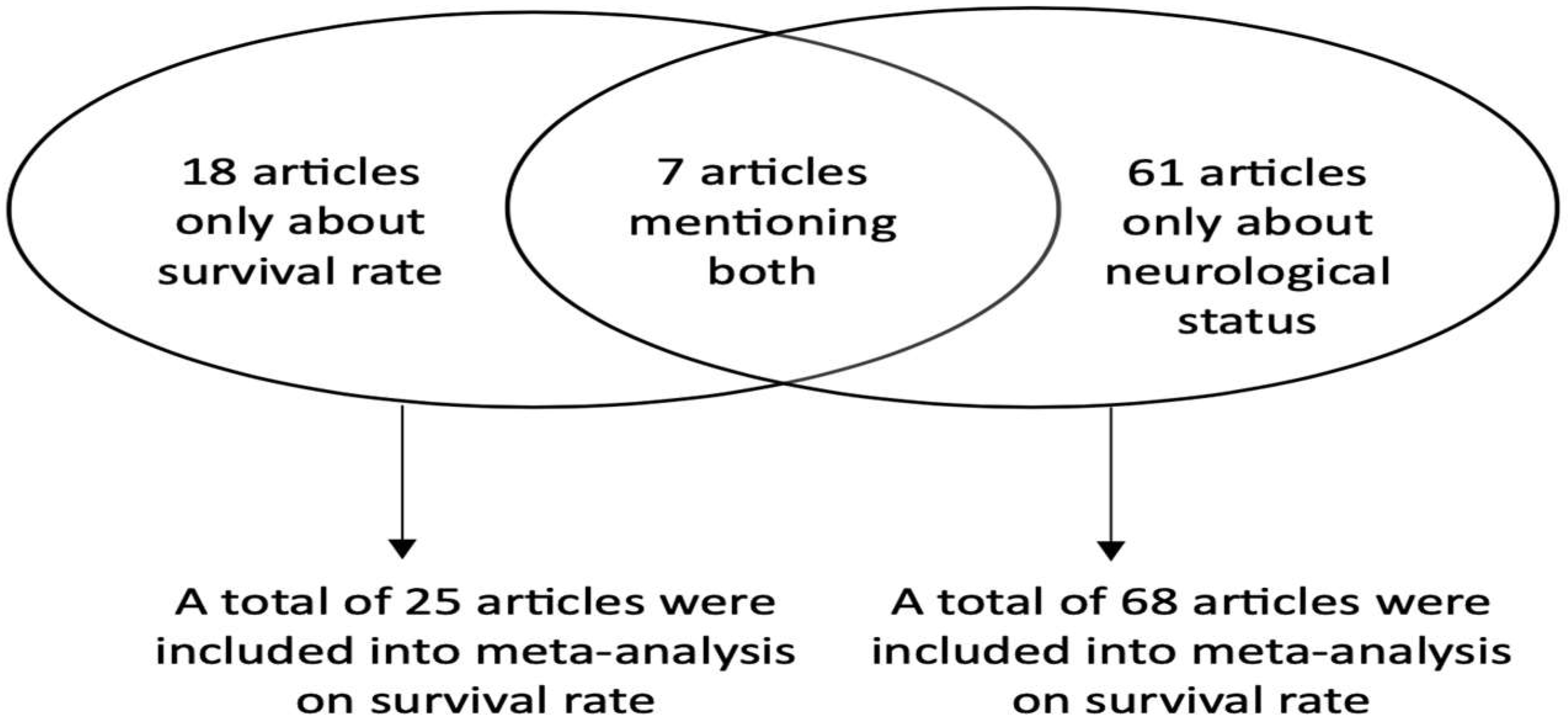
3.1. Study Populations
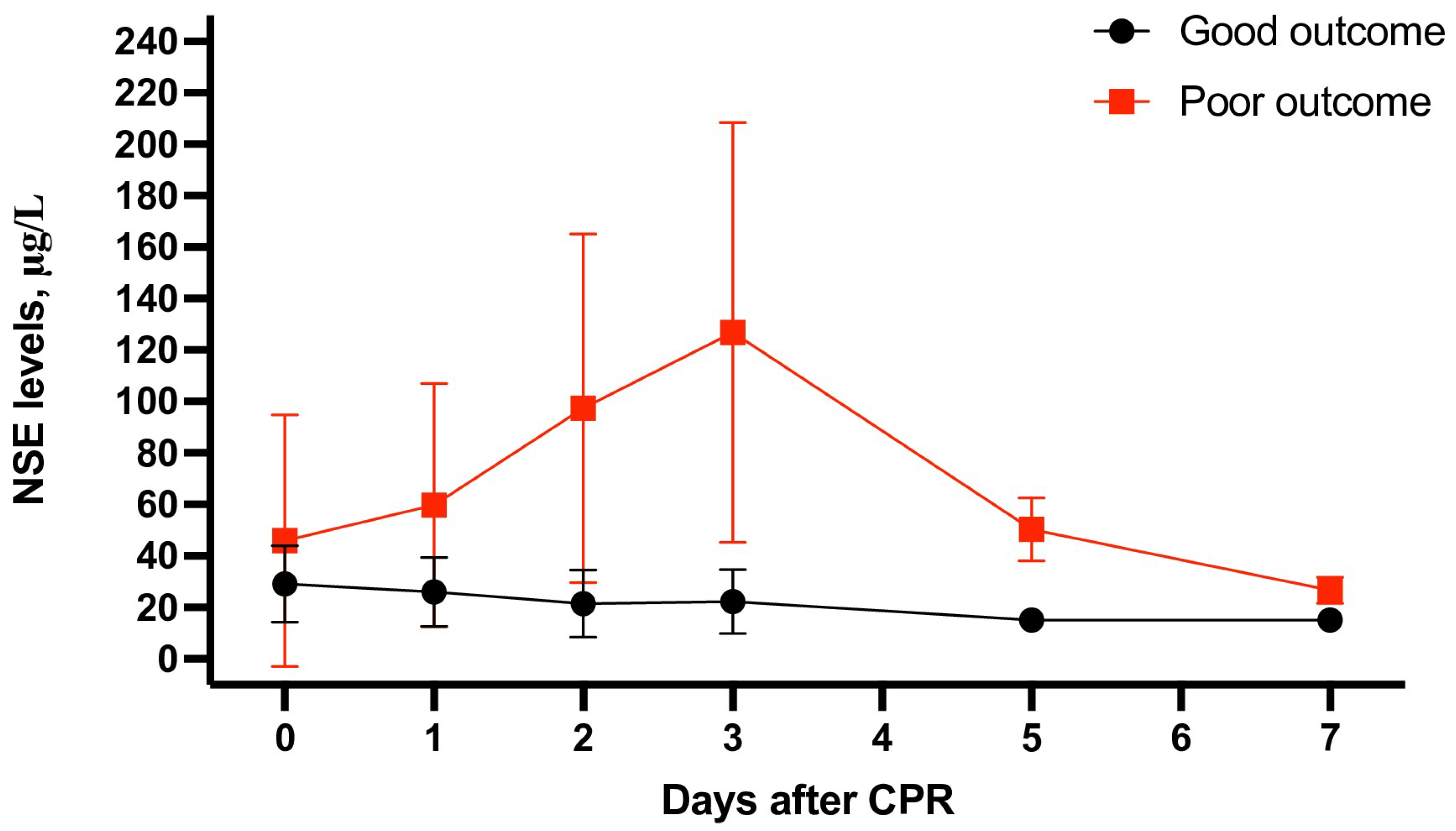
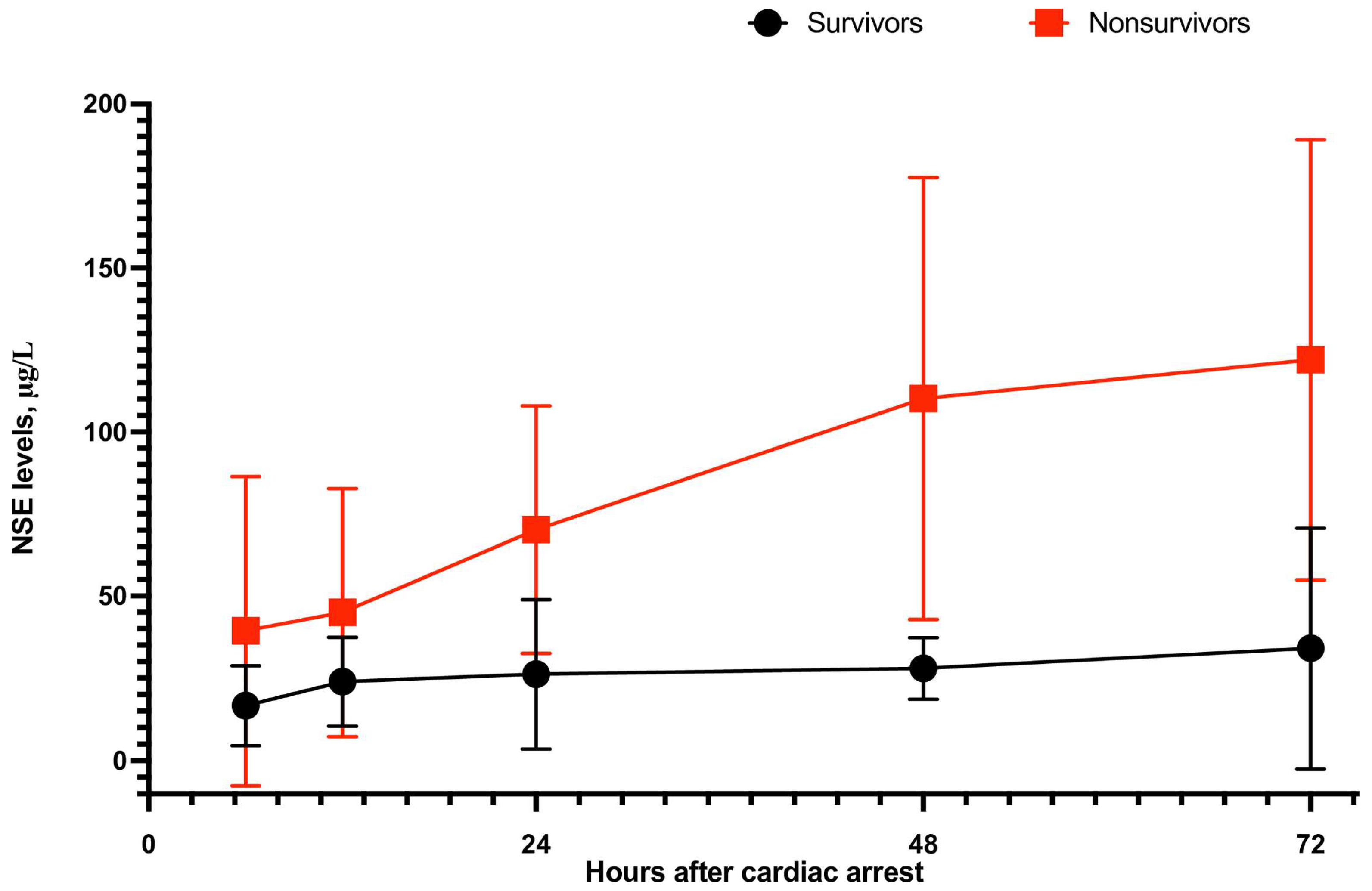
3.2. Meta-Analysis of NSE as a Survival Prognostic Marker
3.3. Meta-Analysis of NSE as a Neurological Prognostic Marker
4. Discussion
4.1. Searching for New Biomarkers and Regulatory Approaches
4.2. Obstacles to Implementing NSE into Routine Clinical Practice
4.3. NSE and Prediction of Neurological Status
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, A.; Käser, D.; Theiler, L.; Greif, R.; Knapp, J.; Berger-Estilita, J. Survival and long-term outcomes following in-hospital cardiac arrest in a Swiss university hospital: A prospective observational study. Scand. J. Trauma. Resusc. Emerg. Med. 2021, 29, 115. [Google Scholar] [CrossRef] [PubMed]
- Bielski, K.; Szarpak, A.; Jaguszewski, M.J.; Kopiec, T.; Smereka, J.; Gasecka, A.; Wolak, P.; Nowak-Starz, G.; Chmielewski, J.; Rafique, Z.; et al. The Influence of COVID-19 on Out-Hospital Cardiac Arrest Survival Outcomes: An Updated Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 5573. [Google Scholar] [CrossRef] [PubMed]
- Widestedt, H.; Giesecke, J.; Karlsson, P.; Jakobsson, J.G. In-hospital cardiac arrest resuscitation performed by the hospital emergency team: A 6-year retrospective register analysis at Danderyd University Hospital, Sweden. F1000Research 2018, 7, 1013. [Google Scholar] [CrossRef] [PubMed]
- Paratz, E.D.; Nehme, E.; Heriot, N.; Bissland, K.; Rowe, S.; Fahy, L.; Anderson, D.; Stub, D.; La Gerche, A.; Nehme, Z. A two-point strategy to clarify prognosis in >80 year olds experiencing out of hospital cardiac arrest. Resuscitation 2023, 191, 109962. [Google Scholar] [CrossRef]
- Mangla, A.; Daya, M.R.; Gupta, S. Post-resuscitation care for survivors of cardiac arrest. Indian Heart J. 2014, 66, S105–S112. [Google Scholar] [CrossRef]
- Sandroni, C.; Cronberg, T.; Sekhon, M. Brain injury after cardiac arrest: Pathophysiology, treatment, and prognosis. Intensive Care Med. 2021, 47, 1393–1414. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Stukas, S.; Hirsch-Reinshagen, V.; Thiara, S.; Schoenthal, T.; Tymko, M.; McNagny, K.M.; Wellington, C.; Hoiland, R. Neuroinflammation and the immune system in hypoxic ischaemic brain injury pathophysiology after cardiac arrest. J. Physiol. 2023. [Google Scholar] [CrossRef]
- Havmöller, R.; Chugh, S.S. Plasma biomarkers for prediction of sudden cardiac death: Another piece of the risk stratification puzzle? Circ. Arrhythm. Electrophysiol. 2012, 5, 237–243. [Google Scholar] [CrossRef]
- Jones, A.; Jarvis, P. Review of the potential use of blood neuro-biomarkers in the diagnosis of mild traumatic brain injury. Clin. Exp. Emerg. Med. 2017, 4, 121–127. [Google Scholar] [CrossRef]
- Wang, K.K.; Yang, Z.; Zhu, T.; Shi, Y.; Rubenstein, R.; Tyndall, J.A.; Manley, G.T. An update on diagnostic and prognostic biomarkers for traumatic brain injury. Expert. Rev. Mol. Diagn. 2018, 18, 165–180. [Google Scholar] [CrossRef]
- Ramont, L.; Thoannes, H.; Volondat, A.; Chastang, F.; Millet, M.C.; Maquart, F.X. Effects of hemolysis and storage condition on neuron-specific enolase (NSE) in cerebrospinal fluid and serum: Implications in clinical practice. Clin. Chem. Lab. Med. 2005, 43, 1215–1217. [Google Scholar] [CrossRef]
- Johnsson, P.; Blomquist, S.; Lührs, C.; Malmkvist, G.; Alling, C.; Solem, J.O.; Ståhl, E. Neuron-specific enolase increases in plasma during and immediately after extracorporeal circulation. Ann. Thorac. Surg. 2000, 69, 750–754. [Google Scholar] [CrossRef]
- Luescher, T.; Mueller, J.; Isenschmid, C.; Kalt, J.; Rasiah, R.; Tondorf, T.; Gamp, M.; Becker, C.; Sutter, R.; Tisljar, K.; et al. Neuron-specific enolase (NSE) improves clinical risk scores for prediction of neurological outcome and death in cardiac arrest patients: Results from a prospective trial. Resuscitation 2019, 142, 50–60. [Google Scholar] [CrossRef]
- Czimmeck, C.; Kenda, M.; Aalberts, N.; Endisch, C.; Ploner, C.J.; Storm, C.; Nee, J.; Streitberger, K.J.; Leithner, C. Confounders for prognostic accuracy of neuron-specific enolase after cardiac arrest: A retrospective cohort study. Resuscitation 2023, 192, 109964. [Google Scholar] [CrossRef]
- Ryczek, R.; Kwasiborski, P.J.; Dymus, J.; Galas, A.; Kaźmierczak-Dziuk, A.; Karasek, A.M.; Mielniczuk, M.; Buksińska-Lisik, M.; Krzesiński, P. Neuron-specific enolase concentrations for the prediction of poor prognosis of comatose patients after out-of-hospital cardiac arrest: An observational cohort study. Kardiol. Pol. 2021, 79, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Andersson, P.; Johnsson, J.; Björnsson, O.; Cronberg, T.; Hassager, C.; Zetterberg, H.; Stammet, P.; Undén, J.; Kjaergaard, J.; Friberg, H.; et al. Predicting neurological outcome after out-of-hospital cardiac arrest with cumulative information; development and internal validation of an artificial neural network algorithm. Crit. Care 2021, 25, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chang, W.T.; Su, K.I.; Huang, C.H.; Tsai, M.S.; Chou, E.; Lu, T.C.; Chen, W.J.; Lee, C.C.; Chen, S.C. Neuroprognostic accuracy of blood biomarkers for post-cardiac arrest patients: A systematic review and meta-analysis. Resuscitation 2020, 148, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Kurek, K.; Tomaszewska, M.; Pruc, M.; Szarpak, L. Role of neuron-specific enolase as a prognostic marker in pediatric cardiac arrest. Am. J. Emerg Med. 2023. [Google Scholar] [CrossRef]
- Sharma, K.; John, M.; Zhang, S.; Gronseth, G. Serum Neuron-Specific Enolase Thresholds for Predicting Postcardiac Arrest Outcome: A Systematic Review and Meta-analysis. Neurology 2022, 98, e62–e72. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Simpson, B.S.; Ball, R.; Freeman, A.; Kirkham, A.; Parry, M.A.; Moore, C.M.; Whitaker, H.C.; Emberton, M. A modified newcastle-ottawa scale for assessment of study quality in genetic urological research. Eur. Urol. 2021, 79, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.; Onur, O.A.; Braumann, S.; Gramespacher, H.; Bittner, S.; Falk, S.; Fink, G.R.; Baldus, S.; Warnke, C. Absolute serum neurofilament light chain levels and its early kinetics predict brain injury after out-of-hospital cardiac arrest. J. Neurol. 2022, 269, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Kang, C.; Ahn, H.J.; You, Y.; Park, J.S.; Min, J.H.; Jeong, W.; Cho, Y.; Ryu, S.; In, Y.N. Serum total cholesterol level as a potential predictive biomarker for neurological outcomes in cardiac arrest survivors who underwent target temperature management. Medicine 2022, 101, e31909. [Google Scholar] [CrossRef]
- Akin, M.; Garcheva, V.; Sieweke, J.T.; Adel, J.; Flierl, U.; Bauersachs, J.; Schäfer, A. Neuromarkers and neurological outcome in out-of-hospital cardiac arrest patients treated with therapeutic hypothermia-experience from the HAnnover COoling REgistry (HACORE). PLoS ONE 2021, 16, e0245210. [Google Scholar] [CrossRef]
- Akin, M.; Sieweke, J.T.; Garcheva, V.; Martinez, C.S.; Adel, J.; Plank, P.; Zandian, P.; Sühs, K.W.; Bauersachs, J.; Schäfer, A. Additive Impact of Interleukin 6 and Neuron Specific Enolase for Prognosis in Patients with Out-of-Hospital Cardiac Arrest—Experience from the HAnnover COoling Registry. Front. Cardiovasc. Med. 2022, 9, 899583. [Google Scholar] [CrossRef]
- Aldesouky Alwassef, A.R.; Ameen Ahmed, A.A.; Shaheen, E.F.; Shaaban, Y.H. Elevated at admission serum neuron specific enolase and hyperglycemia are predictors of poor outcome of post-resuscitation patients. Al-Azhar Med. J. 2016, 45, 331–343. [Google Scholar] [CrossRef]
- Andersson, A.; Arctaedius, I.; Cronberg, T.; Levin, H.; Nielsen, N.; Friberg, H.; Lybeck, A. In-hospital versus out-of-hospital cardiac arrest: Characteristics and outcomes in patients admitted to intensive care after return of spontaneous circulation. Resuscitation 2022, 176, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Auer, J.; Berent, R.; Weber, T.; Porodko, M.; Lamm, G.; Lassnig, E.; Maurer, E.; Mayr, H.; Punzengruber, C.; Eber, B. Ability of neuron-specific enolase to predict survival to hospital discharge after successful cardiopulmonary resuscitation. Can. J. Emerg. Med. 2006, 8, 13–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbella, G.; Lee, J.W.; Alvarez, V.; Novy, J.; Oddo, M.; Beers, L.; Rossetti, A.O. Prediction of regaining consciousness despite an early epileptiform EEG after cardiac arrest. Neurology 2020, 94, e1675–e1683. [Google Scholar] [CrossRef] [PubMed]
- Benghanem, S.; Nguyen, L.S.; Gavaret, M.; Mira, J.P.; Pène, F.; Charpentier, J.; Marchi, A.; Cariou, A. SSEP N20 and P25 amplitudes predict poor and good neurologic outcomes after cardiac arrest. Ann. Intensive Care 2022, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, K.; Ryu, S.; Kang, T.; Kim, H.; Cho, S.; Oh, S. Use of S-100B, NSE, CRP and ESR to predict neurological outcomes in patients with return of spontaneous circulation and treated with hypothermia. Emerg. Med. J. 2016, 33, 690–695. [Google Scholar] [CrossRef]
- Chong, J.Y.; Ahn, H.J.; Park, J.S.; You, Y.; Min, J.H.; Jeong, W.; Cho, Y.; Cho, S.U.; Oh, S.K.; Kang, C.S.; et al. Interleukin-6 as a Potential Predictor of Neurologic Outcomes in Cardiac Arrest Survivors Who Underwent Target Temperature Management. J. Emerg. Med. 2020, 59, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Chung-Esaki, H.M.; Mui, G.; Mlynash, M.; Eyngorn, I.; Catabay, K.; Hirsch, K.G. The Neuron Specific Enolase (NSE) ratio offers benefits over absolute value thresholds in post-cardiac arrest coma prognosis. J. Clin. Neurosci. 2018, 57, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska-Jelonkiewicz, K.; Wood, A.; Bohm, A.; Kwasiborski, P.; Oleksiak, A.; Ryczek, R.; Grand, J.; Tavazzi, G.; Sionis, A.; Stępińska, J. Association between dose of catecholamines and markers of organ injury early after out-of-hospital cardiac arrest. Cardiol. J. 2021, 30, 946–956. [Google Scholar] [CrossRef]
- Dauberschmidt, R.; Zinsmeyer, J.; Mrochen, H.; Meyer, M. Changes of neuron-specific enolase concentration in plasma after cardiac arrest and resuscitation. Mol. Chem. Neuropathol. 1991, 14, 237–245. [Google Scholar] [CrossRef]
- Daubin, C.; Quentin, C.; Allouche, S.; Etard, O.; Gaillard, C.; Seguin, A.; Valette, X.; Parienti, J.J.; Prevost, F.; Ramakers, M.; et al. Serum neuron-specific enolase as predictor of outcome in comatose cardiac-arrest survivors: A prospective cohort study. BMC Cardiovasc. Disord. 2011, 11, 48. [Google Scholar] [CrossRef]
- Deye, N.; Nguyen, P.; Vodovar, N.; Sadoune, M.; Collet, C.; Voicu, S.; Malissin, I.; Gayat, E.; Samuel, J.L.; Delcayre, C.; et al. Protein S100B as a reliable tool for early prognostication after cardiac arrest. Resuscitation 2020, 156, 251–259. [Google Scholar] [CrossRef]
- Einav, S.; Kaufman, N.; Algur, N.; Strauss-Liviatan, N.; Kark, J.D. Brain biomarkers and management of uncertainty in predicting outcome of cardiopulmonary resuscitation: A nomogram paints a thousand words. Resuscitation 2013, 84, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Jeong, K.; Abebe, K.Z.; Guyette, F.X.; Murugan, R.; Callaway, C.W.; Rittenberger, J.C.; on behalf of the Pittsburgh Post-Cardiac Arrest Service. Serum NGAL predicts survival after resuscitation from cardiac arrest. Crit. Care Med. 2016, 44, 111–119. [Google Scholar] [CrossRef]
- Ertl, M.; Weber, S.; Hammel, G.; Schroeder, C.; Krogias, C. Transorbital Sonography for Early Prognostication of Hypoxic-Ischemic Encephalopathy After Cardiac Arrest. J. Neuroimaging 2018, 28, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Gillick, K.; Rooney, K. Serial NSE measurement identifies non-survivors following out of hospital cardiac arrest. Resuscitation 2018, 128, 24–30. [Google Scholar] [CrossRef]
- Grubb, N.R.; Simpson, C.; Sherwood, R.A.; Abraha, H.D.; Cobbe, S.M.; O’Carroll, R.E.; Deary, I.; Fox, K.A. Prediction of cognitive dysfunction after resuscitation from out-of-hospital cardiac arrest using serum neuron-specific enolase and protein S-100. Heart 2007, 93, 1268–1273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haertel, F.; Babst, J.; Bruening, C.; Bogoviku, J.; Otto, S.; Fritzenwanger, M.; Gecks, T.; Ebelt, H.; Moebius-Winkler, S.; Schulze, P.C.; et al. Effect of Hemolysis Regarding the Characterization and Prognostic Relevance of Neuron Specific Enolase (NSE) after Cardiopulmonary Resuscitation with Extracorporeal Circulation (eCPR). J. Clin. Med. 2023, 12, 3015. [Google Scholar] [CrossRef]
- Hasper, D.; von Haehling, S.; Storm, C.; Jörres, A.; Schefold, J.C. Changes in serum creatinine in the first 24 hours after cardiac arrest indicate prognosis: An observational cohort study. Crit. Care 2009, 13, R168. [Google Scholar] [CrossRef]
- Hasslacher, J.; Lehner, G.F.; Harler, U.; Beer, R.; Ulmer, H.; Kirchmair, R.; Fischer-Colbrie, R.; Bellmann, R.; Dunzendorfer, S.; Joannidis, M. Secretoneurin as a marker for hypoxic brain injury after cardiopulmonary resuscitation. Intensive Care Med. 2014, 40, 1518–1527. [Google Scholar] [CrossRef]
- Helwig, K.; Seeger, F.; Hölschermann, H.; Lischke, V.; Gerriets, T.; Niessner, M.; Foerch, C. Elevated Serum Glial Fibrillary Acidic Protein (GFAP) is Associated with Poor Functional Outcome After Cardiopulmonary Resuscitation. Neurocrit. Care 2017, 27, 68–74. [Google Scholar] [CrossRef]
- Hermann, B.; Candia-Rivera, D.; Sharshar, T.; Gavaret, M.; Diehl, J.L.; Cariou, A.; Benghanem, S. Aberrant brain-heart coupling is associated with the severity and prognosis of hypoxic-ischemic brain injury after cardiac arrest. medRxiv 2023. [Google Scholar] [CrossRef]
- Jakkula, P.; Hästbacka, J.; Reinikainen, M.; Pettilä, V.; Loisa, P.; Tiainen, M.; Wilkman, E.; Bendel, S.; Birkelund, T.; Pulkkinen, A.; et al. Near-infrared spectroscopy after out-of-hospital cardiac arrest. Crit. Care 2019, 23, 171. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Fujita, M.; Ogino, Y.; Yamamoto, T.; Tsuruta, R.; Kasaoka, S. Serum neutrophil gelatinase-associated lipocalin levels predict the neurological outcomes of out-of-hospital cardiac arrest victims. BMC Cardiovasc. Disord. 2017, 17, 111. [Google Scholar] [CrossRef]
- Kang, C.; Lee, I.H.; Park, J.S.; You, Y.; Jeong, W.; Ahn, H.J.; Min, J.H. Measuring global impairment of cerebral perfusion using dynamic susceptibility contrast perfusion-weighted imaging in out-of-hospital cardiac arrest survivors: A prospective preliminary study. J. Neuroradiol. 2021, 48, 379–384. [Google Scholar] [CrossRef]
- Kang, C.; In, Y.N.; Park, J.S.; You, Y.; Min, J.H.; Jeong, W.; Ahn, H.J.; Cho, Y.C.; Ryu, S. Prognostic role of serum neutrophil gelatinase-associated lipocalin in cardiac arrest patients A prospective observational study. Medicine 2021, 100, e27463. [Google Scholar] [CrossRef]
- Kim, J.; Choi, B.S.; Kim, K.; Jung, C.; Lee, J.H.; Jo, Y.H.; Rhee, J.E.; Kim, T.; Kang, K.W. Prognostic Performance of Diffusion-Weighted MRI Combined with NSE in Comatose Cardiac Arrest Survivors Treated with Mild Hypothermia. Neurocrit. Care 2012, 17, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Kim, J.M.; Lee, J.S.; Park, S.H.; Jeong, H.B.; Choi, J.K.; Kim, K.; Bae, H.M.; Ko, S.B. Prognostication of neurological outcome after cardiac arrest using wavelet phase coherence analysis of cerebral oxygen. Resuscitation 2020, 150, 41–49. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.J.; Park, K.N.; Choi, S.P.; Lee, B.K.; Oh, S.H.; Jeung, K.W.; Cho, I.S.; Youn, C.S. Neuron-specific enolase and neuroimaging for prognostication after cardiac arrest treated with targeted temperature management. PLoS ONE 2020, 15, e0239979. [Google Scholar] [CrossRef]
- Kim, H.B.; Yang, J.H.; Lee, Y.H. Are serial neuron-specific enolase levels associated with neurologic outcome of ECPR patients: A retrospective multicenter observational study. Am. J. Emerg. Med. 2023, 69, 58–64. [Google Scholar] [CrossRef]
- Kirsch, K.; Heymel, S.; Günther, A.; Vahl, K.; Schmidt, T.; Michalski, D.; Fritzenwanger, M.; Schulze, P.C.; Pfeifer, R. Prognostication of neurologic outcome using gray-white-matter-ratio in comatose patients after cardiac arrest. BMC Neurol. 2021, 21, 456. [Google Scholar] [CrossRef]
- Kwon, W.Y.; Jung, Y.S.; Suh, G.J.; Kim, T.; Kwak, H.; Kim, T.; Kim, J.Y.; Lee, M.S.; Kim, K.S.; Shin, J.; et al. Regional cerebral oxygen saturation in cardiac arrest survivors undergoing targeted temperature management 36C versus 33C: A randomized clinical trial. Resuscitation 2021, 167, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Larsson, I.M.; Wallin, E.; Kristofferzon, M.L.; Niessner, M.; Zetterberg, H.; Rubertsson, S. Post-cardiac arrest serum levels of glial fibrillary acidic protein for predicting neurological outcome. Resuscitation 2014, 85, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Lascarrou, J.B.; Miailhe, A.F.; le Gouge, A.; Cariou, A.; Dequin, P.F.; Reignier, J.; Coupez, E.; Quenot, J.P.; Legriel, S.; Pichon, N.; et al. NSE as a predictor of death or poor neurological outcome after non-shockable cardiac arrest due to any cause: Ancillary study of HYPERION trial data. Resuscitation 2021, 158, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, I.; You, J.S.; Kim, M.J.; Lee, H.S.; Park, Y.S.; Park, H.C.; Chung, S.P. Predictive performance of plasma neutrophil gelatinase-associated lipocalin for neurologic outcomes in out-of-hospital cardiac arrest patients treated with targeted temperature management. A prospective observational study. Medicine 2019, 98, e16930. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.H.; Lee, J.H.; Lee, D.W.; Hwang, S.Y.; Youn, C.S.; Kim, J.H.; Sim, M.S.; Jeung, K.W. Combination of neuron-specific enolase measurement and initial neurological examination for the prediction of neurological outcomes after cardiac arrest. Sci. Rep. 2021, 11, 15067. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.S.; You, Y.; Min, J.H.; Jeong, W.; Ahn, H.J.; In, Y.N.; Cho, Y.C.; Lee, I.H.; Lee, J.K.; et al. Preliminary Prognostication for Good Neurological Outcomes in the Early Stage of Post-Cardiac Arrest Care. Diagnostics 2023, 13, 2174. [Google Scholar] [CrossRef] [PubMed]
- Leão, R.N.; Ávila, P.; Cavaco, R.; Germano, N.; Bento, L. Therapeutic hypothermia after cardiac arrest: Outcome predictors. Rev. Bras. Ter. Intensiva 2015, 27, 322–332. [Google Scholar] [CrossRef]
- Maher, C.; Cadd, M.; Nunn, M.; Worthy, J.; Gray, R.; Boyd, O. The use of neurone specific enolase to prognosticate neurological recovery and long term neurological outcomes in OOHCA patients. J. Intensive Care Soc. 2023, 24, 386–391. [Google Scholar] [CrossRef]
- Martens, P. Serum Neuron-specific Enolase as a Prognostic Marker for Irreversible Brain Damage in Comatose Cardiac Arrest Survivors. Acad. Emerg. Med. 1996, 3, 126–131. [Google Scholar] [CrossRef]
- Martínez-Losas, P.; López de Sá, E.; Armada, E.; Rosillo, S.; Monedero, M.C.; Rey, J.R.; Caro-Codón, J.; Buño Soto, A.; López Sendón, J.L. Neuron-specific enolase kinetics: An additional tool for neurological prognostication after cardiac arrest. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 123–130. [Google Scholar] [CrossRef]
- Müller, J.; Bissmann, B.; Becker, C.; Beck, K.; Loretz, N.; Gross, S.; Amacher, S.A.; Bohren, C.; Pargger, H.; Tisljar, K.; et al. Neuron-Specific Enolase (NSE) Predicts Long-Term Mortality in Adult Patients after Cardiac Arrest: Results from a Prospective Trial. Medicines 2021, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Mörtberg, J.; Salzinger, B.; Lundwall, K.; Edfors, R.; Jacobson, S.H.; Wallén, H.N.; Jernberg, T.; Baron, T.; Erlinge, D.; Andell, P.; et al. Prognostic importance of biomarkers associated with haemostatic, vascular and endothelial disturbances in acute coronary syndrome patients in relation to kidney function. Int. J. Cardiol. 2023, 373, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Nakstad, E.R.; Stær-Jensen, H.; Wimmer, H.; Henriksen, J.; Alteheld, L.H.; Reichenbach, A.; Drægni, T.; Šaltytė-Benth, J.; Wilson, J.A.; Etholm, L.; et al. Late awakening, prognostic factors and long-term outcome in out-of-hospital cardiac arrest—Results of the prospective Norwegian Cardio-respiratory Arrest Study (NORCAST). Resuscitation 2020, 149, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, H.S.; Park, K.N.; Ji, S.; Park, J.Y.; Choi, S.P.; Lim, J.Y.; Kim, H.J.; on Behalf of Crown Investigators. The Levels of Circulating MicroRNAs at 6-Hour Cardiac Arrest Can Predict 6-Month Poor Neurological Outcome. Diagnostics 2021, 11, 1905. [Google Scholar] [CrossRef]
- Oksanen, T.; Tiainen, M.; Skrifvars, M.B.; Varpula, T.; Kuitunen, A.; Castrén, M.; Pettilä, V. Predictive power of serum NSE and OHCA score regarding 6-month neurologic outcome after out-of-hospital ventricular fibrillation and therapeutic hypothermia. Resuscitation 2009, 80, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Wee, J.H.; Choi, S.P.; Oh, J.H.; Cheol, S. Assessment of serum biomarkers and coagulation/fibrinolysis markers for prediction of neurological outcomes of out of cardiac arrest patients treated with therapeutic hypothermia. Clin. Exp. Emerg. Med. 2019, 6, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Petermichl, W.; Philipp, A.; Hiller, K.A.; Foltan, M.; Floerchinger, B.; Graf, B.; Lunz, D. Reliability of prognostic biomarkers after prehospital extracorporeal cardiopulmonary resuscitation with target temperature management. Scand. J. Trauma. Resusc. Emerg. Med. 2021, 29, 147. [Google Scholar] [CrossRef]
- Rafecas, A.; Bañeras, J.; Sans-Roselló, J.; Ortiz-Pérez, J.T.; Rueda-Sobella, F.; Santamarina, E.; Milà, L.; Sionis, A.; Gaig, C.; García-García, C.; et al. Change in neuron specific enolase levels in out-of-hospital cardiopulmonary arrest survivors as a simple and useful tool to predict neurological prognosis. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 232–240. [Google Scholar] [CrossRef]
- Reisinger, J.; Höllinger, K.; Lang, W.; Steiner, C.; Winter, T.; Zeindlhofer, E.; Mori, M.; Schiller, A.; Lindorfer, A.; Wiesinger, K.; et al. Prediction of neurological outcome after cardiopulmonary resuscitation by serial determination of serum neuron-specific enolase. Eur. Heart J. 2007, 28, 52–58. [Google Scholar] [CrossRef]
- Roger, C.; Palmier, L.; Louart, B.; Molinari, N.; Claret, P.G.; de la Coussaye, J.E.; Lefrant, J.Y.; Muller, L. Neuron specific enolase and Glasgow motor score remain useful tools for assessing neurological prognosis after out-of-hospital cardiac arrest treated with therapeutic hypothermia. Anaesth. Crit. Care Pain. Med. 2015, 34, 231–237. [Google Scholar] [CrossRef]
- Rossetti, A.O.; Carrera, E.; Oddo, M. Early EEG correlates of neuronal injury after brain anoxia. Neurology 2012, 78, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, C.; Jesus, C.; Morais, J.; Viana, P. Predictors of death among cardiac arrest patients after therapeutic hypothermia: A non-tertiary care center’s initial experience. Rev. Port. Cardiol. 2016, 35, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Rundgren, M.; Karlsson, T.; Nielsen, N.; Cronberg, T.; Johnsson, P.; Friberg, H. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation 2009, 80, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Ruttmann, E.; Dietl, M.; Kastenberger, T.; El Attal, R.; Ströhle, M.; Ulmer, H.; Mair, P. Characteristics and outcome of patients with hypothermic out-of-hospital cardiac arrest: Experience from a European trauma center. Resuscitation 2017, 120, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.M.; Kim, Y.J.; Sohn, C.H.; Ahn, S.; Seo, D.W.; Kim, W.Y. Prognostic Abilities of Serial Neuron-Specific Enolase and Lactate and their Combination in Cardiac Arrest Survivors During Targeted Temperature Management. J. Clin. Med. 2020, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.; El-Azm, T.A.; Abadier, M.; Rageh, I. Combined Estimation of Plasma Cell-free DNA Level and Neuron Specific Enolase Activity as Outcome Predictors of Post-resuscitation Patients. Bull. Egypt. Soc. Physiol. Sci. 2012, 32, 187–200. [Google Scholar] [CrossRef]
- Schefold, J.C.; Storm, C.; Krüger, A.; Ploner, C.J.; Hasper, D. The Glasgow coma score is a predictor of good outcome in cardiac arrest patients treated with therapeutic hypothermia. Resuscitation 2009, 80, 658–661. [Google Scholar] [CrossRef]
- Schoerkhuber, W.; Kittler, H.; Sterz, F.; Behringer, W.; Holzer, M.; Frossard, M.; Spitzauer, S.; Laggner, A.N. Time Course of Serum Neuron-Specific Enolase A Predictor of Neurological Outcome in Patients Resuscitated from Cardiac Arrest. Stroke 1999, 30, 1598–1603. [Google Scholar] [CrossRef]
- Shin, H.; Lee, Y.; Choi, H.J.; Kim, C.; for the Korean Cardiac Arrest Research Consortium (KoCARC) Investigators. The predictive value of serum procalcitonin level as a prognostic marker for outcomes in out-of- hospital cardiac arrest patients. Hong Kong J. Emerg. Med. 2023, 30, 43–53. [Google Scholar] [CrossRef]
- Shinozaki, K.; Oda, S.; Sadahiro, T.; Nakamura, M.; Abe, R.; Nakada, T.A.; Nomura, F.; Nakanishi, K.; Kitamura, N.; Hirasawa, H. Serum S-100B is superior to neuron-specific enolase as an early prognostic biomarker for neurological outcome following cardiopulmonary resuscitation. Resuscitation 2009, 80, 870–875. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, I.H.; Park, J.S.; Yoo, I.S.; Kim, S.W.; Lee, J.W.; Ryu, S.; You, Y.; Min, J.H.; Cho, Y.C.; et al. Does Combining Biomarkers and Brain Images Provide Improved Prognostic Predictive Performance for Out-Of-Hospital Cardiac Arrest Survivors before Target Temperature Management? J. Clin. Med. 2020, 9, 744. [Google Scholar] [CrossRef]
- Song, H.G.; Park, J.S.; You, Y.; Ahn, H.J.; Yoo, I.; Kim, S.W.; Lee, J.; Ryu, S.; Jeong, W.; Cho, Y.C.; et al. Using Out-of-Hospital Cardiac Arrest (OHCA) and Cardiac Arrest Hospital Prognosis (CAHP) Scores with Modified Objective Data to Improve Neurological Prognostic Performance for Out-of-Hospital Cardiac Arrest Survivors. J. Clin. Med. 2021, 10, 1825. [Google Scholar] [CrossRef]
- Song, H.; Bang, H.J.; You, Y.; Park, J.S.; Kang, C.; Kim, H.J.; Park, K.N.; Oh, S.H.; Youn, C.S. Novel serum biomarkers for predicting neurological outcomes in postcardiac arrest patients treated with targeted temperature management. Crit. Care 2023, 27, 113. [Google Scholar] [CrossRef] [PubMed]
- Stammet, P.; Wagner, D.R.; Gilson, G.; Devaux, Y. Modeling Serum Level of S100b and Bispectral Index to Predict Outcome After Cardiac Arrest. J. Am. Coll. Cardiol. 2013, 62, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Stefanizzi, F.M.; Zhang, L.; Salgado-Somoza, A.; Dankiewicz, J.; Stammet, P.; Hassager, C.; Wise, M.P.; Friberg, H.; Cronberg, T.; Hundt, A.; et al. Circular RNAs to predict clinical outcome after cardiac arrest. Intensive Care Med. Exp. 2022, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Storm, C.; Nee, J.; Jörres, A.; Leithner, C.; Hasper, D.; Ploner, C.J. Serial measurement of neuron specific enolase improves prognostication in cardiac arrest patients treated with hypothermia: A prospective study. Scand. J. Trauma. Resusc. Emerg. Med. 2012, 20, 6. [Google Scholar] [CrossRef]
- Sugita, A.; Kinoshita, K.; Sakurai, A.; Chiba, N.; Yamaguchi, J.; Kuwana, T.; Sawada, N.; Hori, S. Systemic impact on secondary brain aggravation due to ischemia/reperfusion injury in post-cardiac arrest syndrome: A prospective observational study using high-mobility group box 1 protein. Crit. Care 2017, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Tat, R.M.; Golea, A.; Vesa, Ş.C.; Ionescu, D. Resistin-Can it be a new early marker for prognosis in patients who survive after a cardiac arrest? A pilot study. PLoS ONE 2019, 14, e0210666. [Google Scholar] [CrossRef]
- Vondrakova, D.; Kruger, A.; Janotka, M.; Malek, F.; Dudkova, V.; Neuzil, P.; Ostadal, P. Association of neuron-specific enolase values with outcomes in cardiac arrest survivors is dependent on the time of sample collection. Crit. Care 2017, 21, 172. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.F.; Guan, X.L.; Liang, S.S.; Gong, P. Predictive value of soluble CD59 for poor 28-day neurological prognosis and all-cause mortality in patients after cardiopulmonary resuscitation: A prospective observatory study. J. Intensive Care 2023, 11, 3. [Google Scholar] [CrossRef]
- Wessels, T.; Harrer, J.U.; Jacke, C.; Janssens, U.; Klötzsch, C. The prognostic value of early transcranial Doppler ultrasound following cardiopulmonary resuscitation. Ultrasound Med. Biol. 2006, 32, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Wennervirta, J.E.; Ermes, M.J.; Tiainen, S.M.; Salmi, T.K.; Hynninen, M.S.; Särkelä, M.O.; Hynynen, M.J.; Stenman, U.H.; Viertiö-Oja, H.E.; Saastamoinen, K.P.; et al. Hypothermia-treated cardiac arrest patients with good neurological outcome differ early in quantitative variables of EEG suppression and epileptiform activity. Crit. Care Med. 2009, 37, 2427–2435. [Google Scholar] [CrossRef]
- Wihersaari, L.; Reinikainen, M.; Furlan, R.; Mandelli, A.; Vaahersalo, J.; Kurola, J.; Tiainen, M.; Pettilä, V.; Bendel, S.; Varpula, T.; et al. Neurofilament light compared to neuron-specific enolase as a predictor of unfavourable outcome after out-of-hospital cardiac arrest. Resuscitation 2022, 174, 1–8. [Google Scholar] [CrossRef]
- Wolff, B.; Machill, K.; Schumacher, D.; Schulzki, I.; Werner, D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int. J. Cardiol. 2009, 133, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Wurm, R.; Arfsten, H.; Muqaku, B.; Ponleitner, M.; Bileck, A.; Altmann, P.; Rommer, P.; Seidel, S.; Hubner, P.; Sterz, F.; et al. Prediction of Neurological Recovery After Cardiac Arrest Using Neurofilament Light Chain is Improved by a Proteomics-Based Multimarker Panel. Neurocrit. Care 2022, 36, 434–440. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Park, J.S.; Min, J.; Yoo, I.; Ahn, H.J.; Cho, Y.; Ryu, S.; Lee, J.; Kim, S.; Cho, S.; et al. The usefulness of neuron-specific enolase in cerebrospinal fluid to predict neurological prognosis in cardiac arrest survivors who underwent target temperature management: A prospective observational study. Resuscitation 2019, 145, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Zellner, T.; Gärtner, R.; Schopohl, J.; Angstwurm, M. NSE and S-100B are not sufficiently predictive of neurologic outcome after therapeutic hypothermia for cardiac arrest. Resuscitation 2013, 84, 1382–1386. [Google Scholar] [CrossRef]
- Zhai, Q.; Feng, L.; Zhang, H.; Wu, M.; Wang, D.; Ge, H.; Li, S.; Du, L.; Zheng, K.; Li, H.; et al. Serial disseminated intravascular coagulation score with neuron specific enolase predicts the mortality of cardiac arrest—A pilot study. J. Thorac. Dis. 2020, 12, 3573–3581. [Google Scholar] [CrossRef]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef]
- Lagebrant, A.; Lang, M.; Nielsen, N.; Blennow, K.; Dankiewicz, J.; Friberg, H.; Hassager, C.; Horn, J.; Kjaergaard, J.; Kuiper, M.A.; et al. Brain injury markers in blood predict signs of hypoxic ischaemic encephalopathy on head computed tomography after cardiac arrest. Resuscitation 2023, 184, 109668. [Google Scholar] [CrossRef]
- Cronberg, T.; Greer, D.M.; Lilja, G.; Moulaert, V.; Swindell, P.; Rossetti, A.O. Brain injury after cardiac arrest: From prognostication of comatose patients to rehabilitation. Lancet Neurol. 2020, 19, 611–622. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Rikhraj, K.J.K.; Thiara, S.; Fordyce, C.; Kramer, A.H.; Skrifvars, M.B.; Wellington, C.L.; Griesdale, D.E.; Fergusson, N.A.; Sekhon, M.S. Neurologic Prognostication After Cardiac Arrest Using Brain Biomarkers: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.C.; da Rosa, M.M.; Leão, H.I.; Silva, E.D.L.; Ferreira, N.T.; Albuquerque, A.P.B.; Duarte, G.S.; Siqueira, A.M.; Pereira, M.C.; Rêgo, M.J.B.M.; et al. Brain damage serum biomarkers induced by COVID-19 in patients from northeast Brazil. J. Neurovirol. 2023, 29, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kokkoris, S.; Stamataki, E.; Emmanouil, G.; Psachoulia, C.; Ntaidou, T.; Maragouti, A.; Kanavou, A.; Malachias, S.; Christodouli, F.; Papachatzakis, I.; et al. Serum inflammatory and brain injury biomarkers in COVID-19 patients admitted to intensive care unit: A pilot study. eNeurologicalSci 2022, 29, 100434. [Google Scholar] [CrossRef]
- Fink, E.L.; Kochanek, P.M.; Panigrahy, A.; Beers, S.R.; Berger, R.P.; Bayir, H.; Pineda, J.; Newth, C.; Topjian, A.A.; Press, C.A.; et al. Association of Blood-Based Brain Injury Biomarker Concentrations with Outcomes after Pediatric Cardiac Arrest. JAMA Netw. Open 2022, 5, e2230518. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Li, N.; Feng, S.Y.; Li, Y. Serum neurofilament light chain as a predictive marker of neurologic outcome after cardiac arrest: A meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Karantali, E.; Kazis, D.; McKenna, J.; Chatzikonstantinou, S.; Petridis, F.; Mavroudis, I. Neurofilament light chain in patients with a concussion or head impacts: A systematic review and meta-analysis. Eur. J. Trauma. Emerg. Surg. 2022, 48, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zaitseva, D.; Yang, Z.; Forsythe, L.; Joergensen, S.; Zone, A.I.; Shehu, J.; Maghraoui, S.; Ghorbani, A.; Davila, A.; et al. Brain-derived extracellular vesicles as serologic markers of brain injury following cardiac arrest: A pilot feasibility study. Resuscitation 2023, 191, 109937. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.N.; Hwang, J.; Munar, M.; Papa, L.; Hinson, H.E.; Vaughan, A.; Rowell, S.E. Blood-based biomarkers for prediction of intracranial hemorrhage and outcome in patients with moderate or severe traumatic brain injury. J. Trauma. Acute Care Surg. 2020, 89, 80–86. [Google Scholar] [CrossRef]
- Bencsik, C.M.; Kramer, A.H.; Couillard, P.; MacKay, M.; Kromm, J.A. Postarrest Neuroprognostication: Practices and Opinions of Canadian Physicians. Can. J. Neurol. Sci. 2023, 1–12. [Google Scholar] [CrossRef]
- Fordyce, C.B.; Kramer, A.H.; Ainsworth, C.; Christenson, J.; Hunter, G.; Kromm, J.; Lopez Soto, C.; Scales, D.C.; Sekhon, M.; van Diepen, S.; et al. Neuroprognostication in the Post Cardiac Arrest Patient: A Canadian Cardiovascular Society Position Statement. Can. J. Cardiol. 2023, 39, 366–380. [Google Scholar] [CrossRef]
- Carroll, E.; Lewis, A. Neuroprognostication after Cardiac Arrest: Who Recovers? Who Progresses to Brain Death? Semin. Neurol. 2021, 41, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Medicherla, C.B.; Lewis, A. The critically ill brain after cardiac arrest. Ann. N. Y. Acad. Sci. 2022, 1507, 12–22. [Google Scholar] [CrossRef]
- Moseby-Knappe, M.; Levin, H.; Blennow, K.; Ullén, S.; Zetterberg, H.; Lilja, G.; Dankiewicz, J.; Jakobsen, J.C.; Lagebrant, A.; Friberg, H.; et al. Biomarkers of brain injury after cardiac arrest; a statistical analysis plan from the TTM2 trial biobank investigators. Resusc. Plus 2022, 10, 100258. [Google Scholar] [CrossRef] [PubMed]
- Abdi Isse, Y.; Frikke-Schmidt, R.; Wiberg, S.; Grand, J.; Obling, L.E.R.; Meyer, A.S.P.; Kjaergaard, J.; Hassager, C.; Meyer, M.A.S. Predicting poor neurological outcomes following out-of-hospital cardiac arrest using neuron-specific enolase and neurofilament light chain in patients with and without haemolysis. Eur. Heart J. Open 2023, 3, oead078. [Google Scholar] [CrossRef] [PubMed]
- Wihersaari, L.; Ashton, N.J.; Reinikainen, M.; Jakkula, P.; Pettilä, V.; Hästbacka, J.; Tiainen, M.; Loisa, P.; Friberg, H.; Cronberg, T.; et al. Neurofilament light as an outcome predictor after cardiac arrest: A post hoc analysis of the COMACARE trial. Intensive Care Med. 2021, 47, 39–48. [Google Scholar] [CrossRef]


| Measurement Period after Cardiac Arrest | No. of Studies | Mean ± SD | Events | Heterogeneity between Trials | p-Value for Differences across Groups | |||
|---|---|---|---|---|---|---|---|---|
| Good Outcome | Poor Outcome | SMD | 95% CI | p-Value | I2 Statistics | |||
| Neuron-Specific Enolase (NSE) on day 0 (μg/L) | ||||||||
| All trials | 29 | 28.89 ± 15.46 | 45.96 ± 48.01 | −1.26 | −1.59 to −0.93 | <0.001 | 94% | <0.001 |
| OHCA | 17 | 28.68 ± 15.46 | 47.90 ± 38.19 | −1.36 | −1.93 to −0.78 | <0.001 | 95% | <0.001 |
| Neuron-Specific Enolase (NSE) on day 1 (μg/L) | ||||||||
| All trials | 35 | 25.99 ± 13.34 | 59.83 ± 47.24 | −1.99 | −2.36 to −1.62 | <0.001 | 95% | <0.001 |
| OHCA | 16 | 29.33 ± 14.52 | 73.05 ± 58.66 | −2.25 | −2.90 to −1.60 | <0.001 | 96% | <0.001 |
| Neuron-Specific Enolase (NSE) on day 2 (μg/L) | ||||||||
| All trials | 41 | 21.45 ± 13.05 | 97.29 ± 67.79 | −2.88 | −3.30 to −2.46 | <0.001 | 96% | <0.001 |
| OHCA | 21 | 23.09 ± 14.45 | 112.01 ± 70.53 | −3.39 | −4.08 to −2.71 | <0.001 | 97% | <0.001 |
| Neuron-Specific Enolase (NSE) on day 3 (μg/L) | ||||||||
| All trials | 40 | 22.26 ± 12.43 | 126.83 ± 81.63 | −3.09 | −3.52 to −2.45 | <0.001 | 96% | <0.001 |
| OHCA | 23 | 24.82 ± 14.07 | 139.34 ± 88.02 | −3.04 | −3.62 to −2.46 | <0.001 | 97% | <0.001 |
| Neuron-Specific Enolase (NSE) on day 5 (μg/L) | ||||||||
| All trials | 2 | 15.16 ± 3.43 | 50.37 ± 12.23 | −4.16 | −5.01 to −3.32 | 0.02 | 81% | <0.001 |
| OHCA | 2 | 15.16 ± 3.43 | 50.37 ± 12.23 | −4.16 | −5.01 to −3.32 | 0.02 | 81% | <0.001 |
| Neuron-Specific Enolase (NSE) on day 7 (μg/L) | ||||||||
| All trials | 3 | 15.17 ± 3.99 | 26.74 ± 5.08 | −3.24 | −3.60 to −2.88 | 0.19 | 40% | <0.001 |
| OHCA | 3 | 15.17 ± 3.99 | 26.74 ± 5.08 | −3.24 | −3.60 to −2.88 | 0.19 | 40% | <0.001 |
| Peak of Neuron-Specific Enolae (μg/L) | ||||||||
| All trials | 7 | 27.16 ± 11.23 | 111.66 ± 91.79 | −2.14 | −3.13 to −1.15 | <0.0001 | 98% | <0.001 |
| OHCA | 3 | 29.26 ± 12.80 | 119.53 ± 90.34 | −2.59 | −4.81 to −0.37 | <0.001 | 99% | 0.02 |
| IHCA | 2 | 24.78 ± 4.09 | 105.77 ± 110.66 | −1.89 | −3.28 to −0.49 | <0.001 | 92% | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurek, K.; Swieczkowski, D.; Pruc, M.; Tomaszewska, M.; Cubala, W.J.; Szarpak, L. Predictive Performance of Neuron-Specific Enolase (NSE) for Survival after Resuscitation from Cardiac Arrest: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7655. https://doi.org/10.3390/jcm12247655
Kurek K, Swieczkowski D, Pruc M, Tomaszewska M, Cubala WJ, Szarpak L. Predictive Performance of Neuron-Specific Enolase (NSE) for Survival after Resuscitation from Cardiac Arrest: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(24):7655. https://doi.org/10.3390/jcm12247655
Chicago/Turabian StyleKurek, Krzysztof, Damian Swieczkowski, Michal Pruc, Monika Tomaszewska, Wieslaw Jerzy Cubala, and Lukasz Szarpak. 2023. "Predictive Performance of Neuron-Specific Enolase (NSE) for Survival after Resuscitation from Cardiac Arrest: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 24: 7655. https://doi.org/10.3390/jcm12247655
APA StyleKurek, K., Swieczkowski, D., Pruc, M., Tomaszewska, M., Cubala, W. J., & Szarpak, L. (2023). Predictive Performance of Neuron-Specific Enolase (NSE) for Survival after Resuscitation from Cardiac Arrest: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(24), 7655. https://doi.org/10.3390/jcm12247655






