Blood Haemoglobin Concentration Is Directly and Independently Related with Pulse Wave Velocity, a Measure of Large Artery Stiffness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Laboratory Analyses
2.3. Pulse Wave Analysis
2.4. Whole-Body Impedance Cardiography
2.5. Experimental Protocol
2.6. Statistics
3. Results
3.1. Study Population and Laboratory Values
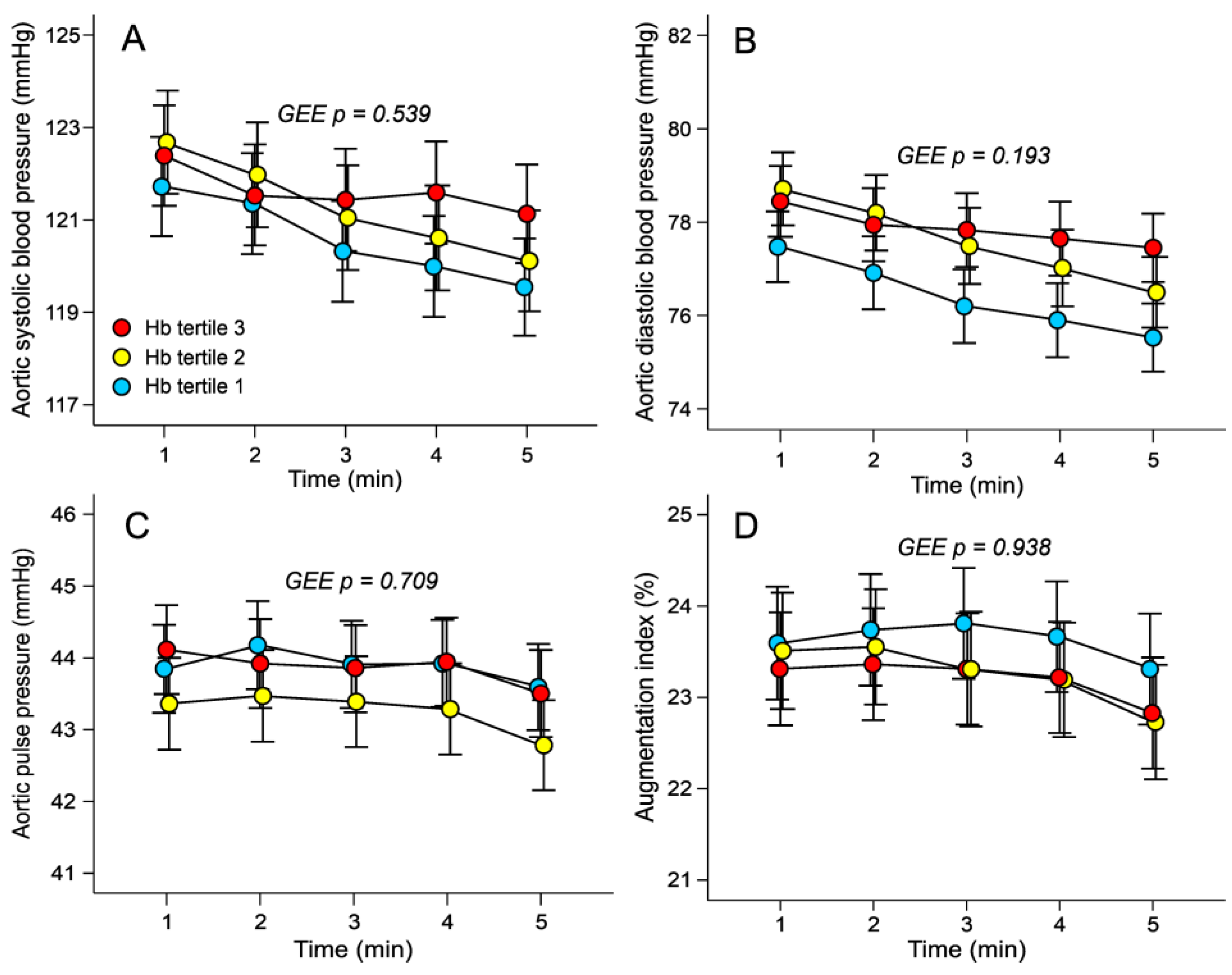
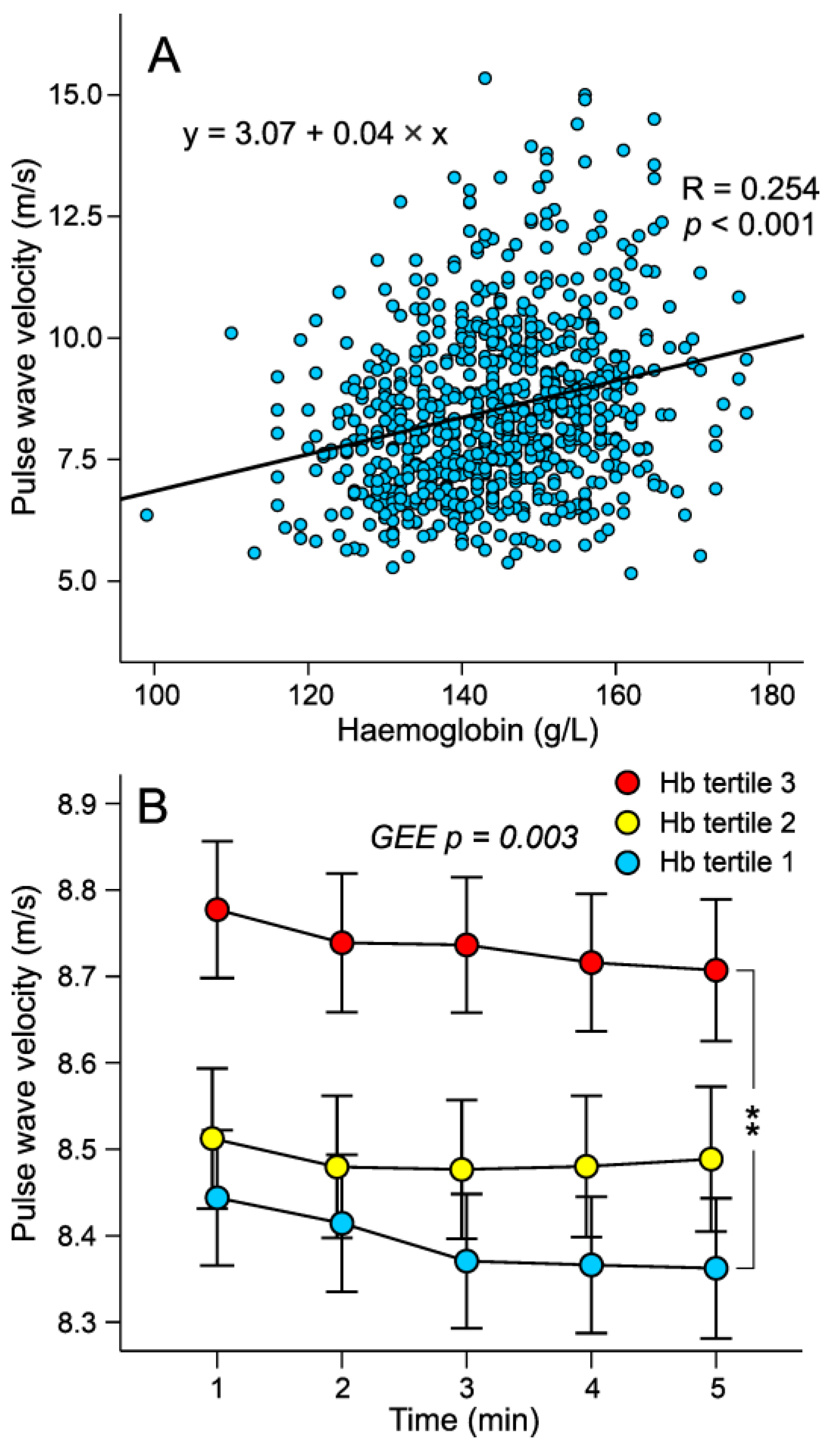
3.2. Haemodynamic Variables in the Tertiles of Haemoglobin Adjusted for Sex
3.3. Factor Influencing Pulse Wave Velocity in Stepwise Linear Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO|Cardiovascular Diseases (CVDs); WHO: Geneva, Switzerland, 2017.
- European Heart Network. European Cardiovascular Disease Statistics. 2017. Available online: https://ehnheart.org/cvd-statistics/cvd-statistics-2017.html (accessed on 18 May 2023).
- Atsma, F.; Veldhuizen, I.; de Kort, W.; van Kraaij, M.; Jong, P.P.-D.; Deinum, J. Hemoglobin Level Is Positively Associated With Blood Pressure in a Large Cohort of Healthy Individuals. Hypertension 2012, 60, 936–941. [Google Scholar] [CrossRef]
- Tapio, J.; Vähänikkilä, H.; Kesäniemi, Y.A.; Ukkola, O.; Koivunen, P. Higher hemoglobin levels are an independent risk factor for adverse metabolism and higher mortality in a 20-year follow-up. Sci. Rep. 2021, 11, 19936. [Google Scholar] [CrossRef]
- Kanbay, M.; Akcay, A.; Delibasi, T.; Uz, B.; Kaya, A.; Koca, C.; Turgut, F.; Bavbek, N.; Uz, E.; Duranay, M.; et al. Comparison of effects of darbepoetin alfa and epoetin alfa on serum endothelin level and blood pressure. Adv. Ther. 2007, 24, 346–352. [Google Scholar] [CrossRef]
- Hoeldtke, R.D.; Streeten, D. Treatment of Orthostatic Hypotension with Erythropoietin. N. Engl. J. Med. 1993, 329, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Lee, J.; Kim, Y.; Kim, K.; Kim, D.; Kim, S.; Shin, D.; Kim, N.; Park, I.; Choi, D.; et al. Relation between insulin resistance and hematological parameters in elderly Koreans—Southwest Seoul (SWS) Study. Diabetes Res. Clin. Prac. 2003, 60, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Facchini, F.S.; Carantoni, M.; Jeppesen, J.; Reaven, G.M. Hematocrit and hemoglobin are independently related to insulin resistance and compensatory hyperinsulinemia in healthy, non-obese men and women. Metabolism 1998, 47, 831–835. [Google Scholar] [CrossRef]
- Winther, S.A.; Finer, N.; Sharma, A.M.; Torp-Pedersen, C.; Andersson, C. Association of anemia with the risk of cardiovascular adverse events in overweight/obese patients. Int. J. Obes. 2014, 38, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.R.; Greenlaw, N.; Ferrari, R.; Ford, I.; Tardif, J.-C.; Tendera, M.; Reid, C.M.; Danchin, N.; Stepinska, J.; Steg, P.G.; et al. Hemoglobin and Change in Hemoglobin Status Predict Mortality, Cardiovascular Events, and Bleeding in Stable Coronary Artery Disease. Am. J. Med. 2017, 130, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.W.; Giles, W.H.; Croft, J.B. Hematocrit and the risk of coronary heart disease mortality. Am. Heart J. 2001, 142, 657–663. [Google Scholar] [CrossRef]
- Kunnas, T.; Solakivi, T.; Huuskonen, K.; Kalela, A.; Renko, J.; Nikkari, S.T. Hematocrit and the risk of coronary heart disease mortality in the TAMRISK study, a 28-year follow-up. Prev. Med. 2009, 49, 45–47. [Google Scholar] [CrossRef]
- Lee, G.; Choi, S.; Kim, K.; Yun, J.; Son, J.S.; Jeong, S.; Kim, S.M.; Park, S.M. Association of Hemoglobin Concentration and Its Change With Cardiovascular and All-Cause Mortality. J. Am. Heart Assoc. 2018, 7, e007723. [Google Scholar] [CrossRef] [PubMed]
- Devesa, A.; Lobo-González, M.; Martínez-Milla, J.; Oliva, B.; García-Lunar, I.; Mastrangelo, A.; España, S.; Sanz, J.; Mendiguren, J.M.; Bueno, H.; et al. Bone marrow activation in response to metabolic syndrome and early atherosclerosis. Eur. Heart J. 2022, 43, 1809–1828. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; O’Rourke, M.F.; Safar, M.E.; Baou, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur. Heart J. 2010, 31, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Butlin, M.; Tan, I.; Avolio, A. Effects of cardiac timing and peripheral resistance on measurement of pulse wave velocity for assessment of arterial stiffness. Sci. Rep. 2017, 7, 5990. [Google Scholar] [CrossRef]
- Devereux, R.B.; Drayer, J.I.; Chien, S.; Pickering, T.G.; Letcher, R.L.; DeYoung, J.L.; Sealey, J.E.; Laragh, J.H. Whole blood viscosity as a determinant of cardiac hypertrophy in systemic hypertension. Am. J. Cardiol. 1984, 54, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Lowe, G.D.O.; Lee, A.J.; Rumley, A.; Price, J.F.; Fowkes, F.G.R. Blood viscosity and risk of cardiovascular events: The Edinburgh Artery Study. Br. J. Haematol. 1997, 96, 168–173. [Google Scholar] [CrossRef]
- Rainer, C.; Kawanishi, D.T.; Chandraratna, P.A.; Bauersachs, R.M.; Reid, C.L.; Rahimtoola, S.H.; Meiselman, H.J. Changes in blood rheology in patients with stable angina pectoris as a result of coronary artery disease. Circulation 1987, 76, 15–20. [Google Scholar] [CrossRef]
- Dormandy, J.A.; Hoare, E.; Colley, J.; Arrowsmith, D.E.; Dormandy, T.L. Clinical, Haemodynamic, Rheological, and Biochemical Findings in 126 Patients with Intermittent Claudication. BMJ 1973, 4, 576–581. [Google Scholar] [CrossRef]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef]
- Fossum, E.; Høieggen, A.; Moan, A.; Nordby, G.; Velund, T.L.; Kjeldsen, S.E. Whole blood viscosity, blood pressure and cardiovascular risk factors in healthy blood donors. Blood Press. 1997, 6, 161–165. [Google Scholar] [CrossRef]
- He, S.; Gu, H.; Yang, J.; Su, Q.; Li, X.; Qin, L. Hemoglobin concentration is associated with the incidence of metabolic syndrome. BMC Endocr. Disord. 2021, 21, 53. [Google Scholar] [CrossRef]
- Kangas, P.; Tikkakoski, A.J.; Tahvanainen, A.M.; Leskinen, M.H.; Viitala, J.M.; Kähönen, M.; Kööbi, T.; Niemelä, O.J.; Mustonen, J.T.; Pörsti, I.H. Metabolic syndrome may be associated with increased arterial stiffness even in the absence of hypertension: A study in 84 cases and 82 controls. Metabolism 2013, 62, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.K.; Eräranta, A.; Tikkakoski, A.J.; Koskela, J.; Hautaniemi, E.J.; Kähönen, M.; Mustonen, J.; Pörsti, I. LDL cholesterol is associated with systemic vascular resistance and wave reflection in subjects naive to cardiovascular drugs. Blood Press. 2019, 28, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F., Jr. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef]
- Young, W.F. Diagnosis and treatment of primary aldosteronism: Practical clinical perspectives. J. Intern. Med. 2019, 285, 126–148. [Google Scholar] [CrossRef]
- Chen, C.H.; Nevo, E.; Fetics, B.; Pak, P.H.; Yin, F.C.; Maughan, W.L.; Kass, D.A. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure: Validation of generalized transfer function. Circulation 1997, 95, 1827–1836. [Google Scholar] [CrossRef]
- Wilenius, M.; Tikkakoski, A.J.; Tahvanainen, A.M.; Haring, A.; Koskela, J.; Huhtala, H.; Kähönen, M.; Kööbi, T.; Mustonen, J.T.; Pörsti, I.H. Central wave reflection is associated with peripheral arterial resistance in addition to arterial stiffness in subjects without antihypertensive medication. BMC Cardiovasc. Disord. 2016, 16, 131. [Google Scholar] [CrossRef]
- Kööbi, T.; Kaukinen, S.; Ahola, T.; Turjanmaa, V.M.H. Non-invasive measurement of cardiac output: Whole-body impedance cardiography in simultaneous comparison with thermodilution and direct oxygen Fick methods. Intensiv. Care Med. 1997, 23, 1132–1137. [Google Scholar] [CrossRef]
- Tahvanainen, A.; Koskela, J.; Tikkakoski, A.; Lahtela, J.; Leskinen, M.; Kähönen, M.; Nieminen, T.; Kööbi, T.; Mustonen, J.; Pörsti, I. Analysis of cardiovascular responses to passive head-up tilt using continuous pulse wave analysis and impedance cardiography. Scand. J. Clin. Lab. Investig. 2009, 69, 128–137. [Google Scholar] [CrossRef]
- Koskela, J.K.; Tahvanainen, A.; Haring, A.; Tikkakoski, A.J.; Ilveskoski, E.; Viitala, J.; Leskinen, M.H.; Lehtimäki, T.; AP Kähönen, M.; Kööbi, T.; et al. Association of resting heart rate with cardiovascular function: A cross-sectional study in 522 Finnish subjects. BMC Cardiovasc. Disord. 2013, 13, 102. [Google Scholar] [CrossRef]
- Kööbi, T.; Kähönen, M.; Iivainen, T.; Turjanmaa, V. Simultaneous non-invasive assessment of arterial stiffness and haemodynamics–a validation study. Clin. Physiol. Funct. Imaging 2003, 23, 31–36. [Google Scholar] [CrossRef]
- Townsend, R.R.; Wilkinson, I.B.; Schiffrin, E.L.; Avolio, A.P.; Chirinos, J.A.; Cockcroft, J.R.; Heffernan, K.S.; Lakatta, E.G.; McEniery, C.M.; Mitchell, G.F.; et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 2015, 66, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Tikkakoski, A.J.; Tahvanainen, A.M.; Leskinen, M.H.; Koskela, J.K.; Haring, A.; Viitala, J.; Kähönen, M.A.; Kööbi, T.; Niemelä, O.; Mustonen, J.T.; et al. Hemodynamic alterations in hypertensive patients at rest and during passive head-up tilt. J. Hypertens. 2013, 31, 906–915. [Google Scholar] [CrossRef] [PubMed]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central blood pressure: Current evidence and clinical importance. Eur. Heart J. 2014, 35, 1719–1725. [Google Scholar] [CrossRef]
- Roman, M.J.; Devereux, R.B.; Kizer, J.R.; Lee, E.T.; Galloway, J.M.; Ali, T.; Umans, J.G.; Howard, B.V. Central Pressure More Strongly Relates to Vascular Disease and Outcome Than Does Brachial Pressure. Hypertension 2007, 50, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, P.; Kong, X.; Mao, W.; Cui, M. Association of hemoglobin with arterial stiffness evaluated by carotid-femoral pulse wave velocity among Chinese adults. Chronic Dis. Transl. Med. 2019, 5, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, R.; Tabara, Y.; Kohara, K.; Miki, T.; Kusunoki, T.; Katoh, T.; Ohtsuka, N.; Takayama, S.; Abe, M. A Slightly Low Hemoglobin Level Is Beneficially Associated with Arterial Stiffness in Japanese Community-Dwelling Women. Clin. Exp. Hypertens. 2012, 34, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Jia, J.; Fan, F.; Zhao, J.; Huo, Y.; Ganesh, S.K.; Zhang, Y. Hemoglobin and erythrocyte count are independently and positively associated with arterial stiffness in a community-based study. J. Hum. Hypertens. 2021, 35, 265–273. [Google Scholar] [CrossRef]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed]
- O’rourke, M.F.; Hashimoto, J. Mechanical Factors in Arterial Aging: A Clinical Perspective. J. Am. Coll. Cardiol. 2007, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kokko, E.; Nevalainen, P.I.; Choudhary, M.K.; Koskela, J.; Tikkakoski, A.; Huhtala, H.; Niemelä, O.; Viukari, M.; Mustonen, J.; Matikainen, N.; et al. Aldosterone-to-renin ratio is related to arterial stiffness when the screening criteria of primary aldosteronism are not met. Sci. Rep. 2020, 10, 19804. [Google Scholar] [CrossRef] [PubMed]
- Bikia, V.; Stergiopulos, N.; Rovas, G.; Pagoulatou, S.; Papaioannou, T.G. The impact of heart rate on pulse wave velocity: An in-silico evaluation. J. Hypertens. 2020, 38, 2451–2458. [Google Scholar] [CrossRef] [PubMed]
- Aladin, A.I.; Al Rifai, M.; Rasool, S.H.; Keteyian, S.J.; Brawner, C.A.; Michos, E.D.; Blaha, M.J.; Al-Mallah, M.H.; McEvoy, J.W. The Association of Resting Heart Rate and Incident Hypertension: The Henry Ford Hospital Exercise Testing (FIT) Project. Am. J. Hypertens. 2016, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Reil, J.-C.; Deedwania, P.; Kim, J.B.; Borer, J.S. Resting heart rate: Risk indicator and emerging risk factor in cardiovascular disease. Am. J. Med. 2015, 128, 219–228. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, E.; Clayton, T.; McEntegart, M.B.; McMurray, J.J.; Lang, C.C.; Roger, S.D.; Young, J.B.; Solomon, S.D.; Granger, C.B.; Ostergren, J.; et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure. Circulation 2006, 113, 986–994. [Google Scholar] [CrossRef]
- Anand, I.S.; Kuskowski, M.A.; Rector, T.S.; Florea, V.G.; Glazer, R.D.; Hester, A.; Chiang, Y.T.; Aknay, N.; Maggioni, A.P.; Opasich, C.; et al. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure. Circulation 2005, 112, 1121–1127. [Google Scholar] [CrossRef]
- McMahon, L.P.; Mason, K.; Skinner, S.L.; Burge, C.M.; Grigg, L.E.; Becker, G.J. Effects of haemoglobin normalization on quality of life and cardiovascular parameters in end-stage renal failure. Nephrol. Dial. Transplant. 2000, 15, 1425–1430. [Google Scholar] [CrossRef]
- Nürnberger, J.; Saez, A.O.; Dammer, S.; Mitchell, A.; Wenzel, R.R.; Philipp, T.; Schäfers, R.F. Left ventricular ejection time: A potential determinant of pulse wave velocity in young, healthy males. J. Hypertens. 2003, 21, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.; Palombo, C.; Salvi, G.M.; Labat, C.; Parati, G.; Benetos, A. Left ventricular ejection time, not heart rate, is an independent correlate of aortic pulse wave velocity. J. Appl. Physiol. 2013, 115, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.; Ragno, E.; Benvenuti, E.; Zito, G.A.; Corsi, A.; Ferrucci, L.; Paolisso, G. New aspects of the insulin resistance syndrome: Impact on haematological parameters. Diabetologia 2001, 44, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Nam, J.S.; Cho, M.H.; Yoo, J.S.; Ahn, C.W.; Jee, S.H.; Lee, H.S.; Cha, B.S.; Kim, K.R.; Lee, H.C. Insulin resistance independently influences arterial stiffness in normoglycemic normotensive postmenopausal women. Menopause 2010, 17, 779–784. [Google Scholar] [CrossRef]
- Kasayama, S.; Saito, H.; Mukai, M.; Koga, M. Insulin sensitivity independently influences brachial-ankle pulse-wave velocity in non-diabetic subjects. Diabet. Med. 2005, 22, 1701–1706. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Chaker, H.; Leaming, R.; Johnson, A.; Brechtel, G.; Baron, A.D. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Investig. 1996, 97, 2601–2610. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Liu, T.; Wang, R. Association between whole blood viscosity and arterial stiffness in patients with type 2 diabetes mellitus. Endocrine 2015, 49, 148–154. [Google Scholar] [CrossRef]
- Levenson, J.; Simon, A.C.; Cambien, F.A.; Beretti, C. Cigarette smoking and hypertension. Factors independently associated with blood hyperviscosity and arterial rigidity. Arteriosclerosis 1987, 7, 572–577. [Google Scholar] [CrossRef]
- Letcher, R.L.; Chien, S.; Pickering, T.G.; Laragh, J.H. Elevated blood viscosity in patients with borderline essential hypertension. Hypertension 1983, 5, 757–762. [Google Scholar] [CrossRef]
- Çınar, Y.; Demir, G.; Paç, M.; Çınar, A.B. Effect of hematocrit on blood pressure via hyperviscosity. Am. J. Hypertens. 1999, 12, 739–743. [Google Scholar] [CrossRef]
- Cabrales, P.; Han, G.; Nacharaju, P.; Friedman, A.J.; Friedman, J.M. Reversal of hemoglobin-induced vasoconstriction with sus-tained release of nitric oxide. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H49–H56. [Google Scholar] [CrossRef]
- Weiss, B.M.; Spahn, D.R.; Rahmig, H.; Rohling, R.; Pasch, T. Radial artery tonometry: Moderately accurate but unpredictable technique of continuous non-invasive arterial pressure measurement. Br. J. Anaesth. 1996, 76, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Nelesen, R.A.; Dimsdale, J.E. Use of radial arterial tonometric continuous blood pressure measurement in cardiovascular reactivity studies. Blood Press. Monit. 2002, 7, 259–263. [Google Scholar] [CrossRef]
- Värri, E.; Suojanen, L.; Koskela, J.K.; Choudhary, M.K.; Tikkakoski, A.; Kähönen, M.; Nevalainen, P.I.; Mustonen, J.; Pörsti, I. Ambulatory daytime blood pressure versus tonometric blood pressure measurements in the laboratory: Effect of posture. Blood Press. Monit. 2023, 28, 199–207. [Google Scholar] [CrossRef]

| Demographics and Clinical Characteristics | Hb Tertile 1 (n = 253) | Hb Tertile 2 (n = 235) | Hb Tertile 3 (n = 255) |
|---|---|---|---|
| Males (%) | 53.8 | 51.0 | 54.1 |
| Age (years) | 46.9 (12.9) | 47.4 (11.5) | 46.8 (11.6) |
| Antihypertensive medication in use (%) | 14.6 | 8.5 | 12.9 |
| Angiotensin-converting enzyme inhibitor (%) | 5.5 | 3.4 | 4.3 |
| Angiotensin receptor blocker (%) | 4.7 | 3.0 | 4.3 |
| Beta blocker (%) | 7.9 | 2.6* | 7.1 |
| Beta and alpha blocker (%) | 0.4 | 0.0 | 0.8 |
| Calcium channel blocker (%) | 6.7 | 3.4 | 4.7 |
| Thiazide (%) | 4.7 | 3.8 | 6.3 |
| Furosemide (%) | 0.8 | 0.0 | 0.8 |
| Potassium-sparing diuretic (%) | 1.6 | 0.4 | 1.2 |
| Prazosin (%) | 0.8 | 0.0 | 0.4 |
| Minoxidil (%) | 0.4 | 0.0 | 0.0 |
| Height (cm) | 173.3 (9.0) | 173.3 (9.8) | 173.5 (9.0) |
| Weight (kg) | 79.9 (16.0) | 81.6 (15.3) | 84.5 (16.8) * |
| Body mass index (kg/m2) | 26.5 (4.4) | 27.1 (4.5) | 27.9 (4.7) * |
| Extracellular water volume (L) | 12.92 (2.02) | 12.98 (1.88) | 13.21 (1.78) |
| Current smokers (%) | 10.7 | 11.5 | 16.5 |
| Alcohol consumption (standard drinks/week) | 3.0 [0.5–7.0] | 2.0 [0.5–6.0] | 3.0 [1.0–6.0] |
| Seated office blood pressure measured by physician | |||
| Systolic (mmHg) | 140 (21) | 141 (20) | 142 (19) |
| Diastolic (mmHg) | 88 (12) | 90 (12) | 91 (11) * |
| Supine brachial blood pressure measured by nurse | |||
| Systolic (mmHg) | 133 (19) | 133 (18) | 134 (18) |
| Diastolic (mmHg) | 80 (11) | 81 (10) | 83 (12) |
| Office blood pressure ≥ 140/90 mmHg † (%) | 55.9 | 61.4 | 64.6 |
| Radial blood pressure in the laboratory ≥ 135/85 mmHg † (%) | 43.9 | 44.3 | 47.4 |
| Aortic blood pressure in the laboratory ≥ 125/85 mmHg † (%) | 39.1 | 39.6 | 42.7 |
| Blood and Plasma Biochemistry | Hb Tertile 1 (n = 253) | Hb Tertile 2 (n = 235) | Hb Tertile 3 (n = 255) |
|---|---|---|---|
| Haemoglobin (g/L) | 135 (9) | 144 (8) * | 154 (9) *† |
| Haematocrit | 0.40 (0.03) | 0.42 (0.02) * | 0.45 (0.03) *† |
| Mean corpuscular haemoglobin (pg) | 30.1 (1.7) | 30.4 (1.4) | 30.8 (1.4) *† |
| Mean corpuscular volume (fl) | 88.9 (4.6) | 88.6 (3.7) | 89.1 (4.2) |
| Erythrocyte count (×1012/L) | 4.50 (0.32) | 4.75/0.32) * | 5.03 (0.38) *† |
| Thrombocyte count (×109/L) | 259 (55) | 263 (255) | 253 (56) |
| Leukocyte count (×109/L) | 5.61 (2.30) | 5.76 (1.33) | 6.33 (1.67) *† |
| Ferritin (µg/L) | 51.5 [22.2–104.9] | 60.5 [26.7–121.1] | 73.5 [39.7–139.3] * |
| Sodium (mmol/L) | 140.6 (2.1) | 140.3 (2.2) | 140.4 (2.1) |
| Potassium (mmol/L) | 3.79 (0.29) | 3.80 (0.30) | 3.79 (0.31) |
| Creatinine (μmol/L) | 74 (13) | 75 (14) | 75 (14) |
| Cystatin C (mg/L) | 0.86 (0.17) | 0.86 (0.16) | 0.88 (0.16) |
| PTH (pmol/L) | 4.6 (1.8) | 4.8 (2.0) | 4.7 (1.9) |
| Phosphate | 0.97 (0.15) | 0.95 (0.17) | 0.96 (0.17) |
| Calcium (mmol/L) | 2.31 (0.11) | 2.31 (0.12) | 2.32 (0.10) |
| 25(OH)D3 (nmol/L) | 74 (38) | 71 (32) | 73 (39) |
| 1,25(OH)2D3 (pmol/L) | 107 (33) | 109 (36) | 105 (30) |
| C-reactive protein (mg/L) | 0.8 [0.5–1.9] | 0.8 [0.5–1.9] | 1.1 [0.5–2.2] *† |
| Uric acid (μmol/L) | 305 (80) | 305 (80) | 325 (75) *† |
| Renin activity (ng Ang I/mL/h) | 0.64 [0.38–1.23] | 0.78 [0.4–1.37] | 1.01 [0.54–1.79] *† |
| Aldosterone (pmol/L) | 416 [301–532] | 429 [323–595] | 489 [373–686] *† |
| Aldosterone to renin ratio | 733 (527) | 696 (460) | 697 (708) |
| Positive screening result for PA (n/%) | 9/3.6% | 11/4.7% | 18/7.0% |
| Total cholesterol (mmol/L) | 5.03 (1.11) | 5.22 (0.98) | 5.22 (1.01) |
| Triglycerides (mmol/L) | 0.99 [0.68–1.42] | 1.07 [0.81–1.55] | 1.17 [0.82–1.75] * |
| HDL cholesterol (mmol/L) | 1.60 (0.48) | 1.57 (0.42) | 1.49 (0.42) * |
| LDL cholesterol (mmol/L) | 2.93 (1.05) | 3.13 (0.89) | 3.19 (0.93) * |
| Glucose (mmol/L) | 5.5 (0.6) | 5.5 (0.6) | 5.6 (0.7) * |
| Insulin (mU/L) | 7.9 (5.7) | 10.5 (26.4) | 10.5 (9.1) |
| QUICKI | 0.359 (0.035) | 0.353 (0.043) | 0.348 (0.047) * |
| Pulse Wave Velocity, Model (R2 = 0.581) | B | Beta | p-Value |
|---|---|---|---|
| (constant) | 1.097 | ||
| Age | 0.074 | 0.478 | <0.001 |
| Mean aortic pressure | 0.024 | 0.178 | <0.001 |
| Uric acid | 0.003 | 0.150 | <0.001 |
| Mean heart rate | 0.028 | 0.148 | <0.001 |
| Aldosterone to renin ratio | 0.00041 | 0.123 | <0.001 |
| Mean ejection duration | −0.009 | −0.100 | 0.008 |
| Triglycerides | 0.278 | 0.098 | 0.001 |
| Smoking (present) | −0.396 | −0.073 | 0.007 |
| Haemoglobin | 0.011 | 0.070 | 0.028 |
| Vitamin 25(OH)D3 | −0.003 | −0.057 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, M.K.; Bouquin, H.; Hytönen, J.; Koskela, J.K.; Niemelä, O.; Nevalainen, P.I.; Mustonen, J.; Pörsti, I. Blood Haemoglobin Concentration Is Directly and Independently Related with Pulse Wave Velocity, a Measure of Large Artery Stiffness. J. Clin. Med. 2023, 12, 7623. https://doi.org/10.3390/jcm12247623
Choudhary MK, Bouquin H, Hytönen J, Koskela JK, Niemelä O, Nevalainen PI, Mustonen J, Pörsti I. Blood Haemoglobin Concentration Is Directly and Independently Related with Pulse Wave Velocity, a Measure of Large Artery Stiffness. Journal of Clinical Medicine. 2023; 12(24):7623. https://doi.org/10.3390/jcm12247623
Chicago/Turabian StyleChoudhary, Manoj Kumar, Heidi Bouquin, Jere Hytönen, Jenni K. Koskela, Onni Niemelä, Pasi I. Nevalainen, Jukka Mustonen, and Ilkka Pörsti. 2023. "Blood Haemoglobin Concentration Is Directly and Independently Related with Pulse Wave Velocity, a Measure of Large Artery Stiffness" Journal of Clinical Medicine 12, no. 24: 7623. https://doi.org/10.3390/jcm12247623
APA StyleChoudhary, M. K., Bouquin, H., Hytönen, J., Koskela, J. K., Niemelä, O., Nevalainen, P. I., Mustonen, J., & Pörsti, I. (2023). Blood Haemoglobin Concentration Is Directly and Independently Related with Pulse Wave Velocity, a Measure of Large Artery Stiffness. Journal of Clinical Medicine, 12(24), 7623. https://doi.org/10.3390/jcm12247623






