Visual Acuity Examination Methodology in Keratoconus
Abstract
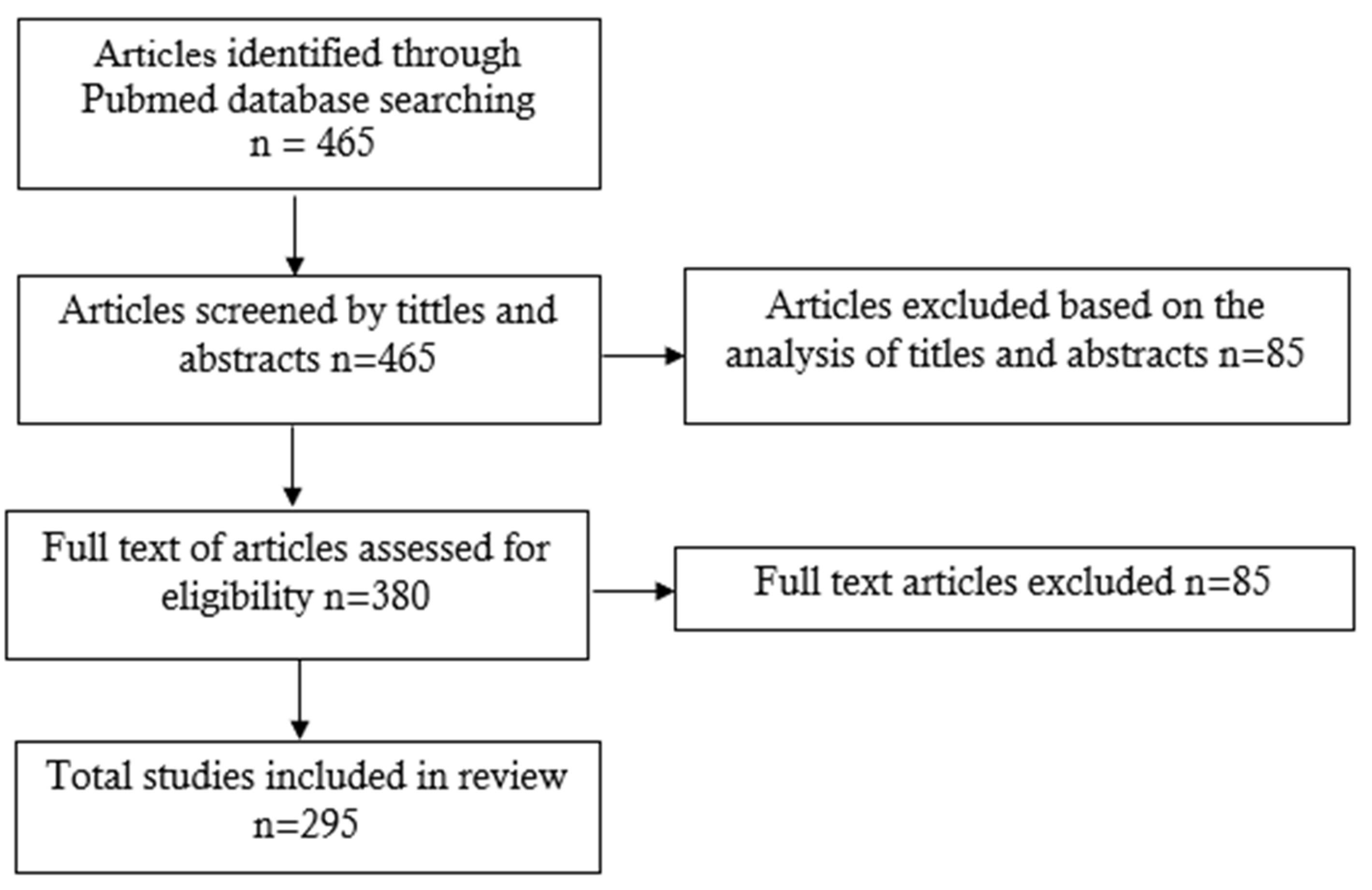
:1. Introduction
2. Materials and Methods
3. Results
3.1. Analysis of Visual Acuity Testing Methodology
3.2. Analysis of Visual Acuity Results Presentation Methodology in Articles
3.3. Conversion of Visual Acuity Values Examined Using Snellen Charts to LogMAR in Articles
3.4. Actual Scale of Practice of Converting Visual Acuity Measured with Snellen Charts to LogMAR Scale
4. Discussion
5. Conclusions
- If possible, use standardized visual acuity charts;
- If you do not use standardized tables to test visual acuity, try to describe the test methodology well. Specify the type of table used (logarithmic or decimal). Provide the manufacturer of the optotype table, etc.;
- If you care about the quality of your examinations, try not to convert visual acuity values tested using Snellen charts into the logMAR scale and vice versa, unless it is necessary;
- If you convert visual acuity values, e.g., from the Snellen fraction to the logMAR scale and vice versa, please inform about it in the text of the article.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kniestedt, C.; Stamper, R.L. Visual acuity and its measurement. Ophthalmol. Clin. N. Am. 2003, 16, 155–170. [Google Scholar] [CrossRef]
- Azzam, D.; Ronquillo, Y. Snellen Chart. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Engin, Ö.; Despriet, D.D.G.; Van Der Meulen-Schot, H.M.; Romers, A.; Slot, X.; Sang, M.T.F.; Fronius, M.; Kelderman, H.; Simonsz, H.J. Comparison of optotypes of Amsterdam Picture Chart with those of Tumbling-E, LEA Symbols, ETDRS, and Landolt-C in non-amblyopic and amblyopic patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 2013–2020. [Google Scholar] [CrossRef]
- Lovie-Kitchin, J.E. Is it time to confine Snellen charts to the annals of history? Ophthalmic Physiol. Opt. 2015, 35, 631–636. [Google Scholar] [CrossRef]
- McMonnies, C.W. Chart construction and letter legibility/readability. Ophthalmic Physiol. Opt. 1999, 19, 498–506. [Google Scholar] [CrossRef]
- Bailey, I.L.; Lovie, J.E. New Design Principles for Visual Acuity Letter Charts. Optom. Vis. Sci. 1976, 53, 740–745. [Google Scholar] [CrossRef]
- Rosser, D.A.; Laidlaw, D.A.H.; Murdoch, I.E. The development of a “reduced logMAR” visual acuity chart for use in routine clinical practice. Br. J. Ophthalmol. 2001, 85, 432–436. [Google Scholar] [CrossRef]
- Ferris, F.L.; Bailey, I. Standardizing the Measurement of Visual Acuity for Clinical Research Studies. Ophthalmology 1996, 103, 181–182. [Google Scholar] [CrossRef]
- McMonnies, C.W.; Ho, A. Letter legibility and chart equivalence. Ophthalmic Physiol. Opt. 2000, 20, 142–152. [Google Scholar] [CrossRef]
- Radner, W. Reading charts in ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1465–1482. [Google Scholar] [CrossRef]
- Hussain, B.; Saleh, G.M.; Sivaprasad, S.; Hammond, C.J. Changing from Snellen to LogMAR: Debate or delay? Clin. Exp. Ophthalmol. 2006, 34, 6–8. [Google Scholar] [CrossRef]
- Pajić, S.P.; Petrović, T.; Stojković, M.; Anđelić, S. Has snellen chart lost the battle to etdrs in cataract surgery visual acuity evaluation? Acta Clin. Croat. 2022, 60, 441–449. [Google Scholar]
- Romero-Jiménez, M.; Santodomingo-Rubido, J.; Wolffsohn, J.S. Keratoconus: A review. Contact Lens Anterior Eye 2010, 33, 157–166. [Google Scholar] [CrossRef]
- Tur, V.M.; MacGregor, C.; Jayaswal, R.; O’Brart, D.; Maycock, N. A review of keratoconus: Diagnosis, pathophysiology, and genetics. Surv. Ophthalmol. 2017, 62, 770–783. [Google Scholar]
- Asimellis, G.; Kaufman, E.J. Keratoconus; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470435/ (accessed on 29 December 2022).
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef]
- Vohra, V.; Tuteja, S.; Gurnani, B.; Chawla, H. Collagen Cross Linking for Keratoconus; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562271/ (accessed on 29 December 2022).
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Santodomingo-Rubido, J.; Carracedo, G.; Suzaki, A.; Villa-Collar, C.; Vincent, S.J.; Wolffsohn, J.S. Keratoconus: An updated review. Contact Lens Anterior Eye 2022, 45, 101559. [Google Scholar] [CrossRef]
- Ansari, M.S.A.; Zulfiqar, N.; Nafees, K.; Shaheen, M. Improvement in Visual Acuity Six Months After Penetrating Keratoplasty In Patients Of Keratoconus. J. Ayub Med. Coll. Abbottabad-Pak. 2021, 33, 289–292. [Google Scholar]
- Muijzer, M.B.; Claessens, J.L.; Cassano, F.; Godefrooij, D.A.; Prevoo, Y.F.; Wisse, R.P. The evaluation of a web-based tool for measuring the uncorrected visual acuity and refractive error in keratoconus eyes: A method comparison study. PLoS ONE 2021, 16, e0256087. [Google Scholar]
- Griševi, S.; Gilevska, F.; Biščević, A.; Ahmedbegović-Pjano, M.; Pidro, A.; Patel, S.; Bohač, M. Cross-linking treatment for better visual acuity. Med. Glas. 2020, 17, 123–128. [Google Scholar]
- Amanzadeh, K.; Elham, R.; Jafarzadepur, E. Effects of single-segment Intacs implantation on visual acuity and corneal topographic indices of keratoconus. J. Curr. Ophthalmol. 2017, 29, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Jafarzadehpur, E.; Mirzajani, A.; Yekta, A.; Khabazkhoob, M. A comparison of the visual acuity outcome between Clearkone and RGP lenses. J. Curr. Ophthalmol. 2018, 30, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Liduma, S.; Luguzis, A.; Krumina, G. The impact of irregular corneal shapeparameters on visual acuity and contrast sensitivity. BMC Ophthalmol. 2020, 20, 466. [Google Scholar] [CrossRef] [PubMed]
- Vejdani, A.; Zarei-Ghanavati, S.; Khakshour, H.; Vejdani, M.; Ghooshkhanei, H. Evaluation of changes in visual acuity, contrast sensitivity and aberrations in patients with keratoconus after corneal collagen cross-linking. J. Ophthalmic Vis. Res. 2017, 12, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Arej, N.; Chanbour, W.; Zaarour, K.; Amro, M.; El-Rami, H.; Harb, F.; Jarade, E. Management of cataract in keratoconus: Early visual outcomes of different treatment modalities. Int. J. Ophthalmol. 2019, 12, 1654–1658. [Google Scholar] [CrossRef]
- Iqbal, M.; Elmassry, A.; E Badawi, A.; Gharieb, H.M.; Said, O.M. Visual and Refractive Long-Term Outcomes Following Standard Cross-Linking in Progressive Keratoconus Management. Clin. Ophthalmol. 2019, 13, 2477–2488. [Google Scholar] [CrossRef]
- Godefrooij, D.A.; Kandoussi, M.E.; Soeters, N.; Wisse, R.P. Higher order optical aberrations and visual acuity in a randomized controlled trial comparing transepithelial versus epithelium-off corneal crosslinking for progressivekeratoconus. Clin. Ophthalmol. 2017, 11, 1931–1936. [Google Scholar] [CrossRef]
- Jabbarvand, M.; Khodaparast, M.; Jamali, A.; Ahmadzadeh, H.; Bordbar, S. Changes in the Optical Corneal Densitometry, Visual Acuity, and Refractive Error after the Annular Intracorneal Inlay Implantation. J. Curr. Ophthalmol. 2021, 33, 23–30. [Google Scholar] [CrossRef]
- Csorba, A.; Kránitz, K.; Dormán, P.; Popper-Sachetti, A.; Kiss, H.; Szalai, I.; Nagy, Z.Z. Factors influencing haze formation and corneal flattening, and the impact ofhaze on visual acuity after conventional collagen cross-linking: A 12-monthretrospective study. BMC Ophthalmol. 2021, 21, 306. [Google Scholar] [CrossRef]
- Amer, I.; Elaskary, A.; Mostafa, A.; Hazem, H.A.; Omar, A.; Abdou, A. Long-Term Visual, Refractive and Topographic Outcomes of “Epi-off” Corneal Collagen Cross-Linkingin Pediatric Keratoconus: Standard versus Accelerated Protocol. Clin. Ophthalmol. 2020, 14, 3747–3754. [Google Scholar] [CrossRef]
- Yüksel, B.; Kandemir, B.; Uzunel, U.D.; Çelik, O.; Ceylan, S.; Küsbeci, T. Comparison of visual and topographic outcomes of deep-anterior lamellar keratoplasty andpenetrating keratoplasty in keratoconus. Int. J. Ophthalmol. 2017, 10, 385–390. [Google Scholar]
- Heikal, M.A.; Abdelshafy, M.; Soliman, T.T.; Hamed, A.M. Refractive and visual outcomesafter Keraring intrastromal corneal ring segment implantation for keratoconusassisted by femtosecond laser at 6 months follow-up. Clin. Ophthalmol. 2016, 11, 81–86. [Google Scholar] [CrossRef] [PubMed]
- El-Khoury, S.; Abdelmassih, Y.; Amro, M.; Dirani, A.; Cherfan, C.; Jarade, E. The Effect of Age on Corneal Topographic Indices, Keratometry and Visual Acuity After Combined Intrastromal Corneal Ring Segment (ICRS) Implantation and Corneal Crosslinking. Med. Hypothesis Discov. Innov. Ophthalmol. 2020, 9, 135–142. [Google Scholar] [PubMed]
- Tognon, T.; Campos, M.; Wengrzynovski, J.P.; Barella, K.A.; Pasqualotti, A.; Martins, L.A.d.B.; Forseto, A.d.S.; de Sousa, L.B. Indications and visual outcomes of intrastromal corneal ring segment implantation in a large patient series. Clinics 2017, 72, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska-Bil, D.; Czarnota-Nowakowska, B.; Krysik, K.; Lyssek-Boroń, A.; Dobrowolski, D.; Grabarek, B.O.; Wylęgała, E. Comparison of Long-Term Outcomes of the Lamellar and Penetrating Keratoplasty Approaches in Patients with Keratoconus. J. Clin. Med. 2021, 10, 2421. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.J.; Abell, R.G.; McGuinness, M.B.; Vajpayee, R.B. Comparative Evaluation of Clinical Characteristics And Visual Outcomes Of Traumatic And Non-Traumatic Graft Dehiscence Following Corneal Transplantation Surgery. Clin. Ophthalmol. 2019, 13, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Krysik, K.; Lyssek-Boron, A.; Janiszewska-Bil, D.; Wylegala, E.; Dobrowolski, D. Impact of ultrasound and optical biometry on refractive outcomes of cataractsurgery after penetrating keratoplasty in keratoconus. Int. J. Ophthalmol. 2019, 18, 949–953. [Google Scholar]
- Buzzonetti, L.; Petrocelli, G.; Valente, P.; Petroni, S.; Parrilla, R.; Iarossi, G. Refractive outcome of keratoconus treated by big-bubble deep anterior lamellarkeratoplasty in pediatric patients: Two-year follow-up comparison betweenmechanical trephine and femtosecond laser assisted techniques. Eye Vis. 2019, 6, 1–5. [Google Scholar] [CrossRef]
- Bozkurt, E.; Ozgurhan, E.B.; Akcay, B.I.S.; Kurt, T.; Yildirim, Y.; Günaydin, Z.K.; Demirok, A. Refractive, Topographic, and Aberrometric Results at 2-Year Follow-Up forAccelerated Corneal Cross-Link for Progressive Keratoconus. J. Ophthalmol. 2017, 2017, 5714372. [Google Scholar] [CrossRef]
- Hashemian, S.J.; Farshchian, N.; Foroutam-Jazi, A.; Jafari, M.E.; Hashemian, M.S.; Hashemian, S.M. Visual and Refractive Outcomes and Tomographic Changes afterFemtosecond Laser-assisted Intrastromal Corneal Ring Segment Implantation inPatients with Keratoconus. J. Ophthalmic Vis. Res. 2018, 13, 376–382. [Google Scholar] [CrossRef]
- Nicula, C.; Nicula, D.; Suciu, C. Results in keratoconus correction with Kerasoft 3 Contact lenses. Rom. J. Ophthalmol. 2020, 64, 122–127. [Google Scholar] [CrossRef]
- Williams, M.A.; Moutray, T.N.; Jackson, A.J. Uniformity of Visual Acuity Measures in Published Studies. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4321–4327. [Google Scholar] [CrossRef] [PubMed]
- Daiber, H.F.; Gnugnoli, D.M. Visual Acuity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hannah, J.Y.; Kaiser, P.K.; Zamora, D.; Bocanegra, M.; Cone, C.; Brown, D.M.; Sadda, S.R.; Wykoff, C.C. Visual Acuity Variability: Comparing Discrepancies between Snellen and ETDRS Measurements among Subjects Entering Prospective Trials. Ophthalmol. Retin. 2021, 5, 224–233. [Google Scholar]
- Kaiser, P.K. Prospective evaluation of visual acuity assessment: A comparison of snellen versus ETDRS charts in clinical practice (An AOS Thesis). Trans. Am. Ophthalmol. Soc. 2009, 107, 311–324. [Google Scholar] [PubMed]
- Falkenstein, I.A.; Cochran, D.E.; Azen, S.P.; Dustin, L.; Tammewar, A.M.; Kozak, I.; Freeman, W.R. Comparison of Visual Acuity in Macular Degeneration Patients Measured with Snellen and Early Treatment Diabetic Retinopathy Study Charts. Ophthalmology 2008, 115, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.W.; Josic, K.; Maguire, M.G.; Jampol, L.M.; Martin, D.F.; Rofagha, S.; Sun, J.K.; DRCR Retina Network. Comparison of Snellen Visual Acuity Measurements in Retinal Clinical Practice to Electronic ETDRS Protocol Visual Acuity Assessment. Ophthalmology 2023, 130, 533–541. [Google Scholar] [CrossRef]
- Kalpana, S.K.J.; Jayarajini, S. Comparison of Static Visual Acuity between Snellen and Early Treatment Diabetic Retinopathy Study Charts. Int. J. Educ. Res. Dev. 2013, 2, 82–88. [Google Scholar]
- Elliott, D.B. The good (logMAR), the bad (Snellen) and the ugly (BCVA, number of letters read) of visual acuity measurement. Ophthalmic Physiol. Opt. 2016, 36, 355–358. [Google Scholar] [CrossRef]
- Rakhshandadi, T.; Sedaghat, M.R.; Askarizadeh, F.; Momeni-Moghaddam, H.; Khabazkhoob, M.; Yekta, A. Refractive characteristics of keratoconus eyes with corneal Vogt’s striae: A contralateral eye study. J. Optometry 2021, 14, 183–188. [Google Scholar] [CrossRef]
- Salabati, M.; Huang, C.; Kamalipour, A.; Hannah, J.Y.; Mahmoudzadeh, R.; Jeng-Miller, K.; Chen, E.; Shah, C.P.; Wykoff, C.C.; Hsu, J. Magnitude of Visual Acuity Change with ETDRS versus Snellen Testing in Clinical Trials: Implications for Clinic-Based Outcomes. Ophthalmol. Sci. 2024, 4, 100372. [Google Scholar] [CrossRef]
| Type of Chart | Number of Papers |
|---|---|
| Snellen | 103 |
| ETDRS | 35 |
| Tumbling E | 2 |
| Landolt C | 2 |
| Bailey–Lovie | 1 |
| Sloan | 1 |
| COMPlog, Medisoft Inc. | 2 |
| Not specified | 150 |
| Methods of Presenting the Results of Visual Acuity Testing | Number of Papers |
|---|---|
| LogMAR scale | 215 |
| Fraction or decimal scale | 62 |
| Increments of letters or rows of letters | 7 |
| LogMAR scale and fraction or decimal scale | 11 |
| Number of Papers in Which Acuity Tested with Snellen Chart Was Converted to LogMAR Scale | Number of Other Papers |
|---|---|
| 68 | 227 |
| Number of Papers | |
|---|---|
| Publications in which visual acuity was tested using the Snellen chart and was converted to logMAR | 68 |
| Publications in which the testing methodology was not stated but the result was presented on the logMAR scale (converted or tested using ETDRS?) | 106 |
| Other publications | 121 |
| Article | Design | Number of Participants | Chart Used, Results |
|---|---|---|---|
| Aneeq Ansari et al. [20] | retrospective | 65 eyes of 65 patients | Snellen chart, results in fraction notation |
| Muijzer et al. [21] | prospective | 100 eyes of 50 patients | ETDRS chart, results in logMAR scale |
| Grišević et al. [22] | prospective | 44 eyes of 34 patients | Snellen chart, results in decimal notation |
| Amanzadeh et al. [23] | prospective | 42 eyes of 32 patients | visual acuity test method not indicated, results in logMAR scale |
| Hassani et al. [24] | prospective | 28 eyes | visual acuity test method not indicated, results in logMAR scale |
| Liduma et al. [25] | prospective | 77 eyes of 44 patients | visual acuity test method not indicated, results in decimal notation |
| Zarei-Ghanavati et al. [26] | prospective | 22 eyes of 11 patients | ETDRS chart, results in logMAR scale |
| Arej et al. [27] | retrospective | 31 eyes | ETDRS chart, results in logMAR scale |
| Iqbal et al. [28] | retrospective | 28 patients | visual acuity test method not indicated, results in logMAR scale |
| Godeefrooij et al. [29] | prospective | 61 patients | visual acuity test method not indicated, results in logMAR scale |
| Jabbarvand et al. [30] | retrospective | 34 patients | Snellen chart, visual acuity converted to logMAR, results in logMAR scale |
| Csorba et al. [31] | retrospective | 47 eyes of 47 patients | Snellen chart, visual acuity converted to logMAR, results in logMAR scale |
| Amer et al. [32] | prospective | 68 eyes of 35 pediatric patients | Snellen chart, results in decimal notation |
| Yüksel et al. [33] | prospective | 76 eyes of 71 patients | visual acuity test method not indicated, results in logMAR scale |
| Heikal et al. [34] | retrospective | 30 eyes of 20 patients | visual acuity test method not indicated, results in logMAR scale |
| El-Khoury et al. [35] | retrospective | 26 eyes of patients ≤ 18 years, and 26 eyes of adult patients as controls. | visual acuity test method not indicated, results in logMAR scale |
| Tognon et al. [36] | retrospective | 1222 eyes of 1196 patients | Snellen chart, visual acuity converted to logMAR, results in logMAR scale |
| Janiszewska-Bil et al. [37] | prospective | 90 eyes of 90 patients | Snellen chart, results in decimal notation and logMAR scale |
| Stevenson et al. [38] | retrospective | 71 eyes of 71 patients | Snellen chart, visual acuity converted to logMAR, results in logMAR scale |
| Krysik et al. [39] | retrospective | 42 eyes of 34 patients | Snellen chart, results in decimal notation |
| Buzzonetti et al. [40] | retrospective | 20 eyes of 20 patient | visual acuity test method not indicated, results in logMAR scale |
| Bozkurt et al. [41] | retrospective | 47 eyes of 41 patients | ETDRS chart, results in logMAR scale |
| Hashemian et al. [42] | prospective | 71 eyes of 52 patients | visual acuity test method not indicated, results in logMAR scale and fraction notation |
| Nicula et al. [43] | retrospective | 61 eyes of 35 patients | Snellen chart, results in logMAR scale |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandzik, M.; Wylęgała, E.; Wylęgała, A.; Szkodny, D.; Roszkowska, A.M.; Wróblewska-Czajka, E. Visual Acuity Examination Methodology in Keratoconus. J. Clin. Med. 2023, 12, 7620. https://doi.org/10.3390/jcm12247620
Nandzik M, Wylęgała E, Wylęgała A, Szkodny D, Roszkowska AM, Wróblewska-Czajka E. Visual Acuity Examination Methodology in Keratoconus. Journal of Clinical Medicine. 2023; 12(24):7620. https://doi.org/10.3390/jcm12247620
Chicago/Turabian StyleNandzik, Magdalena, Edward Wylęgała, Adam Wylęgała, Dominika Szkodny, Anna Maria Roszkowska, and Ewa Wróblewska-Czajka. 2023. "Visual Acuity Examination Methodology in Keratoconus" Journal of Clinical Medicine 12, no. 24: 7620. https://doi.org/10.3390/jcm12247620
APA StyleNandzik, M., Wylęgała, E., Wylęgała, A., Szkodny, D., Roszkowska, A. M., & Wróblewska-Czajka, E. (2023). Visual Acuity Examination Methodology in Keratoconus. Journal of Clinical Medicine, 12(24), 7620. https://doi.org/10.3390/jcm12247620






