Mesenchymal Stromal Cell Healing Outcomes in Clinical and Pre-Clinical Models to Treat Pressure Ulcers: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Collection Process
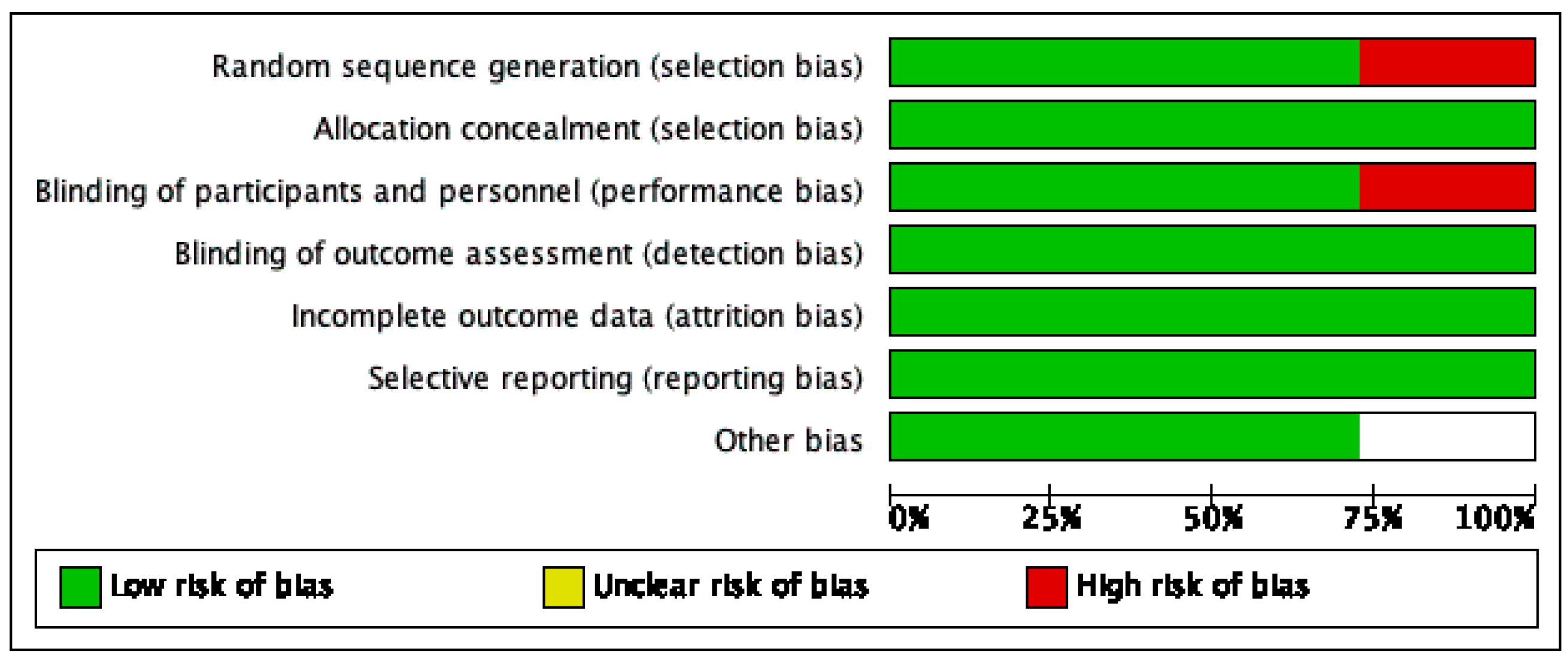
2.4. Risk of Bias of Individual and Across Studies
3. Results
3.1. Study Selection and Characteristics
3.2. Use of MSCs to Treat Pressure Ulcers in Pre-Clinical Studies
3.3. Use of MSCs to Treat Pressure Ulcers in Clinical Trials
4. Discussion
4.1. MSC Mechanisms in the Treatment of Pressure Ulcers
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal stromal cell |
| BMSC | Bone-marrow-derived stromal cell |
| ADSC | Adipose-derived stromal cell |
| cADSCs | Cryopreserved adipose-derived stem cells |
| hUC-MSCs | Human umbilical cord mesenchymal stromal cells |
| hAEC | Human amniotic epithelial cell |
| I/R | Ischemia-reperfusion |
| TGF-3 | Transforming growth factor B3 |
| dhADSC | Human adipose-derived stromal cells extracted from diabetic subjects |
| nhADSCs | Human adipose-derived stromal cells extracted from nondiabetic subjects |
| Lcn2 | Lipocalin 2 |
| VDR | Vitamin D receptor |
| HGF | Hepatocyte growth factor |
| VEGF | Vascular endothelial growth factor |
| TNF | Tumor necrosis factor |
| TSG-6 | Tumor necrosis factor stimulated gene-6 |
| IL-1RA | Interleukin-1 receptor antagonist |
| NFkB | Nuclear factor kappa B |
References
- Cushing, C.A.; Phillips, L.G. Evidence-based medicine: Pressure sores. Plast. Reconstr. Surg. 2013, 132, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Gill, S.S.; Rochon, P.A. Preventing Pressure Ulcers: A Systematic Review. JAMA 2006, 296, 974–984. [Google Scholar] [CrossRef]
- Woodbury, M.G.; Houghton, P.E. Prevalence of Pressure Ulcers in Canadian Healthcare Settings. Ostomy Wound Manag. 2004, 50, 22–39. [Google Scholar]
- Bauer, K.; Rock, K.; Nazzal, M.; Jones, O.; Qu, W. Pressure Ulcers in The United States’ Inpatient Population from 2008 to 2012: Results of a Retrospective Nationwide Study. Ostomy Wound Manag. 2016, 62, 30–38. [Google Scholar]
- Xiao, S.; Liu, Z.; Yao, Y.; Wei, Z.R.; Wang, D.; Deng, C. Diabetic Human Adipose-Derived Stem Cells Accelerate Pressure Ulcer Healing by Inducing Angiogenesis and Neurogenesis. Stem Cells Dev. 2019, 28, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, A.; Nagpal, P.S.; Chhabra, H.S. Experimental Animal Modelling for Pressure Injury: A Systematic Review. J. Clin. Orthop. Trauma 2021, 17, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Pelizzo, G.; Avanzini, M.A.; Mantelli, M.; Croce, S.; Maltese, A.; Vestri, E.; De Silvestri, A.; Percivalle, E.; Calcaterra, V. Granulation Tissue-Derived Mesenchymal Stromal Cells: A Potential Application for Burn Wound Healing in Pediatric Patients. J. Stem Cells Regen. Med. 2018, 14, 53–58. [Google Scholar]
- Portas, M.; Mansilla, E.; Drago, H.; Dubner, D.; Radl, A.; Coppola, A.; Di Giorgio, M. Use Of Human Cadaveric Mesenchymal Stem Cells for Cell Therapy of a Chronic Radiation-Induced Skin Lesion: A Case Report. Radiat. Prot. Dosim. 2016, 171, 99–106. [Google Scholar] [CrossRef]
- Wu, Q.; Lei, X.; Chen, L.; Zheng, Y.; Huang, H.; Qian, C.; Liang, Z. Autologous Platelet-Rich Gel Combined with In Vitro Amplification of Bone Marrow Mesenchymal Stem Cell Transplantation to Treat the Diabetic Foot Ulcer: A Case Report. Ann. Transl. Med. 2018, 6, 307. [Google Scholar] [CrossRef]
- Kühl, T.; Mezger, M.; Hausser, I.; Handgretinger, R.; Bruckner-Tuderman, L.; Nyström, A. High Local Concentrations of Intradermal Mscs Restore Skin Integrity and Facilitate Wound Healing in Dystrophic Epidermolysis Bullosa. Mol. Ther. 2015, 23, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C.; Gagne, P.J.; Jacobowitz, G.R.; Levine, J.P.; Gurtner, G.C. Human Endothelial Progenitor Cells from Type II Diabetics Exhibit Impaired Proliferation, Adhesion, and Incorporation into Vascular Structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef]
- Strong, A.L.; Bowles, A.C.; Maccrimmon, C.P.; Frazier, T.P.; Lee, S.J.; Wu, X.; Katz, A.J.; Gawronska-Kozak, B.; Bunnell, B.A.; Gimble, J.M. Adipose Stromal Cells Repair Pressure Ulcers in Both Young and Elderly Mice: Potential Role of Adipogenesis in Skin Repair. Stem Cells Transl. Med. 2015, 4, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal Cells from The Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/Stem Cells: A Joint Statement of The International Federation for Adipose Therapeutics and Science (Ifats) and The International Society for Cellular Therapy (Isct). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Mcintosh, K.R.; Frazier, T.; Rowan, B.G.; Gimble, J.M. Evolution and Future Prospects of Adipose-Derived Immunomodulatory Cell Therapeutics. Expert. Rev. Clin. Immunol. 2013, 9, 175–184. [Google Scholar] [CrossRef] [PubMed]
- De La Garza-Rodea, A.S.; Knaan-Shanzer, S.; Van Bekkum, D.W. Pressure Ulcers: Description of a New Model and Use OF Mesenchymal Stem Cells for Repair. Dermatology 2011, 223, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Jiang, Z.X.; Zhou, A.T.; Yu, L.M.; Quan, M.T.; Cheng, H.G. Pathologic Changes of Wound Tissue in Rats with Stage III Pressure Ulcers Treated by Transplantation of Human Amniotic Epithelial Cells. Int. J. Clin. Exp. Pathol. 2015, 8, 12284–12291. [Google Scholar]
- Motegi, S.; Sekiguchi, A.; Uchiyama, A.; Uehara, A.; Fujiwara, C.; Yamazaki, S.; Perera, B.; Nakamura, H.; Ogino, S.; Yokoyama, Y.; et al. Protective Effect of Mesenchymal Stem Cells on The Pressure Ulcer Formation by The Regulation of Oxidative and Endoplasmic Reticulum Stress. Sci. Rep. 2017, 7, 17186. [Google Scholar] [CrossRef]
- Feldman, D.S.; Mccauley, J.E. Mesenchymal Stem Cells and Transforming Growth Factor-(3) (Tgf-(3)) to Enhance The Regenerative Ability of an Albumin Scaffold in Full Thickness Wound Healing. J. Funct. Biomater. 2018, 9, 65. [Google Scholar] [CrossRef]
- Bukowska, J.; Alarcon Uquillas, A.; Wu, X.; Frazier, T.; Walendzik, K.; Vanek, M.; Gaupp, D.; Bunnell, B.A.; Kosnik, P.; Mehrara, B.; et al. Safety and Efficacy of Human Adipose-Derived Stromal/Stem Cell Therapy in an Immunocompetent Murine Pressure Ulcer Model. Stem Cells Dev. 2020, 29, 440–451. [Google Scholar] [CrossRef]
- Pourmohammadi-Bejarpasi, Z.; Sabzevari, R.; Mohammadi Roushandeh, A.; Ebrahimi, A.; Mobayen, M.; Jahanian-Najafabadi, A.; Darjani, A.; Habibi Roudkenar, M. Combination Therapy of Metadichol Nanogel and Lipocalin-2 Engineered Mesenchymal Stem Cells Improve Wound Healing in Rat Model of Excision Injury. Adv. Pharm. Bull. 2022, 12, 550–560. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Mitsuno, H.; Nonaka, I.; Sen, Y.; Kawanishi, K.; Inada, Y.; Takakura, Y.; Okuchi, K.; Nonomura, A. Wound Therapy by Marrow Mesenchymal Cell Transplantation. Plast. Reconstr. Surg. 2008, 121, 860–877. [Google Scholar] [CrossRef]
- Sarasua, J.G.; Lopez, S.P.; Viejo, M.A.; Basterrechea, M.P.; Rodriguez, A.F.; Gutierrez, A.F.; Gala, J.G.; Menendez, Y.M.; Augusto, D.E.; Arias, A.P.; et al. Treatment of Pressure Ulcers with Autologous Bone Marrow Nuclear Cells in Patients with Spinal Cord Injury. J. Spinal Cord Med. 2011, 34, 301–307. [Google Scholar] [CrossRef]
- Wettstein, R.; Savic, M.; Pierer, G.; Scheufler, O.; Haug, M.; Halter, J.; Gratwohl, A.; Baumberger, M.; Schaefer, D.J.; Kalbermatten, D.F. Progenitor Cell Therapy for Sacral Pressure Sore: A Pilot Study with a Novel Human Chronic Wound Model. Stem Cell Res. Ther. 2014, 5, 7. [Google Scholar] [CrossRef]
- Feldman, D. Designing A Biomaterial Approach to Control the Adaptive Response to a Skin Injury. Materials 2022, 15, 6366. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Mishra, R.K. Pressure Ulcers: Current Understanding and Newer Modalities of Treatment. Indian J. Plast. Surg. 2015, 48, 4–16. [Google Scholar] [CrossRef]
- Moreno-Camacho, C.A.; Montoya-Torres, J.R.; Jaegler, A.; Gondran, N. Sustainability Metrics For Real Case Applications of the Supply Chain Network Design Problem: A Systematic Literature Review. J. Clean. Prod. 2019, 231, 600–618. [Google Scholar] [CrossRef]
- Schatteman, G.C.; Ma, N. Old Bone Marrow Cells Inhibit Skin Wound Vascularization. Stem Cells 2006, 24, 717–721. [Google Scholar] [CrossRef]
- Nayeri, F.; Xu, J.; Abdiu, A.; Nayeri, T.; Aili, D.; Liedberg, B.; Carlsson, U. Autocrine Production of Biologically Active Hepatocyte Growth Factor (Hgf) by Injured Human Skin. J. Dermatol. Sci. 2006, 43, 49–56. [Google Scholar] [CrossRef]
- Amos, P.J.; Kapur, S.K.; Stapor, P.C.; Shang, H.; Bekiranov, S.; Khurgel, M.; Rodeheaver, G.T.; Peirce, S.M.; Katz, A.J. Human Adipose-Derived Stromal Cells Accelerate Diabetic Wound Healing: Impact of Cell Formulation and Delivery. Tissue Eng. Part. A 2010, 16, 1595–1606. [Google Scholar] [CrossRef]
- Kilroy, G.E.; Foster, S.J.; Wu, X.; Ruiz, J.; Sherwood, S.; Heifetz, A.; Ludlow, J.W.; Stricker, D.M.; Potiny, S.; Green, P.; et al. Cytokine Profile of Human Adipose-Derived Stem Cells: Expression of Angiogenic, Hematopoietic, and Pro-Inflammatory Factors. J. Cell. Physiol. 2007, 212, 702–709. [Google Scholar] [CrossRef]
- Uchiyama, A.; Motegi, S.I.; Sekiguchi, A.; Fujiwara, C.; Perera, B.; Ogino, S.; Yokoyama, Y.; Ishikawa, O. Mesenchymal Stem Cells-Derived Mfg-E8 Accelerates Diabetic Cutaneous Wound Healing. J. Dermatol. Sci. 2017, 86, 187–197. [Google Scholar] [CrossRef]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.A.; Wei, L. Transplantation of Hypoxia-Preconditioned Mesenchymal Stem Cells Improves Infarcted Heart Function via Enhanced Survival of Implanted Cells and Angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [CrossRef]
- Michaels, J.T.; Churgin, S.S.; Blechman, K.M.; Greives, M.R.; Aarabi, S.; Galiano, R.D.; Gurtner, G.C. Db/Db Mice Exhibit Severe Wound-Healing Impairments Compared with Other Murine Diabetic Strains in a Silicone-Splinted Excisional Wound Model. Wound Repair. Regen. 2007, 15, 665–670. [Google Scholar] [CrossRef]
- Keswani, S.G.; Katz, A.B.; Lim, F.Y.; Zoltick, P.; Radu, A.; Alaee, D.; Herlyn, M.; Crombleholme, T.M. Adenoviral Mediated Gene Transfer of Pdgf-B Enhances Wound Healing in Type I and Type II Diabetic Wounds. Wound Repair. Regen. 2004, 12, 497–504. [Google Scholar] [CrossRef]
- Langer, S.; Born, F.; Breidenbach, A.; Schneider, A.; Uhl, E.; Messmer, K. Effect Of C-Peptide on Wound Healing and Microcirculation in Diabetic Mice. Eur. J. Med. Res. 2002, 7, 502–508. [Google Scholar]
- Greenwald, D.P.; Shumway, S.; Zachary, L.S.; Labarbera, M.; Albear, P.; Temaner, M.; Gottlieb, L.J. Endogenous Versus Toxin-Induced Diabetes in Rats: A Mechanical Comparison of Two Skin Wound-Healing Models. Plast. Reconstr. Surg. 1993, 91, 1087–1093. [Google Scholar] [CrossRef]
- Trinh, N.T.; Yamashita, T.; Ohneda, K.; Kimura, K.; Salazar, G.T.; Sato, F.; Ohneda, O. Increased Expression of Egr-1 in Diabetic Human Adipose Tissue-Derived Mesenchymal Stem Cells Reduces Their Wound Healing Capacity. Stem Cells Dev. 2016, 25, 760–773. [Google Scholar] [CrossRef]
- Zografou, A.; Papadopoulos, O.; Tsigris, C.; Kavantzas, N.; Michalopoulos, E.; Chatzistamatiou, T.; Papassavas, A.; Stavropoulou-Gioka, C.; Dontas, I.; Perrea, D. Autologous Transplantation of Adipose-Derived Stem Cells Enhances Skin Graft Survival and Wound Healing in Diabetic Rats. Ann. Plast. Surg. 2013, 71, 225–232. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Szlapka-Kosarzewska, J.; Weiss, C. Excessive Endoplasmic Reticulum Stress Correlates with Impaired Mitochondrial Dynamics, Mitophagy and Apoptosis, in Liver and Adipose Tissue, but not in Muscles in Ems Horses. Int. J. Mol. Sci. 2018, 19, 165. [Google Scholar] [CrossRef]
- Kornicka, K.; Houston, J.; Marycz, K. Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use. Stem Cell Rev. Rep. 2018, 14, 337–345. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Basinska, K.; Czyrek, A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into Eqascs Isolated from Ems Horses in the Context of Their Aging. Oxid. Med. Cell. Longev. 2016, 2016, 4710326. [Google Scholar] [CrossRef]
- Kornicka, K.; Marycz, K.; Marędziak, M.; Tomaszewski, K.A.; Nicpoń, J. The Effects of the DNA Methyltranfserases Inhibitor 5-Azacitidine on Ageing, Oxidative Stress and DNA Methylation of Adipose Derived Stem Cells. J. Cell. Mol. Med. 2017, 21, 387–401. [Google Scholar] [CrossRef]
- Marycz, K.; Kornicka, K.; Irwin-Houston, J.M.; Weiss, C. Combination of Resveratrol and 5-Azacytydine improves Osteogenesis of Metabolic Syndrome Mesenchymal Stem Cells. J. Cell. Mol. Med. 2018, 22, 4771–4793. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Gou, M.; Da, L.C.; Zhang, W.Q.; Xie, H.Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part. B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef]
- Sanchez-Diaz, M.; Quiñones-Vico, M.I.; Sanabria De La Torre, R.; Montero-Vílchez, T.; Sierra-Sánchez, A.; Molina-Leyva, A.; Arias-Santiago, S. Biodistribution of Mesenchymal Stromal Cells After Administration in Animal Models and Humans: A Systematic Review. J. Clin. Med. 2021, 10, 2925. [Google Scholar] [CrossRef]
- Sanabria-De La Torre, R.; Quiñones-Vico, M.I.; Fernández-González, A.; Sánchez-Díaz, M.; Montero-Vílchez, T.; Sierra-Sánchez, Á.; Arias-Santiago, S. Alloreactive Immune Response Associated to Human Mesenchymal Stromal Cells Treatment: A Systematic Review. J. Clin. Med. 2021, 10, 2991. [Google Scholar] [CrossRef]


| Model/Participants | Animal | Type of Study | Type of Cell | Number of Cells Administered | Method of Cell Administration | Reference |
|---|---|---|---|---|---|---|
| 19 men and 3 women with type IV chronic pressure ulcers; mean age: 56.41 years (range 29–79 years) | Human | Clinical | Human BMSCs | ≈60 million cells | Intradermal injection in ulcer margins | [24] |
| 9 men and 11 women; average age, 64.8 years; range, 22 to 91 years (n = 20) | Human | Clinical | Human BMSCs | 1.0 × 105 cells/mL | Artificial dermis composite graft | [23] |
| Complete para- or tetraplegic patients with a primary sacral pressure sore grade III–IV (n = 36) | Human | Clinical | hBMSCs | Between 17.4 × 106 cells and 25.5 × 106 cells/mL | Intradermal injection half of the ulcer | [25] |
| Female C57BL/6 wild-type mice (aged 6–8 weeks) n = 24 | Mice | Pre-clinical | Human ADSCs | 1.0 × 106 cells | Intradermal injection in ulcer margins | [5] |
| Male and female C57BL/6 mice (aged 8 weeks to 22 months old) | Mice | Pre-clinical | Human ADSCs | Not stated | Intradermal injection in ulcer margins | [21] |
| Male and female NOD/SCID mice (aged 8–12 weeks). | Mice | Pre-clinical | Human BMSCs | 5.0 × 105 cells | Intradermal injection in ulcer margins | [17] |
| OKD48 mice with a transgene encoding a modified nuclear factor (erythroid-derived 2)-related factor 2 (Nrf2) protein | Mice | Pre-clinical | BMSCs | 2.0 × 106 cells/200 μL | Intradermal injection in ulcer margins | [19] |
| Female C57BL/6 wild-type mice | Mice | Pre-clinical | ADSCs | Between 1.0 × 105 cells and 1.0 × 106 cells | Intradermal injection in ulcer margins | [13] |
| New Zealand White rabbits weighing approximately 3 kg. | Rabbit | Pre-clinical | BMSCs | 4.0 × 105 cells/mL | Intradermal injection in ulcer margins | [26] |
| Male Wistar rats weighing 200–220 g (n = 74) | Rats | Pre-clinical | hUC-MSCs | 1.0 × 106 cells | Intradermal injection in ulcer margins | [22] |
| Male Sprague–Dawley rats, weighing between 120 and 150 g with stage III pressure ulcers (n = 96) | Rats | Pre-clinical | hAECs | 5.0 × 105 cells/mL | Subcutaneous injection | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Guzman, R.A.; Avila, F.R.; Maita, K.; Garcia, J.P.; De Sario, G.D.; Borna, S.; Eldaly, A.S.; Quinones-Hinojosa, A.; Zubair, A.C.; Ho, O.A.; et al. Mesenchymal Stromal Cell Healing Outcomes in Clinical and Pre-Clinical Models to Treat Pressure Ulcers: A Systematic Review. J. Clin. Med. 2023, 12, 7545. https://doi.org/10.3390/jcm12247545
Torres-Guzman RA, Avila FR, Maita K, Garcia JP, De Sario GD, Borna S, Eldaly AS, Quinones-Hinojosa A, Zubair AC, Ho OA, et al. Mesenchymal Stromal Cell Healing Outcomes in Clinical and Pre-Clinical Models to Treat Pressure Ulcers: A Systematic Review. Journal of Clinical Medicine. 2023; 12(24):7545. https://doi.org/10.3390/jcm12247545
Chicago/Turabian StyleTorres-Guzman, Ricardo A., Francisco R. Avila, Karla Maita, John P. Garcia, Gioacchino D. De Sario, Sahar Borna, Abdullah S. Eldaly, Alfredo Quinones-Hinojosa, Abba C. Zubair, Olivia A. Ho, and et al. 2023. "Mesenchymal Stromal Cell Healing Outcomes in Clinical and Pre-Clinical Models to Treat Pressure Ulcers: A Systematic Review" Journal of Clinical Medicine 12, no. 24: 7545. https://doi.org/10.3390/jcm12247545
APA StyleTorres-Guzman, R. A., Avila, F. R., Maita, K., Garcia, J. P., De Sario, G. D., Borna, S., Eldaly, A. S., Quinones-Hinojosa, A., Zubair, A. C., Ho, O. A., & Forte, A. J. (2023). Mesenchymal Stromal Cell Healing Outcomes in Clinical and Pre-Clinical Models to Treat Pressure Ulcers: A Systematic Review. Journal of Clinical Medicine, 12(24), 7545. https://doi.org/10.3390/jcm12247545