Intensive Care Unit-Acquired Weakness after Liver Transplantation: Analysis of Seven Cases and a Literature Review
Abstract
:1. Background
2. Analysis of Seven Cases
3. Literature Review and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latronico, N.; Bolton, C.F. Critical illness polyneuropathy and myopathy: A major cause of muscle weakness and paralysis. Lancet Neurol. 2011, 10, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.; O’Brien, J.M., Jr.; Hoffmann, S.P.; Phillips, G.; Garland, A.; Finley, J.C.; Almoosa, K.; Hejal, R.; Wolf, K.M.; Lemeshow, S.; et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am. J. Respir. Crit. Care Med. 2008, 178, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Bolton, C.F.; Gilbert, J.J.; Hahn, A.F.; Sibbald, W.J. Polyneuropathy in critically ill patients. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1223–1231. [Google Scholar] [CrossRef]
- Stevens, R.D.; Marshall, S.A.; Cornblath, D.R.; Hoke, A.; Needham, D.M.; de Jonghe, B.; Ali, N.A.; Sharshar, T. A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit. Care Med. 2009, 37, S299–S308. [Google Scholar] [CrossRef]
- Damian, M.S.; Wijdicks, E.F.M. The clinical management of neuromuscular disorders in intensive care. Neuromuscul. Disord. 2019, 29, 85–96. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, L.; Ni, F.; Ji, W.; Wu, J.; Zhang, H. Critical illness polyneuropathy and myopathy: A systematic review. Neural. Regen. Res. 2014, 9, 101–110. [Google Scholar] [PubMed]
- De Jonghe, B.; Sharshar, T.; Lefaucheur, J.P.; Authier, F.J.; Durand-Zaleski, I.; Boussarsar, M.; Cerf, C.; Renaud, E.; Mesrati, F.; Carlet, J.; et al. Paresis acquired in the intensive care unit: A prospective multi-center study. JAMA 2002, 288, 2859–2867. [Google Scholar] [CrossRef]
- Patel, B.K.; Pohlman, A.S.; Hall, J.B.; Kress, J.P. Impact of early mobilization on glycemic control and ICU-acquired weakness in critically ill patients who are mechanically ventilated. Chest 2014, 146, 583–589. [Google Scholar] [CrossRef]
- Yang, T.; Li, Z.; Jiang, L.; Wang, Y.; Xi, X. Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol. Scand. 2018, 138, 104–114. [Google Scholar] [CrossRef]
- Hermans, G.; Casaer, M.P.; Clerckx, B.; Güiza, F.; Vanhullebusch, T.; Derde, S.; Meersseman, P.; Derese, I.; Mesotten, D.; Wouters, P.J.; et al. Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: A subanalysis of the EPaNIC trial. Lancet Respir. Med. 2013, 1, 621–629. [Google Scholar] [CrossRef]
- Wolfe, K.S.; Patel, B.K.; MacKenzie, E.L.; Giovanni, S.P.; Pohlman, A.S.; Churpek, M.M.; Hall, J.B.; Kress, J.P. Impact of vasoactive medications on ICU-acquired weakness in mechanically ventilated patients. Chest 2018, 154, 781–787. [Google Scholar] [CrossRef]
- Yang, T.; Li, Z.; Jiang, L.; Xi, X. Corticosteroid use and intensive care unit-acquired weakness: A systematic review and meta-analysis. Crit. Care 2018, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Forel, J.M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.M.; Perez, D.; Seghboyan, J.M.; et al. ACURASYS Study Investigators Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Huang, D.T.; Brower, R.G.; Ferguson, N.D.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Gundel, S.; Hayden, D.; Hite, R.D.; et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar] [PubMed]
- Bourenne, J.; Hraiech, S.; Roch, A.; Gainnier, M.; Papazian, L.; Forel, J.M. Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Ann. Transl. Med. 2017, 5, 291. [Google Scholar] [CrossRef]
- Foster, J. Complications of sedation in critical illness: An update. Crit. Care Nurs. Clin. N. Am. 2016, 28, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Latronico, N.; Fenzi, F.; Recupero, D.; Guarneri, B.; Tomelleri, G.; Tonin, P.; De Maria, G.; Antonini, L.; Rizzuto, N.; Candiani, A. Critical illness myopathy and neuropathy. Lancet 1996, 347, 1579–1582. [Google Scholar] [CrossRef]
- Stibler, H.; Edström, L.; Ahlbeck, K.; Remahl, S.; Ansved, T. Electrophoretic determination of the myosin/actin ratio in the diagnosis of critical illness myopathy. Intensive Care Med. 2003, 29, 1515–1527. [Google Scholar] [CrossRef]
- De Jounghe, B.; Lacherade, J.C.; Sharshar, T.; Outin, H. Intensive care unit-acquired weakness: Risk factors and prevention. Crit. Care Med. 2009, 37, S309–S315. [Google Scholar] [CrossRef]
- Appleton, R.T.; Kinsella, J.; Quasim, T. The incidence of intensive care unit-acquired weakness syndromes: A systematic review. J. Intensive Care Soc. 2015, 16, 126–136. [Google Scholar] [CrossRef]
- Vanhorebeek, I.; Latronico, N.; Van den Berghe, G. ICU-acquired weakness. Intensive Care Med. 2020, 46, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Derde, S.; Hermans, G.; Derese, I.; Güiza, F.; Hedström, Y.; Wouters, P.J.; Bruyninckx, F.; D’Hoore, A.; Larsson, L.; Van den Berghe, G.; et al. Muscle atrophy and preferential loss of myosin in prolonged critically ill patients. Crit. Care Med. 2012, 40, 79–89. [Google Scholar] [CrossRef]
- Latronico, N.; Rasulo, F.A.; Eikermann, M.; Piva, S. Illness Weakness, Polyneuropathy and Myopathy: Diagnosis, treatment, and long-term outcomes. Crit. Care 2023, 27, 439. [Google Scholar] [CrossRef] [PubMed]
- Latronico, N.; Herridge, M.; Hopkins, R.O.; Angus, D.; Hart, N.; Hermans, G.; Iwashyna, T.; Arabi, Y.; Citerio, G.; Ely, E.W.; et al. The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 2017, 43, 1270–1281. [Google Scholar] [CrossRef]
- Miró, O.; Salmerón, J.M.; Masanés, F.; Alonso, J.R.; Graus, F.; Mas, A.; Grau, J.M. Acute quadriplegic myopathy with myosin-deficient muscle fibres after liver transplantation: Defining the clinical picture and delimiting the risk factors. Transplantation 1999, 67, 1144–1151. [Google Scholar] [PubMed]
- Wijdicks, E.F.; Litchy, W.J.; Wiesner, R.H.; Krom, R.A. Neuromuscular complications associated with liver transplantation. Muscle Nerve 1996, 19, 696–700. [Google Scholar] [CrossRef]
- Rezaiguia-Delclaux, S.; Lefaucheur, J.P.; Zakkouri, M.; Duvoux, C.; Duvaldestin, P.; Stéphan, F. Severe acute polyneuropathy complicating orthotopic liver allograft failure. Transplantation 2002, 74, 880–882. [Google Scholar] [CrossRef]
- Jang, M.H.; Yoon, M.H.; Ahn, S.J.; Lee, J.W.; Shin, M.J. Diagnosis of Critical Illness Myopathy after Liver Transplantation and Muscle Condition Monitoring: A Case Report. Transplant. Proc. 2018, 50, 4023–4027. [Google Scholar] [CrossRef]
- Watanabe, J.; Ito, E.; Hatano, M.; Tohyama, T.; Okada, Y.; Takada, Y. Recovery after critical illness polyneuropathy in a patient with orthotopic liver transplantation: A case report. Transplant. Proc. 2016, 48, 3207–3209. [Google Scholar] [CrossRef]
- Campellone, J.V.; Lacomis, D.; Giuliani, M.J.; Kramer, D.J. Mononeuropathies associated with liver transplantation. Muscle Nerve 1998, 21, 896–901. [Google Scholar] [CrossRef]
- Kimura, J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 4th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Avolio, A.W.; Lai, Q.; Cillo, U.; Romagnoli, R.; De Simone, P. L-GrAFT and EASE scores in liver transplantation: Need for reciprocal external validation and comparison with other scores. J. Hepatol. 2021, 75, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Avolio, A.W.; Agnes, S.; Chirico, A.S.; Castagneto, M. Primary dysfunction after liver transplantation: Donor or recipient fault? Transplant. Proc. 1999, 31, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza-García, I.; Arias-Rivera, S.; Frade-Mera, M.J.; Martí, J.D.; Gallart, E.; San José-Arribas, A.; Velasco-Sanz, T.R.; Blazquez-Martínez, E.; Raurell-Torredà, M. Enteral nutrition management in critically ill adult patients and its relationship with intensive care unit-acquired muscle weakness: A national cohort study. PLoS ONE 2023, 18, e0286598. [Google Scholar] [CrossRef]
- Hermans, G.; De Jonghe, B.; Bruyninckx, F.; Van den Berghe, G. Clinical review: Critical illness polyneuropathy and myopathy. Crit. Care 2008, 12, 238. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Phadke, R.; Rawal, J.; McPhail, M.J.; Sidhu, P.S.; Rowlerson, A.; Moxham, J.; Harridge, S.; Hart, N.; Montgomery, H.E. Qualitative Ultrasound in Acute Critical Illness Muscle Wasting. Crit. Care Med. 2015, 43, 1603–1611. [Google Scholar] [CrossRef]
- Avolio, A.W.; Gaspari, R.; Teofili, L.; Bianco, G.; Spinazzola, G.; Soave, P.M.; Paiano, G.; Francesconi, A.G.; Arcangeli, A.; Nicolotti, N.; et al. Postoperative respiratory failure in liver transplantation: Risk factors and effect on prognosis. PLoS ONE 2019, 14, e0211678. [Google Scholar] [CrossRef] [PubMed]
- Avolio, A.W.; Agnes, S.; Cillo, U.; Lirosi, M.C.; Romagnoli, R.; Baccarani, U.; Zamboni, F.; Nicolini, D.; Donataccio, M.; Perrella, A.; et al. http://www.D-MELD.com, the Italian survival calculator to optimize donor to recipient matching and to identify the unsustainable matches in liver transplantation. Transpl. Int. 2012, 25, 294–301. [Google Scholar] [CrossRef]
- Annicchiarico, B.E.; Siciliano, M.; Avolio, A.W.; Caracciolo, G.; Gasbarrini, A.; Agnes, S.; Castagneto, M. Treatment of chronic hepatitis C virus infection with pegylated interferon and ribavirin in cirrhotic patients awaiting liver transplantation. Transplant. Proc. 2008, 4, 1918–1920. [Google Scholar] [CrossRef]
- Gaspari, R.; Teofili, L.; Aceto, P.; Valentini, C.G.; Punzo, G.; Sollazzi, L.; Agnes, S.; Avolio, A.W. Thromboelastography does not reduce transfusion requirements in liver transplantation: A propensity score-matched study. J. Clin. Anesth. 2021, 69, 110154. [Google Scholar] [CrossRef]
- Alhamar, M.; Uzuni, A.; Mehrotra, H.; Elbashir, J.; Galusca, D.; Nagai, S.; Yoshida, A.; Abouljoud, M.S.; Otrock, Z.K. Predictors of intraoperative massive transfusion in orthotopic liver transplantation. Transfusion 2023. [Google Scholar] [CrossRef] [PubMed]
- Lapisatepun, W.; Ma, C.; Lapisatepun, W.; Agopian, V.; Wray, C.; Xia, V.W. Super-massive transfusion during liver transplantation. Transfusion 2023, 63, 1677–1684. [Google Scholar] [CrossRef]
- Massicotte, L.; Carrier, F.M.; Denault, A.Y.; Karakiewicz, P.; Hevesi, Z.; McCormack, M.; Thibeault, L.; Nozza, A.; Tian, Z.; Dagenais, M.; et al. Development of a Predictive Model for Blood Transfusions and Bleeding During Liver Transplantation: An Observational Cohort Study. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1722–1730. [Google Scholar] [CrossRef]
- Moore, H.B.; LaRiviere, W.; Rodriguez, I.; Brown, K.; Hadley, K.; Pomposelli, J.J.; Adams, M.A.; Wachs, M.E.; Conzen, K.D.; Kennealey, P.T.; et al. Early predictors of prolonged intensive care utilization following liver transplantation. Am. J. Surg. 2023, 226, 829–834. [Google Scholar] [CrossRef]
- Moore, H.B.; Saben, J.; Rodriguez, I.; Bababekov, Y.J.; Pomposelli, J.J.; Yoeli, D.; Ferrell, T.; Adams, M.A.; Pshak, T.J.; Kaplan, B.; et al. Postoperative fibrinolytic resistance is associated with early allograft dysfunction in liver transplantation: A prospective observational study. Liver Transpl. 2023, 29, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Duehrkop, C.; Rieben, R. Refinement of tourniquet-induced peripheral ischemia/reperfusion injury in rats: Comparison of 2 h vs 24 h reperfusion. Lab. Anim. 2014, 48, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Goossens, C.; Weckx, R.; Derde, S.; Dufour, T.; Vander Perre, S.; Pauwels, L.; Thiessen, S.E.; Van Veldhoven, P.P.; Van den Berghe, G.; Langouche, L. Adipose tissue protects against sepsis-induced muscle weakness in mice: From lipolysis to ketones. Crit. Care 2019, 23, 236. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Zanetto, A.; Piano, S.; Heimbach, J.K.; Dasarathy, S. Liver transplantation in the patient with physical frailty. J. Hepatol. 2023, 78, 1105–1117. [Google Scholar] [CrossRef]
- Hermans, G.; Clerckx, B.; Vanhullebusch, T.; Segers, J.; Vanpee, G.; Robbeets, C.; Casaer, M.P.; Wouters, P.; Gosselink, R.; Van Den Berghe, G. Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve 2012, 45, 18–25. [Google Scholar] [CrossRef]
- Vanpee, G.; Segers, J.; Van Mechelen, H.; Wouters, P.; Van den Berghe, G.; Hermans, G.; Gosselink, R. The interobserver agreement of handheld dynamometry for muscle strength assessment in critically ill patients. Crit. Care Med. 2011, 39, 1929–1934. [Google Scholar] [CrossRef]
- Hermans, G.; De Jonghe, B.; Bruyninckx, F.; Van den Berghe, G. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst. Rev. 2014, 30, CD006832. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 2015, 19, 274. [Google Scholar] [CrossRef] [PubMed]
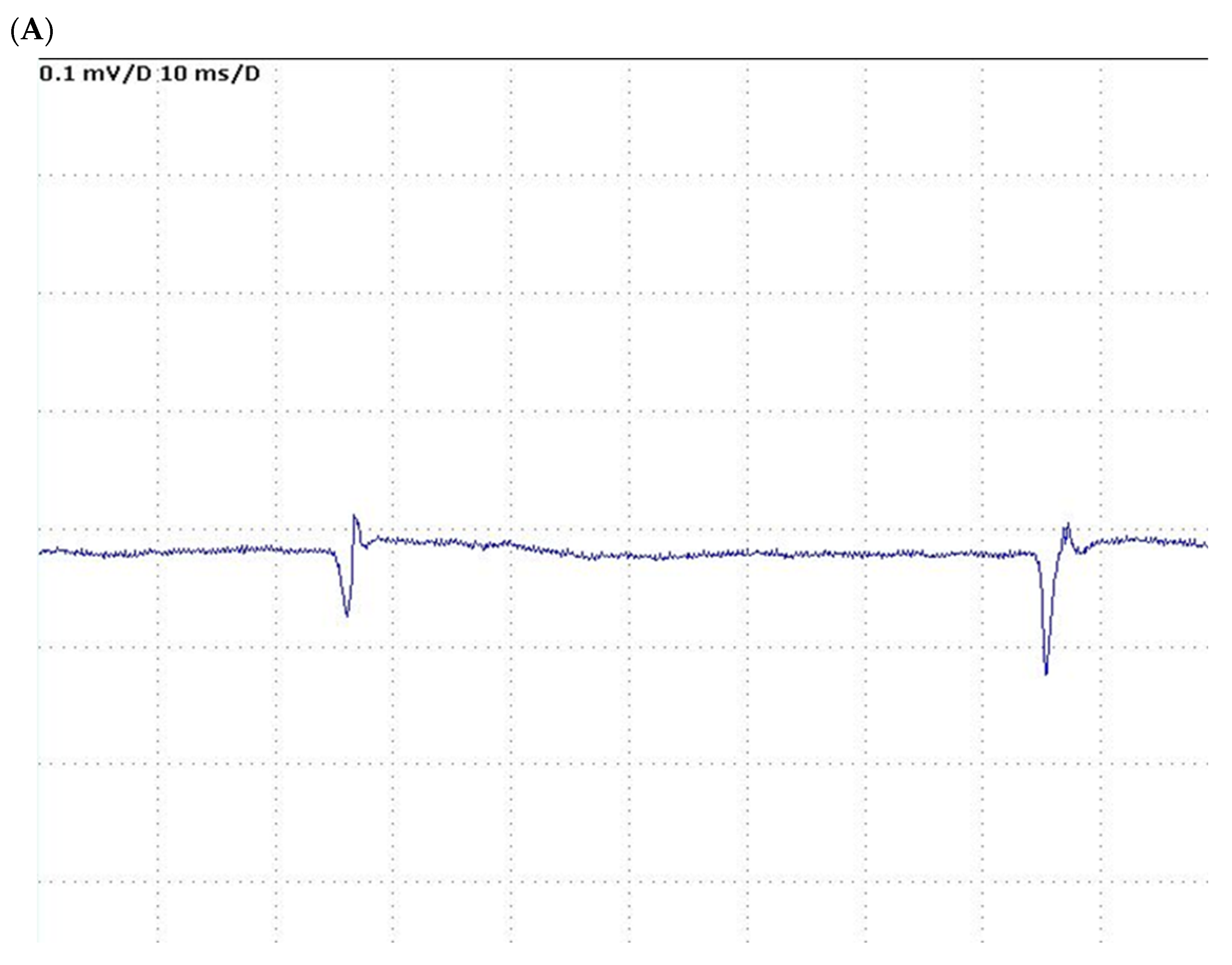
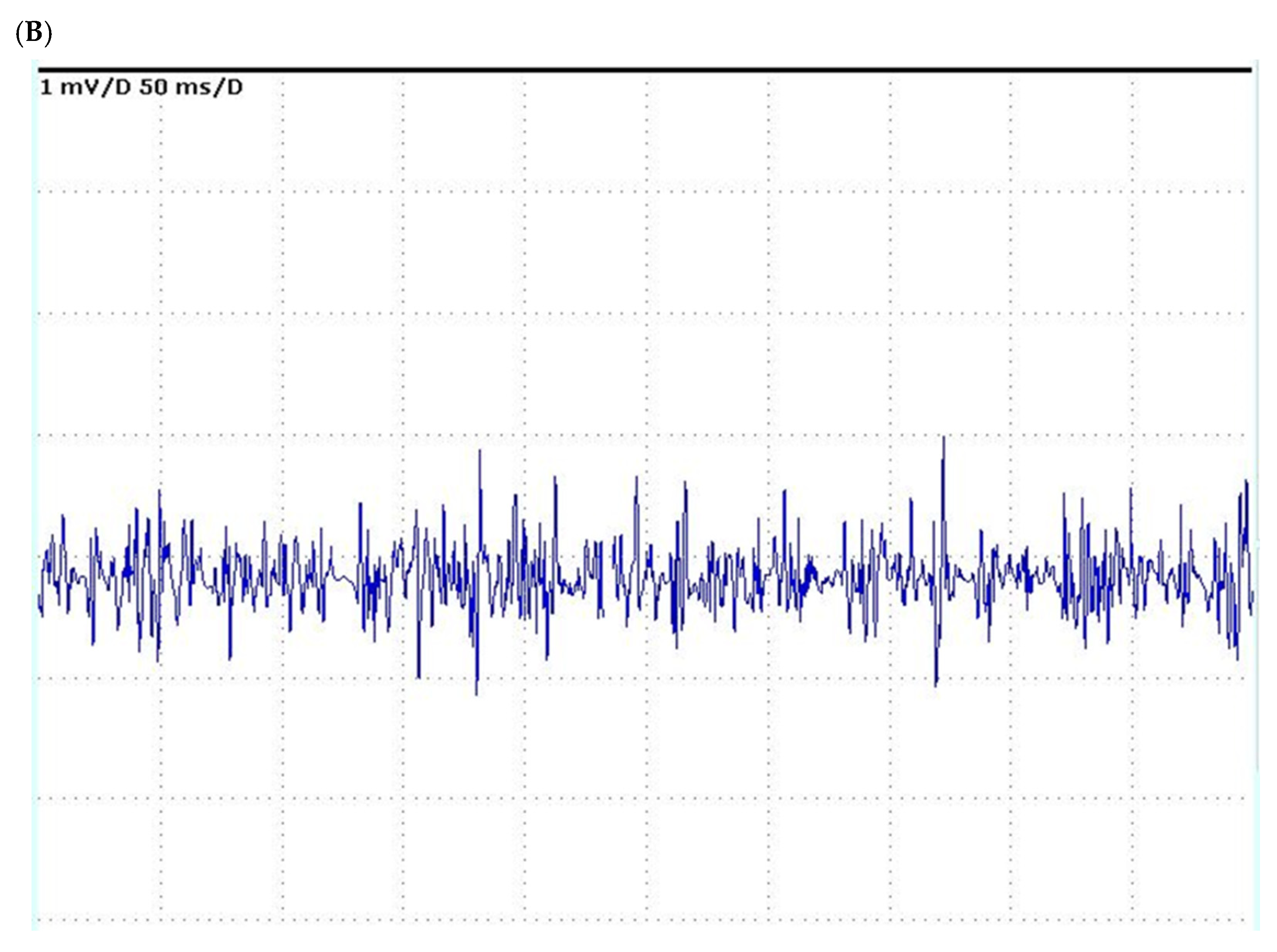
| Author, Year | Age, Years | Gender, M/F | Transplant Donor | Primary Disease | Child-Pugh/ MELD | Graft Function | ^ RBC Units; * RBC Units | AKI | Re-Transplant | Discharge |
|---|---|---|---|---|---|---|---|---|---|---|
| Mirò O, 1999 [26] Incidence 4/281 (1.4%) | ||||||||||
| Case 1 | 51 | M | DD | ALC | CP 6 (A) | Poor | 9; 11 | Yes | Yes | 89 POD |
| Case 2 | 57 | M | DD | HCV | CP 8 (B) | PNF | 21; 46 | Yes | Yes | 229 POD |
| Case 3 | 41 | F | DD | HCV | CP 13 (C) | Good | 68; 9 | Yes | Yes | 48 POD |
| Case 4 | 59 | M | DD | HCV | CP 5 (A) | Poor | 4; 26 | Yes | Yes | 46 POD |
| Watanabe J, 2016 [30] | 43 | M | LD | HCV | MELD 20 | Poor | NS | Yes | Yes | 150 POD |
| Jang MH, 2018 [29] | 47 | M | LD | HBV | MELD 25 | NS | NS | HS | No | 91 POD |
| Rezaiguia-Delclaux S, 2022 [28] Incidence 3/30 (10%) | ||||||||||
| Case 1 | 59 | M | DD | ALC | CP 10 (C) | PNF | NS | Yes | Yes | Discharged § |
| Case 2 | 42 | M | DD | ALC | CP 10 (C) | PNF | NS | Yes | Yes | Death (157th day) |
| Case 3 | 60 | M | DD | ALC | CP 11 (C) | PVT | NS | No | RS | Discharged § |
| Parameters | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Age, years | 64 | 56 | 58 | 38 | 35 | 46 | 53 |
| Gender, M/F | M | M | F | F | M | M | F |
| BMI, Kg/m2 | 26.6 | 22.2 | 34.2 | 33.5 | 26.1 | 25.2 | 24.6 |
| Alcoholic cirrhosis | No | Yes | No | Yes | No | No | No |
| HBV/HCV Cirrhosis | No | Yes | No | No | No | No | No |
| Other causes of LD | Cryptogenic | - | Polycystosis | - | Cryptogenic | Trauma | AID |
| Hepatocarcinoma | No | Yes | No | No | No | No | No |
| Diabetes mellitus | No | No | Yes | No | No | No | No |
| MELD score | 30 | 38 | 40 | 40 | 40 | 31 | 33 |
| D-MELD score | 810 | 912 | 3105 | 3160 | 2420 | 2139 | 2234 |
| ^ RBCs, units | 36 | 18 | 19 | 11 | 19 | 6 | 4 |
| ^ FFP, units | 23 | 14 | 8 | 5 | 20 | 5 | 3 |
| ^ Platelets, units | 9 | 5 | 3 | 2 | 3 | 0 | 1 |
| SAPS II score | 46 | 76 | 49 | 60 | 41 | 67 | 34 |
| EASE score | −0.9 | +1.9 | +1.7 | −1.3 | −1.9 | −1.7 | −2.1 |
| P.o. Hyperglycemia | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Tracheostomy | Yes | No | No | No | No | Yes | Yes |
| Norepinephrine | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Serum CPK, UI/L | 74 | 113 | 239 | 15 | 2000 | 80 | 120 |
| Pre-OLT ICU stay, days | 0 | 4 | 5 | 9 | 1 | 13 | 3 |
| Pre-OLT CRRT | No | Yes | No | Yes | Yes | Yes | No |
| Post-OLT CRRT | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| EAF | No | Yes | Yes | No | No | No | No |
| * RBCs ≥ 15, units | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Graft Rejection | No | No | No | No | No | No | Yes |
| Infection site | Blood | Blood/Bal | Blood | Urine | Blood/Bal | Blood/ascites | Blood |
| Duration of MV, hours | 168 | 75 | 144 | 336 | 192 | 180 | 240 |
| ICU-AW post-OLT, days | 14 | 8 | 8 | 8 | 10 | 13 | 9 |
| ICU-AW recovery | Yes | Yes | Yes | Yes | Yes | Yes | No |
| ICU-LOS, days | 79 | 14 | 20 | 20 | 33 | 53 | 40 |
| Hospital LOS, days | 187 | 53 | 68 | 85 | 33 | 177 | 40 |
| 90 days outcome | Alive | Alive | Alive | Alive | Dead | Alive | Dead |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspari, R.; Spinazzola, G.; Aceto, P.; Avolio, A.W.; Delli Compagni, M.; Postorino, S.; Michi, T.; Fachechi, D.C.; Modoni, A.; Antonelli, M. Intensive Care Unit-Acquired Weakness after Liver Transplantation: Analysis of Seven Cases and a Literature Review. J. Clin. Med. 2023, 12, 7529. https://doi.org/10.3390/jcm12247529
Gaspari R, Spinazzola G, Aceto P, Avolio AW, Delli Compagni M, Postorino S, Michi T, Fachechi DC, Modoni A, Antonelli M. Intensive Care Unit-Acquired Weakness after Liver Transplantation: Analysis of Seven Cases and a Literature Review. Journal of Clinical Medicine. 2023; 12(24):7529. https://doi.org/10.3390/jcm12247529
Chicago/Turabian StyleGaspari, Rita, Giorgia Spinazzola, Paola Aceto, Alfonso Wolfango Avolio, Manuel Delli Compagni, Stefania Postorino, Teresa Michi, Daniele Cosimo Fachechi, Anna Modoni, and Massimo Antonelli. 2023. "Intensive Care Unit-Acquired Weakness after Liver Transplantation: Analysis of Seven Cases and a Literature Review" Journal of Clinical Medicine 12, no. 24: 7529. https://doi.org/10.3390/jcm12247529
APA StyleGaspari, R., Spinazzola, G., Aceto, P., Avolio, A. W., Delli Compagni, M., Postorino, S., Michi, T., Fachechi, D. C., Modoni, A., & Antonelli, M. (2023). Intensive Care Unit-Acquired Weakness after Liver Transplantation: Analysis of Seven Cases and a Literature Review. Journal of Clinical Medicine, 12(24), 7529. https://doi.org/10.3390/jcm12247529






