Intravascular Imaging in Ultra-Low or Zero-Contrast Percutaneous Coronary Interventions: The Time Is Now?
Abstract
:1. Introduction
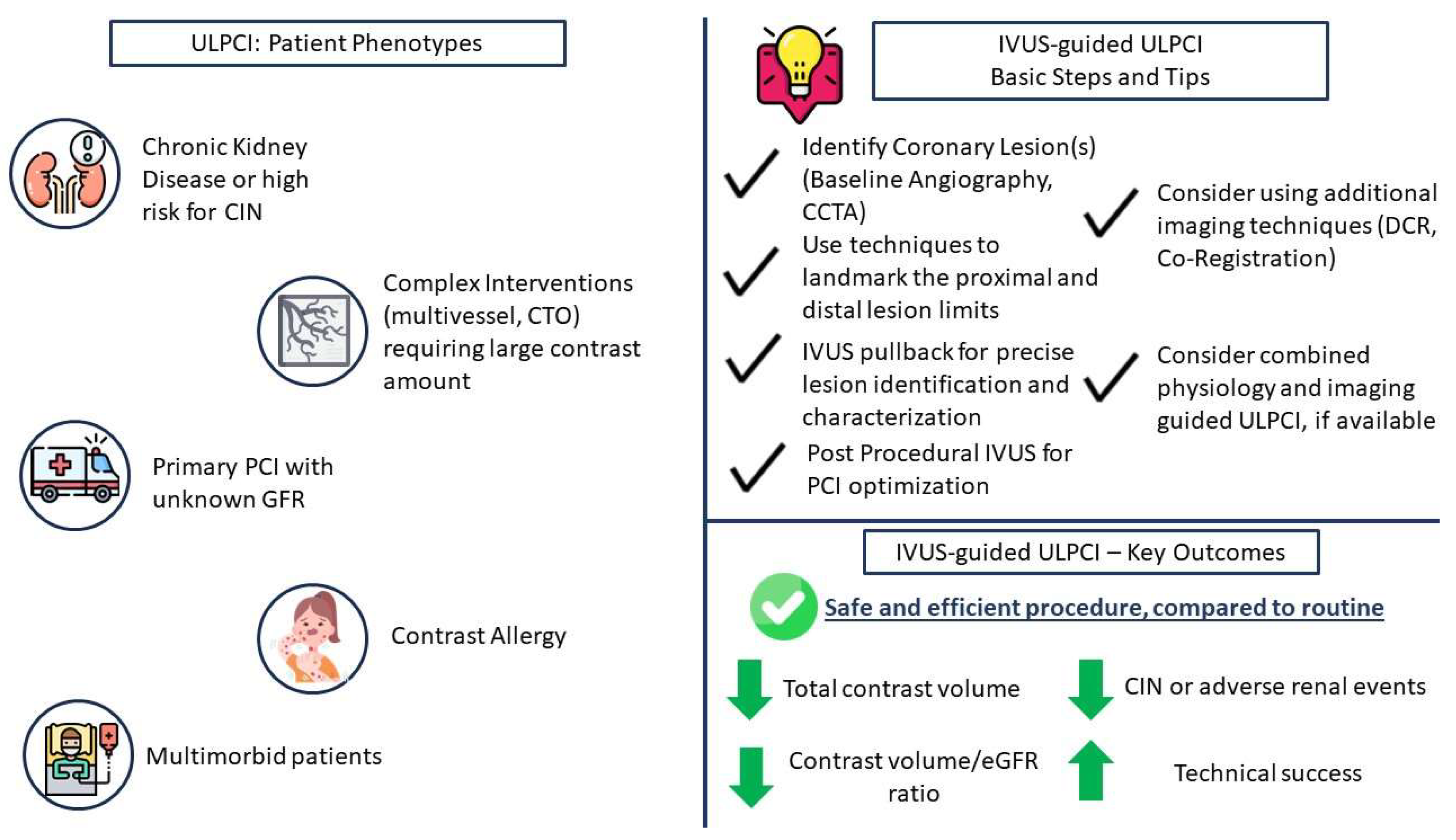
2. The Need for ULPCI—Patient Characteristics
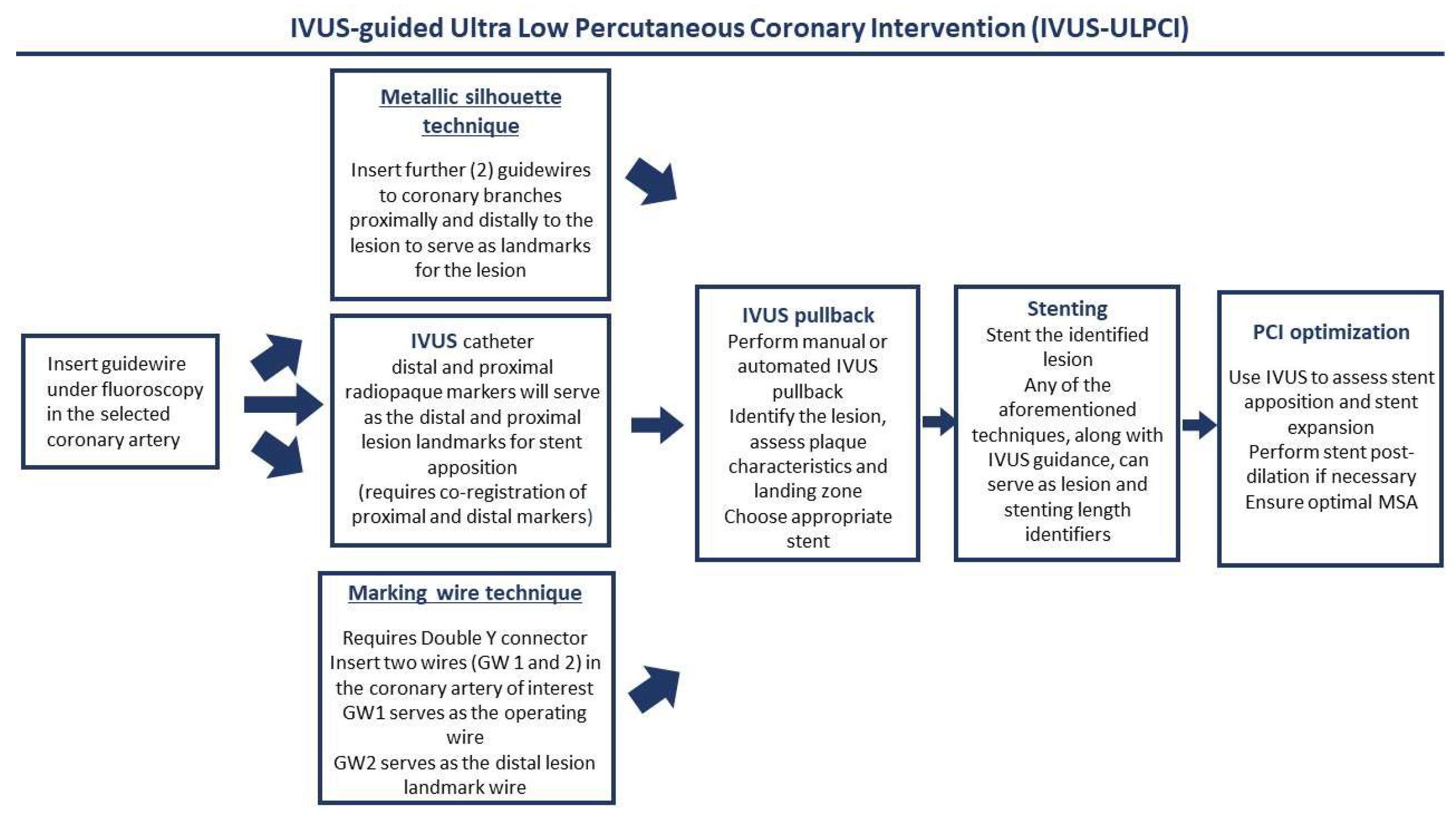
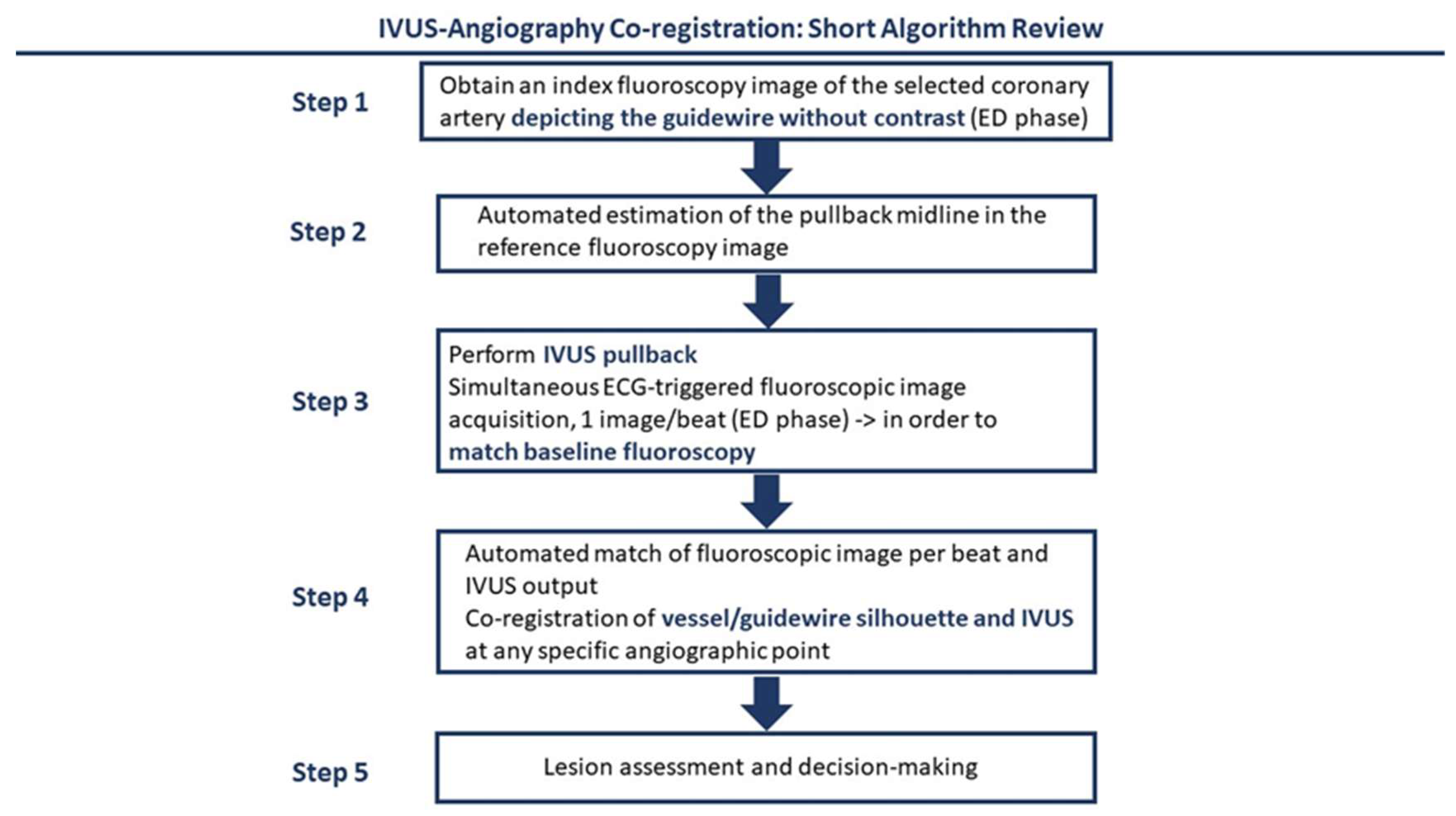
3. Intravascular Imaging for ULPCI—Technical and Procedural Considerations
4. Intravascular Imaging for ULPCI—Clinical Data
5. Future Perspectives
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klein, L.W.; Sheldon, M.W.; Brinker, J.; Mixon, T.A.; Skelding, K.; Strunk, A.O.; Tommaso, C.L.; Weiner, B.; Bailey, S.R.; Uretsky, B.; et al. The use of radiographic contrast media during PCI: A focused review. Catheter. Cardiovasc. Interv. 2009, 74, 728–746. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Solomon, R.; Tasanarong, A. Side Effects of Radiographic Contrast Media: Pathogenesis, Risk Factors, and Prevention. Biomed. Res. Int. 2014, 2014, 741018. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Fang, H.-Y.; Hussein, H.; Fang, C.Y.; Chen, Y.L.; Hsueh, S.K.; Cheng, C.I.; Yang, C.H.; Chen, C.J.; Hang, C.L.; et al. Predictors of contrast-induced nephropathy in chronic total occlusion percutaneous coronary intervention. EuroIntervention 2014, 9, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, B.; Shabbir, A.; Travieso, A.; Gonzalo, N.; Escaned, J. Procedural and Technological Innovations Facilitating Ultra-low Contrast Percutaneous Coronary Interventions. Interv. Cardiol. Rev. Res. Resour. 2023, 18, e09. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E4–E17. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Piayda, K.; Kleinebrecht, L.; Afzal, S.; Bullens, R.; Ter Horst, I.; Polzin, A.; Veulemans, V.; Dannenberg, L.; Wimmer, A.C.; Jung, C.; et al. Dynamic coronary roadmapping during percutaneous coronary intervention: A feasibility study. Eur. J. Med. Res. 2018, 23, 36. [Google Scholar] [CrossRef]
- Yabe, T.; Muramatsu, T.; Tsukahara, R.; Nakano, M.; Takimura, H.; Kawano, M.; Hada, T.; Ikeda, T. The impact of percutaneous coronary intervention using the novel dynamic coronary roadmap system. Heart Vessels 2020, 35, 323–330. [Google Scholar] [CrossRef]
- Nijjer, S.S.; Sen, S.; Petraco, R.; Escaned, J.; Echavarria-Pinto, M.; Broyd, C.; Al-Lamee, R.; Foin, N.; Foale, R.A.; Malik, I.S.; et al. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Provides Virtual Intervention and Predicts Hemodynamic Outcome for Serial Lesions and Diffuse Coronary Artery Disease. JACC Cardiovasc. Interv. 2014, 7, 1386–1396. [Google Scholar] [CrossRef]
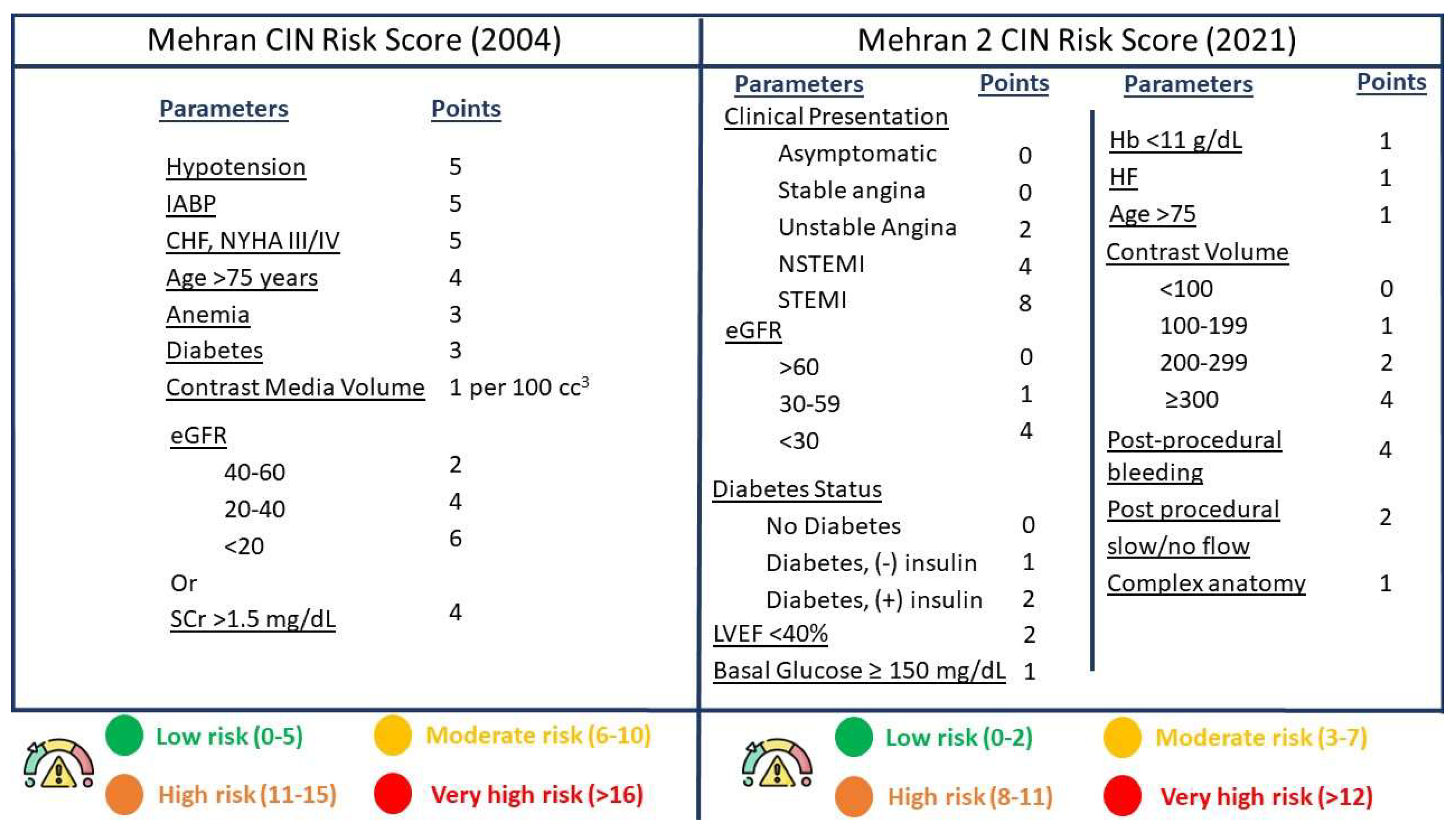
- Mehran, R.; Dangas, G.D.; Weisbord, S.D. Contrast-Associated Acute Kidney Injury. N. Engl. J. Med. 2019, 380, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Lo, W.-C.; Wu, Y.-C.; Lin, T.C.; Lin, C.H.; Wu, M.S.; Tu, Y.K. The Incidence of Contrast-Induced Nephropathy and the Need of Dialysis in Patients Receiving Angiography: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 862534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Liu, Z.; Bai, Y. Risk factors of contrast-induced nephropathy after percutaneous coronary intervention: A retrospective analysis. J. Int. Med. Res. 2021, 49, 030006052110059. [Google Scholar] [CrossRef] [PubMed]
- Khalfallah, M.; Allaithy, A.; Maria, D.A. Incidence, Predictors and Outcomes of Contrast Induced Nephropathy in Patients with ST Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Glob. Heart 2021, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Patel, U.D.; Chang, T.I.; Kennedy, K.F.; Masoudi, F.A.; Matheny, M.E.; Kosiborod, M.; Amin, A.P.; Messenger, J.C.; Rumsfeld, J.S.; et al. Contemporary Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Interventions. JACC Cardiovasc. Interv. 2014, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ix, J.H.; Shlipak, M.G.; Liu, H.H.; Schiller, N.B.; Whooley, M.A. Association between Renal Insufficiency and Inducible Ischemia in Patients with Coronary Artery Disease. J. Am. Soc. Nephrol. 2003, 14, 3233–3238. [Google Scholar] [CrossRef]
- Malleshappa, P.; Shah, B. Prevalence of chronic kidney disease and the incidence of acute kidney injury in patients with coronary artery disease in Mumbai, India. Heart Views Off. J. Gulf Heart Assoc. 2015, 16, 47. [Google Scholar] [CrossRef]
- Liu, H.; Yu, J.; Chen, F.; Li, J.; Hu, D. Inpatients with coronary heart disease have a high prevalence of chronic kidney disease based on estimated glomerular filtration rate (eGFR) in China. Heart Vessels 2007, 22, 223–228. [Google Scholar] [CrossRef]
- Patel, B.; Shah, M.; Dusaj, R.; Maynard, S.; Patel, N. Percutaneous Coronary Intervention and Inpatient Mortality in Patients with Advanced Chronic Kidney Disease Presenting with Acute Coronary Syndrome. Bayl. Univ. Med. Cent. Proc. 2017, 30, 400–403. [Google Scholar] [CrossRef]
- He, H.; Chen, X.-R.; Chen, Y.-Q.; Niu, T.-S.; Liao, Y.-M. Prevalence and Predictors of Contrast-Induced Nephropathy (CIN) in Patients with ST-Segment Elevation Myocardial Infarction (STEMI) Undergoing Percutaneous Coronary Intervention (PCI): A Meta-Analysis. J. Interv. Cardiol. 2019, 2019, 2750173. [Google Scholar] [CrossRef]
- Sedhai, Y.R.; Golamari, R.; Timalsina, S.; Basnyat, S.; Koirala, A.; Asija, A.; Choksi, T.; Kushwah, A.; Geovorgyan, D.; Dar, T.; et al. Contrast-Induced Nephropathy After Cardiac Catheterization: Culprits, Consequences and Predictors. Am. J. Med. Sci. 2017, 354, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Owen, R.; Chiarito, M.; Baber, U.; Sartori, S.; Cao, D.; Nicolas, J.; Pivato, C.A.; Nardin, M.; Krishnan, P.; et al. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention: Derivation and validation from an observational registry. Lancet 2021, 398, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Inohara, T.; Kohsaka, S.; Abe, T.; Miyata, H.; Numasawa, Y.; Ueda, I.; Nishi, Y.; Naito, K.; Shibata, M.; Hayashida, K.; et al. Development and Validation of a Pre-Percutaneous Coronary Intervention Risk Model of Contrast-Induced Acute Kidney Injury with an Integer Scoring System. Am. J. Cardiol. 2015, 115, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Fu, N.-K.; Xu, J.; Yang, S.C.; Li, S.; Liu, Y.Y.; Cong, H.L. A simple preprocedural score for risk of contrast-induced acute kidney injury after percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2014, 83, E8–E16. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Aymong, E.D.; Nikolsky, E.; Lasic, Z.; Iakovou, I.; Fahy, M.; Mintz, G.S.; Lansky, A.J.; Moses, J.W.; Stone, G.W.; et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2004, 44, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Choi, J.P.; Feghali, G.A.; Schussler, J.M.; Stoler, R.M.; Vallabahn, R.C.; Mehta, A. Contrast-Induced Acute Kidney Injury. J. Am. Coll. Cardiol. 2016, 68, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Dinh, D.T.; Brennan, A.; Batchelor, R.; Duffy, S.J.; Shaw, J.A.; Chan, W.; Layland, J.; van Gaal, W.J.; Reid, C.M.; et al. Incidence, predictors and clinical implications of new renal impairment following percutaneous coronary intervention. Open Heart 2022, 9, e001876. [Google Scholar] [CrossRef] [PubMed]
- Mohebi, R.; Karimi Galougahi, K.; Garcia, J.J.; Horst, J.; Ben-Yehuda, O.; Radhakrishnan, J.; Chertow, G.M.; Jeremias, A.; Cohen, D.J.; Cohen, D.J.; et al. Long-Term Clinical Impact of Contrast-Associated Acute Kidney Injury Following PCI. JACC Cardiovasc. Interv. 2022, 15, 753–766. [Google Scholar] [CrossRef]
- Narula, A.; Mehran, R.; Weisz, G.; Dangas, G.D.; Yu, J.; Généreux, P.; Nikolsky, E.; Brener, S.J.; Witzenbichler, B.; Guagliumi, G.; et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: Results from the HORIZONS-AMI substudy. Eur. Heart J. 2014, 35, 1533–1540. [Google Scholar] [CrossRef]
- Jha, K.K.; Thakur, L.; Yost, G.; Berger, A.; Green, S.; Agarwal, S.; Scott, T.D.; Bauch, T.D.; Blankenship, J.C. Clinical Efficacy of Emergency Premedication Regimen for Contrast Allergy Before Percutaneous Coronary Interventions. Circ. Cardiovasc. Interv. 2020, 13, e008672. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Huang, W.; Liu, J.; Cao, Y. Zero-contrast percutaneous coronary intervention for chronic total occlusions guided by intravascular ultrasound with ChromaFlo mode: A case report. Eur. Heart J. Case Rep. 2020, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Tsumuraya, N.; Nie, M.; Ikari, Y. Zero Contrast Coronary Intervention Using Intravascular Ultrasound Guidance in a Patient with Allergy to Contrast Medium. Tokai J. Exp. Clin. Med. 2016, 41, 152–155. [Google Scholar] [PubMed]
- Gupta, A.; Neupane, S.; Basir, M.; Alaswad, K. Zero-iodinated contrast retrograde percutaneous coronary interventions of chronic total occlusions using gadolinium and imaging guidance: A case report of a patient with severe anaphylaxis to iodinated contrast. Eur. Heart J. Case Rep. 2020, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Okura, H.; Nezuo, S.; Yoshida, K. Successful stent implantation guided by intravascular ultrasound and a Doppler guidewire without contrast injection in a patient with allergy to iodinated contrast media. J. Invasive Cardiol. 2011, 23, 297–299. [Google Scholar] [PubMed]
- Mariani, J.; Guedes, C.; Soares, P.; Zalc, S.; Campos, C.M.; Lopes, A.C.; Spadaro, A.G.; Perin, M.A.; Filho, A.E.; Takimura, C.K.; et al. Intravascular Ultrasound Guidance to Minimize the Use of Iodine Contrast in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2014, 7, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Jino, B.; Roy, S.; Shafeeq, A.; Rajendran, M. Absolute zero-contrast percutaneous coronary intervention under intravascular ultrasound guidance in chronic kidney disease patients—From despair to hope? IJC Heart Vasc. 2022, 40, 101052. [Google Scholar] [CrossRef]
- Shibata, K.; Wakabayashi, K.; Ishinaga, T.; Morimura, M.; Aizawa, N.; Suzuki, T.; Furuya, T.; Sato, C.; Nishikura, T.; Ikeda, N.; et al. Feasibility, Safety, and Long-Term Outcomes of Zero-Contrast Percutaneous Coronary Intervention in Patients with Chronic Kidney Disease. Circ. J. 2022, 86, 787–796. [Google Scholar] [CrossRef]
- Azzalini, L.; Laricchia, A.; Regazzoli, D.; Mitomo, S.; Hachinohe, D.; Bellini, B.; Demir, O.M.; Poletti, E.; Maccagni, D.; Colombo, A. Ultra-Low Contrast Percutaneous Coronary Intervention to Minimize the Risk for Contrast-Induced Acute Kidney Injury in Patients with Severe Chronic Kidney Disease. J. Invasive Cardiol. 2019, 31, 176–182. [Google Scholar] [CrossRef]
- Truesdell, A.G.; Alasnag, M.A.; Kaul, P.; Rab, S.T.; Riley, R.F.; Young, M.N.; Batchelor, W.B.; Maehara, A.; Welt, F.G.; Kirtane, A.J.; et al. Intravascular Imaging During Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2023, 81, 590–605. [Google Scholar] [CrossRef]
- Baruś, P.; Modrzewski, J.; Gumiężna, K.; Dunaj, P.; Głód, M.; Bednarek, A.; Wańha, W.; Roleder, T.; Kochman, J.; Tomaniak, M. Comparative Appraisal of Intravascular Ultrasound and Optical Coherence Tomography in Invasive Coronary Imaging: 2022 Update. J. Clin. Med. 2022, 11, 4055. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Karimi Galougahi, K.; Nazif, T.; Maehara, A.; Hardy, M.A.; Cohen, D.J.; Ratner, L.E.; Collins, M.B.; Moses, J.W.; Kirtane, A.J.; et al. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure: A feasibility, safety, and outcome study. Eur. Heart J. 2016, 37, 3090–3095. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Cassar, A.; Fetterly, K.A.; Bell, M.; Theessen, H.; Ecabert, O.; Bresnahan, J.F.; Lerman, A. Co-registration of angiography and intravascular ultrasound images through image-based device tracking. Catheter. Cardiovasc. Interv. 2016, 88, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Sacha, J.; Gierlotka, M.; Lipski, P.; Feusette, P.; Dudek, D. Zero-contrast percutaneous coronary interventions to preserve kidney function in patients with severe renal impairment and hemodialysis subjects. Adv. Interv. Cardiol. 2019, 15, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Nath, R.K.; Mahapatra, H.S.; Pandit, B.N.; Raj, A.; Sharma, A.K.; Kumar, T.; Kuber, D.; Aggarwal, P. Ultra-low CONtraSt PCI vs conVEntional PCI in patients of ACS with increased risk of CI-AKI (CONSaVE-AKI). Indian Heart J. 2022, 74, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Nandhakumar, V.; Pakshirajan, B.; Chopra, A.; Anandan, H.; Janakiraman, E.; Uthayakumaran, K.; Kalidoss, L.; Victor, S.M.; Ajit, M.S. Safety and feasibility of intravascular ultrasound guided zero-contrast percutaneous coronary intervention–A prospective study. Int. J. Cardiol. 2022, 353, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yin, Z.; Liang, C.; He, J.; Wang, C.L.; Peng, X.; Zhang, Y.; Zheng, Z.F.; Pan, H.W. Zero contrast optical coherence tomography-guided percutaneous coronary intervention in patients with non-ST segment elevation myocardial infarction and chronic kidney disease. Catheter. Cardiovasc. Interv. 2021, 97, 1072–1079. [Google Scholar] [CrossRef]
- Frick, K.; Michael, T.T.; Alomar, M.; Mohammed, A.; Rangan, B.V.; Abdullah, S.; Grodin, J.; Hastings, J.L.; Banerjee, S.; Brilakis, E.S. Low molecular weight dextran provides similar optical coherence tomography coronary imaging compared to radiographic contrast media. Catheter. Cardiovasc. Interv. 2014, 84, 727–731. [Google Scholar] [CrossRef]
- Almendarez, M.; Gurm, H.S.; Mariani, J.; Montorfano, M.; Brilakis, E.S.; Mehran, R.; Azzalini, L. Procedural Strategies to Reduce the Incidence of Contrast-Induced Acute Kidney Injury During Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1877–1888. [Google Scholar] [CrossRef]
| Clinical Trial | Type of Study | Year | Patient Characteristics | Technique | Outcomes |
|---|---|---|---|---|---|
| Mariani et al. [36] | Randomized controlled trial | 2014 | 83 CKD patients undergoing either angiography-guided PCI (n = 41) or ULPCI | ULCPI, IVUS guided |
|
| Sacha et al. [44] | Observational | 2019 | 20 patients with advanced CKD and hemodialysis (29 lesions) undergoing ULPCI | Zero-contrast IVUS guided |
|
| Azallini et al. [39] | Observational | 2019 | 111 patients with CKD (ULPCI: n = 8, conventional angiography: n = 103) | ULPCI, IVUS and/or OCT guided |
|
| Shrivastava et al. (CONSAVE-AKI) [45] | Randomized controlled trial | 2022 |
| ULPCI, including contrast dilution and IVUS techniques |
|
| Ali et al. [42] | Observational | 2016 | 31 patients with advanced CKD, who had PCI indication in a prior coronary angiogram | Zero-contrast, IVUS and physiology guided |
|
| Kumar et al. [37] | Observational | 2022 | 42 CKD patients (66 vessels) with an indication for absolute zero-contrast PCI | Zero-contrast, IVUS guided |
|
| Shibata et al. [38] | Observational | 2022 | 100 propensity matched CKD patients undergoing PCI (50 patients in each of the following strategies: IVUS-guided zero-contrast PCI, conventional angiography) | Zero-contrast, IVUS guided |
|
| Nandhakumar et al. [46] | Observational | 2022 | 27 patients with CKD (31 vessels) | Zero-contrast, IVUS guided |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, K.; Pyrpyris, N.; Papanikolaou, A.; Beneki, E.; Tsioufis, P.; Antonopoulos, A.; Fragoulis, C.; Tatakis, F.; Koutsopoulos, G.; Aznaouridis, K.; et al. Intravascular Imaging in Ultra-Low or Zero-Contrast Percutaneous Coronary Interventions: The Time Is Now? J. Clin. Med. 2023, 12, 7499. https://doi.org/10.3390/jcm12237499
Dimitriadis K, Pyrpyris N, Papanikolaou A, Beneki E, Tsioufis P, Antonopoulos A, Fragoulis C, Tatakis F, Koutsopoulos G, Aznaouridis K, et al. Intravascular Imaging in Ultra-Low or Zero-Contrast Percutaneous Coronary Interventions: The Time Is Now? Journal of Clinical Medicine. 2023; 12(23):7499. https://doi.org/10.3390/jcm12237499
Chicago/Turabian StyleDimitriadis, Kyriakos, Nikolaos Pyrpyris, Aggelos Papanikolaou, Eirini Beneki, Panagiotis Tsioufis, Alexios Antonopoulos, Christos Fragoulis, Fotis Tatakis, Georgios Koutsopoulos, Konstantinos Aznaouridis, and et al. 2023. "Intravascular Imaging in Ultra-Low or Zero-Contrast Percutaneous Coronary Interventions: The Time Is Now?" Journal of Clinical Medicine 12, no. 23: 7499. https://doi.org/10.3390/jcm12237499
APA StyleDimitriadis, K., Pyrpyris, N., Papanikolaou, A., Beneki, E., Tsioufis, P., Antonopoulos, A., Fragoulis, C., Tatakis, F., Koutsopoulos, G., Aznaouridis, K., Aggeli, K., & Tsioufis, K. (2023). Intravascular Imaging in Ultra-Low or Zero-Contrast Percutaneous Coronary Interventions: The Time Is Now? Journal of Clinical Medicine, 12(23), 7499. https://doi.org/10.3390/jcm12237499









