Quantitative Association between Computed-Tomography-Based L1 Skeletal Muscle Indices and Major Adverse Clinical Events Following Percutaneous Coronary Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Enrollment
2.2. Data Collection, Peri-Procedural Management, and Clinical Follow-Up
2.3. CT-Based Method of Skeletal Muscle Measurement
2.4. Study Outcome Definitions
2.5. Statistical Analysis
3. Results
3.1. Baseline Demographic Characteristics
3.2. Correlation between L1 and L3 Skeletal Muscle Mass Measurements
3.3. Procedural Characteristics and Peri-Procedural Medications
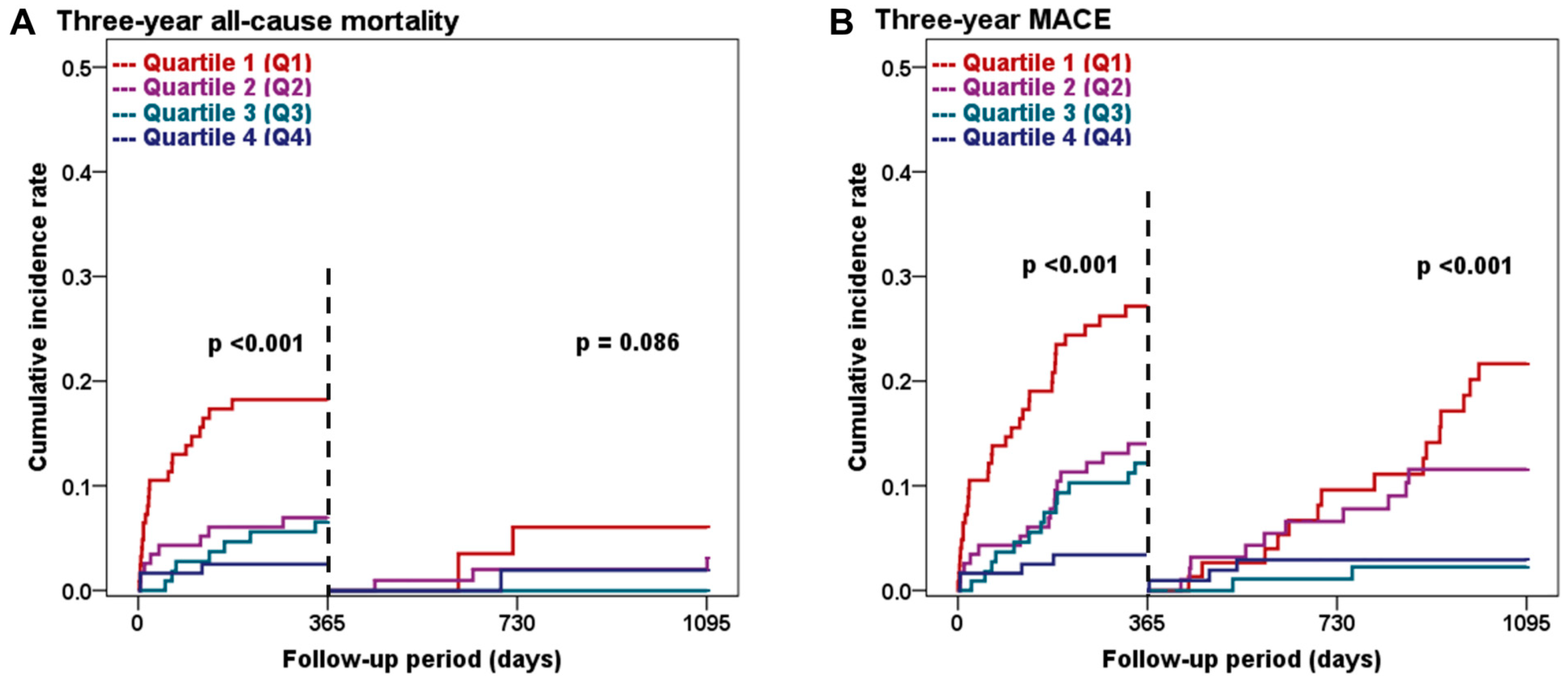
3.4. Three-Year Clinical Outcomes Based on the Sex-Specific L1 SMI Quartiles
3.5. Landmark Analysis at the One-Year Follow-Up
3.6. Stepwise Multivariate Analysis Based on the L1 SMI Quartiles
4. Discussion
4.1. CT-Based L1 SMI Measurement as a Quantitative Prognostic Marker for CAD
4.2. Biological Mechanism Linking Sarcopenia and Future Cardiovascular Risk
4.3. Clinical and Therapeutic Implication of Assessing Sarcopenia in CAD
4.4. Limitation and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990s–991s. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyère, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing, 2018; epub ahead of print. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef]
- Jun, J.E.; Choi, M.S.; Park, S.W.; Kim, G.; Jin, S.M.; Kim, K.; Hwang, Y.C.; Ahn, K.J.; Chung, H.Y.; Jeong, I.K.; et al. Low Skeletal Muscle Mass Is Associated With the Presence, Incidence, and Progression of Coronary Artery Calcification. Can. J. Cardiol. 2021, 37, 1480–1488. [Google Scholar] [CrossRef]
- Xia, M.F.; Chen, L.Y.; Wu, L.; Ma, H.; Li, X.M.; Li, Q.; Aleteng, Q.; Hu, Y.; He, W.Y.; Gao, J.; et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: A cross-sectional study. Clin. Nutr. 2021, 40, 571–580. [Google Scholar] [CrossRef]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef]
- Garg, L.; Agrawal, S.; Pew, T.; Hanzel, G.S.; Abbas, A.E.; Gallagher, M.J.; Shannon, F.L.; Hanson, I.D. Psoas Muscle Area as a Predictor of Outcomes in Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2017, 119, 457–460. [Google Scholar] [CrossRef]
- Damluji, A.A.; Rodriguez, G.; Noel, T.; Davis, L.; Dahya, V.; Tehrani, B.; Epps, K.; Sherwood, M.; Sarin, E.; Walston, J.; et al. Sarcopenia and health-related quality of life in older adults after transcatheter aortic valve replacement. Am. Heart J. 2020, 224, 171–181. [Google Scholar] [CrossRef]
- Lee, S.A.; Jang, I.Y.; Park, S.Y.; Kim, K.W.; Park, D.W.; Kim, H.J.; Kim, J.B.; Jung, S.H.; Choo, S.J.; Chung, C.H.; et al. Benefit of Sarcopenia Screening in Older Patients Undergoing Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2022, 113, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Drudi, L.M.; Phung, K.; Ades, M.; Zuckerman, J.; Mullie, L.; Steinmetz, O.K.; Obrand, D.I.; Afilalo, J. Psoas Muscle Area Predicts All-Cause Mortality after Endovascular and Open Aortic Aneurysm Repair. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2016, 52, 764–769. [Google Scholar] [CrossRef]
- Newton, D.H.; Kim, C.; Lee, N.; Wolfe, L.; Pfeifer, J.; Amendola, M. Sarcopenia predicts poor long-term survival in patients undergoing endovascular aortic aneurysm repair. J. Vasc. Surg. 2018, 67, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Matsumoto, T.; Inoue, K.; Matsuda, D.; Yoshiga, R.; Yoshiya, K.; Furuyama, T.; Maehara, Y. Sarcopenia is a risk factor for cardiovascular events experienced by patients with critical limb ischemia. J. Vasc. Surg. 2017, 65, 1390–1397. [Google Scholar] [CrossRef]
- Juszczak, M.T.; Taib, B.; Rai, J.; Iazzolino, L.; Carroll, N.; Antoniou, G.A.; Neequaye, S.; Torella, F. Total psoas area predicts medium-term mortality after lower limb revascularization. J. Vasc. Surg. 2018, 68, 1114–1125.e1. [Google Scholar] [CrossRef]
- Pizzimenti, M.; Meyer, A.; Charles, A.L.; Giannini, M.; Chakfé, N.; Lejay, A.; Geny, B. Sarcopenia and peripheral arterial disease: A systematic review. J. Cachexia Sarcopenia Muscle 2020, 11, 866–886. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.O.; Park, S.Y.; Choi, B.G.; Na, J.O.; Choi, C.U.; Kim, E.J.; Rha, S.W.; Park, C.G.; Hong, S.J.; Seo, H.S. Prognostic Impact of Low Skeletal Muscle Mass on Major Adverse Cardiovascular Events in Coronary Artery Disease: A Propensity Score-Matched Analysis of a Single Center All-Comer Cohort. J. Clin. Med. 2019, 8, 712. [Google Scholar] [CrossRef]
- Okamura, H.; Kimura, N.; Mieno, M.; Yuri, K.; Yamaguchi, A. Preoperative sarcopenia is associated with late mortality after off-pump coronary artery bypass grafting. Eur. J. Cardiothorac. Surg. 2020, 58, 121–129. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ Can. Med. Assoc. J. J. L’association Medicale Can. 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Ryan, T.J.; Faxon, D.P.; Gunnar, R.M.; Kennedy, J.W.; King, S.B., 3rd; Loop, F.D.; Peterson, K.L.; Reeves, T.J.; Williams, D.O.; Winters, W.L., Jr.; et al. Guidelines for percutaneous transluminal coronary angioplasty. A report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty). Circulation 1988, 78, 486–502. [Google Scholar] [PubMed]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Kobayashi, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition 2016, 32, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Shiraki, M.; Hiramatsu, A.; Moriya, K.; Hino, K.; Nishiguchi, S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016, 46, 951–963. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Nurmohamed, S.A.; van der Pant, K.; Blauwhoff-Buskermolen, S.; Wierdsma, N.J. Percentiles for skeletal muscle index, area and radiation attenuation based on computed tomography imaging in a healthy Caucasian population. Eur. J. Clin. Nutr. 2018, 72, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Perez, A.A.; Garrett, J.W.; Graffy, P.M.; Zea, R.; Summers, R.M. Fully Automated Deep Learning Tool for Sarcopenia Assessment on CT: L1 Versus L3 Vertebral Level Muscle Measurements for Opportunistic Prediction of Adverse Clinical Outcomes. AJR Am. J. Roentgenol. 2022, 218, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Recio-Boiles, A.; Galeas, J.N.; Goldwasser, B.; Sanchez, K.; Man, L.M.W.; Gentzler, R.D.; Gildersleeve, J.; Hollen, P.J.; Gralla, R.J. Enhancing evaluation of sarcopenia in patients with non-small cell lung cancer (NSCLC) by assessing skeletal muscle index (SMI) at the first lumbar (L1) level on routine chest computed tomography (CT). Support. Care Cancer Off. J. Multinatl. Assoc. Support Care Cancer 2018, 26, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Mitsiopoulos, N.; Baumgartner, R.N.; Heymsfield, S.B.; Lyons, W.; Gallagher, D.; Ross, R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J. Appl. Physiol. 1998, 85, 115–122. [Google Scholar] [CrossRef]
- Rush, E.C.; Goedecke, J.H.; Jennings, C.; Micklesfield, L.; Dugas, L.; Lambert, E.V.; Plank, L.D. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int. J. Obes. 2007, 31, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.J.; Hasni, S. Pathogenesis and Management of Sarcopenia. Clin. Geriatr. Med. 2017, 33, 17–26. [Google Scholar] [CrossRef]
- Sakuma, K.; Aoi, W.; Yamaguchi, A. Molecular mechanism of sarcopenia and cachexia: Recent research advances. Pflug. Arch. 2017, 469, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.-Y.; Kuo, H.-K.; Wu, Y.-T. Sarcopenia, Cardiopulmonary Fitness, and Physical Disability in Community-Dwelling Elderly People. Phys. Ther. 2010, 90, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.; O’Doherty, A.F.; Taylor, C.; Clark, A.L.; Carroll, S.; Ingle, L. Low skeletal muscle mass is associated with low aerobic capacity and increased mortality risk in patients with coronary heart disease—A CARE CR study. Clin. Physiol. Funct. Imaging, 2018; epub ahead of print. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.; Kiens, B.; Hargreaves, M.; Richter, E.A. Influence of active muscle mass on glucose homeostasis during exercise in humans. J. Appl. Physiol. 1991, 71, 552–557. [Google Scholar] [CrossRef]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Després, J.-P. Body Fat Distribution and Risk of Cardiovascular Disease. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Kliemann, N.; Noll, M.; Sarrafzadegan, N.; de Oliveira, C. Visceral obesity and incident cancer and cardiovascular disease: An integrative review of the epidemiological evidence. Obes. Rev. 2021, 22, e13088. [Google Scholar] [CrossRef] [PubMed]
- Erul, E.; Guven, D.C.; Onur, M.R.; Yazici, G.; Aksoy, S. Role of sarcopenia on survival and treatment-related toxicity in head and neck cancer: A narrative review of current evidence and future perspectives. Eur. Arch. Otorhinolaryngol. 2023, 280, 3541–3556. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, D.W.; Ko, Y.; Ha, J.; Shin, Y.B.; Lee, J.; Sung, Y.S.; Kim, K.W. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia. Ageing Res. Rev. 2021, 70, 101398. [Google Scholar] [CrossRef] [PubMed]



| L1 SMI Q1 (n = 124) | L1 SMI Q2 (n = 116) | L1 SMI Q3 (n = 112) | L1 SMI Q4 (n = 123) | p-Value | |
|---|---|---|---|---|---|
| Demographic feature | |||||
| Age (years) | 72.41 ± 9.36 | 66.15 ± 9.70 | 63.57 ± 8.42 | 61.33 ± 9.86 | <0.001 |
| Gender (male) | 88 (70.9) | 78 (67.2) | 78 (69.6) | 86 (69.9) | 0.937 |
| BMI (kg/m2) | 21.58 ± 2.64 | 23.21 ± 2.32 | 24.36 ± 2.92 | 26.62 ± 2.58 | <0.001 |
| Clinical presentation | |||||
| Myocardial infarction | 38 (30.6) | 31 (26.7) | 43 (38.3) | 31 (25.2) | 0.127 |
| Unstable angina | 32 (25.8) | 39 (33.6) | 28 (25.0) | 47 (38.2) | 0.078 |
| Stable angina | 45 (36.2) | 41 (35.3) | 37 (33.0) | 40 (32.5) | 0.913 |
| Past medical history | |||||
| Previous CAD | 78 (62.9) | 73 (62.9) | 69 (61.6) | 88 (71.5) | 0.344 |
| Hypertension | 61 (49.1) | 55 (47.4) | 44 (39.2) | 53 (43.0) | 0.422 |
| Diabetes | 14 (11.2) | 23 (19.8) | 23 (20.5) | 23 (18.6) | 0.204 |
| Diabetes with insulin therapy | 23 (18.5) | 27 (23.3) | 17 (15.2) | 20 (16.3) | 0.392 |
| Dyslipidemia | 11 (8.8) | 9 (7.7) | 7 (6.2) | 13 (10.5) | 0.678 |
| Cerebrovascular accident | 27 (21.7) | 17 (14.6) | 15 (13.3) | 21 (17) | 0.320 |
| Peripheral artery disease | 21 (16.9) | 10 (8.6) | 6 (5.3) | 9 (7.3) | 0.013 |
| Heart failure | |||||
| LVEF < 50% | 41 (33.1) | 27 (23.3) | 26 (23.2) | 16 (13.0) | 0.003 |
| LVEF < 40% | 20 (16.1) | 13 (11.2) | 11 (9.8) | 8 (6.5) | 0.109 |
| Atrial fibrillation | 10 (8.1) | 2 (1.7) | 6 (5.4) | 7 (5.7) | 0.153 |
| Significant valvular disease | 6 (4.8) | 2 (1.7) | 1 (0.9) | 3 (2.4) | 0.285 |
| Chronic kidney disease (stage ≥ 3) | 68 (54.8) | 38 (32.8) | 28 (25.0) | 21 (17.1) | <0.001 |
| Renal replacement therapy | 8 (6.5) | 3 (2.6) | 1 (0.9) | 4 (3.3) | 0.114 |
| Previous malignancy | 11 (8.8) | 8 (6.8) | 5 (4.4) | 8 (6.5) | 0.607 |
| Current smoker | 41 (33.0) | 41 (35.3) | 37 (33.0) | 44 (35.7) | 0.953 |
| Frailty (CFS ≥ 5) | 44 (35.5) | 31 (26.7) | 24 (21.4) | 22 (17.9) | 0.010 |
| Mild to moderate (CFS 5–6) | 33 (26.6) | 29 (25.0) | 21 (18.8) | 22 (17.9) | |
| Severe (CFS 7) | 2 (1.7) | 3 (2.7) | 0 (0.0) | 16 (3.4) | |
| Laboratory data | |||||
| Total cholesterol (mg/dL) | 158.0 (128.2–185.7) | 156.0 (129.0–187.0) | 163.0 (136.0–198.0) | 173.5 (144.2–203.0) | 0.055 |
| LDL-c (mg/dL) | 104.0 (77.5–125) | 94.0 (75.8–128.0) | 95.0 (74.0–130.0) | 110.5 (85.5–140.7) | 0.184 |
| hs-CRP (mg/L) | 6.0 (1.0–17.5) | 3.0 (1.0–12.3) | 2.0 (1.0–9.0) | 2.0 (1.0–7.5) | 0.035 |
| HbA1c (%) | 6.40 ± 1.46 | 6.34 ± 1.70 | 6.61 ± 1.92 | 6.72 ± 1.63 | 0.301 |
| Serum creatinine (mg/dL) | 1.46 ± 2.17 | 1.19 ± 1.32 | 1.29 ± 2.10 | 1.16 ± 1.40 | 0.573 |
| CrCl (mL/min) | 57.99 ± 27.27 | 70.37 ± 29.24 | 78.98 ± 30.42 | 88.07 ± 30.84 | <0.001 |
| LVEF (%) | 50.17 ± 11.47 | 53.02 ± 11.10 | 53.56 ± 10.15 | 55.84 ± 6.98 | <0.001 |
| CT scan information | |||||
| Average days from PCI to CT scan | −1.89 ± 11.72 | −3.58 ± 10.79 | −3.52 ± 11.02 | −4.40 ± 13.19 | 0.407 |
| L1 SMA (cm2) | 65.40 ± 15.34 | 81.69 ± 15.06 | 92.93 ± 16.06 | 111.33 ± 22.23 | <0.001 |
| Male | 72.28 ± 12.21 | 91.30 ± 6.57 | 102.37 ± 7.95 | 123.93 ± 11.88 | <0.001 |
| Female | 48.57 ± 6.82 | 61.96 ± 4.50 | 70.98 ± 3.93 | 82.38 ± 9.81 | <0.001 |
| L1 SMI (cm2/m2) | 24.64 ± 3.97 | 30.65 ± 2.91 | 35.02 ± 3.17 | 41.49 ± 5.20 | <0.001 |
| Male | 25.98 ± 3.66 | 32.49 ± 1.19 | 36.92 ± 1.38 | 43.99 ± 3.82 | <0.001 |
| Female | 21.37 ± 2.56 | 26.89 ± 1.34 | 30.61 ± 0.92 | 35.76 ± 2.87 | <0.001 |
| Available for L3 assessment | 93 (75.0) | 74 (63.8) | 76 (67.9) | 83 (67.5) | 0.295 |
| L3 SMA (cm2) | 78.96 ± 17.49 | 102.93 ± 20.76 | 114.75 ± 23.74 | 134.78 ± 28.75 | <0.001 |
| Male | 87.28 ± 15.19 | 114.69 ± 16.73 | 127.99 ± 17.30 | 149.96 ± 20.00 | <0.001 |
| Female | 63.46 ± 8.80 | 82.93 ± 9.80 | 89.03 ± 10.35 | 101.52 ± 14.80 | <0.001 |
| L3 SMI (cm2/m2) | 29.96 ± 4.77 | 38.84 ± 4.71 | 43.38 ± 5.78 | 50.44 ± 7.22 | <0.001 |
| Male | 31.38 ± 4.80 | 40.55 ± 4.99 | 45.97 ± 5.16 | 53.04 ± 6.63 | <0.001 |
| Female | 27.73 ± 3.68 | 36.07 ± 3.68 | 38.29 ± 3.59 | 44.37 ± 5.02 | <0.001 |
| L1 SMI Q1 (n = 124) | L1 SMI Q2 (n = 116) | L1 SMI Q3 (n = 112) | L1 SMI Q4 (n = 123) | p-Value | |
|---|---|---|---|---|---|
| PCI procedural profiles | |||||
| Number of treated lesions | 1.89 ± 1.14 | 1.71 ± 0.98 | 1.53 ± 0.90 | 1.71 ± 1.04 | 0.049 |
| Number of treated vessels | 1.37 ± 0.63 | 1.27 ± 0.50 | 1.24 ± 0.47 | 1.29 ± 0.59 | 0.280 |
| Treated vessels | |||||
| Left main | 6 (4.8) | 3 (2.5) | 5 (4.4) | 3 (2.4) | 0.677 |
| LAD | 77 (62.0) | 70 (60.3) | 61 (54.4) | 70 (56.9) | 0.636 |
| LCX | 36 (29.0) | 21 (18.1) | 36 (32.1) | 41 (33.3) | 0.039 |
| RCA | 46 (37.0) | 53 (45.6) | 39 (34.8) | 43 (34.9) | 0.271 |
| Lesion type B2C | 115 (92.7) | 110 (94.8) | 107 (95.5) | 115 (93.4) | 0.796 |
| Multivessel disease | 31 (25.0) | 29 (25.0) | 31 (27.6) | 26 (21.1) | 0.709 |
| Left main disease | 9 (7.2) | 9 (7.7) | 11 (9.8) | 8 (6.5) | 0.807 |
| Diffuse lesion (>30 mm) | 46 (37) | 47 (40.5) | 49 (43.7) | 44 (35.7) | 0.595 |
| Small vessel disease (<2.25 mm) | 7 (5.6) | 7 (6.0) | 11 (9.8) | 9 (7.3) | 0.605 |
| Intravascular imaging | 13 (10.5) | 9 (7.8) | 13 (12.6) | 10 (8.1) | 0.706 |
| Number of inserted stents | 1.85 ± 1.01 | 1.70 ± 0.96 | 1.50 ± 0.80 | 1.68 ± 0.97 | 0.043 |
| Average stent diameter (mm) | 2.90 ± 0.39 | 3.01 ± 0.46 | 3.02 ± 0.37 | 2.98 ± 0.46 | 0.128 |
| Total stent length (mm) | 43.67 ± 27.48 | 40.14 ± 27.69 | 36.49 ± 23.01 | 39.26 ± 28.28 | 0.210 |
| Bare metal stents | 2 (1.6) | 5 (4.3) | 2 (1.7) | 1 (0.8) | 0.361 |
| Drug eluting stents | 122 (98.3) | 113 (97.4) | 110 (98.2) | 123 (100.0) | 0.404 |
| 1st generation | 25 (20.1) | 28 (24.1) | 23 (20.5) | 17 (13.8) | 0.239 |
| 2nd generation | 97 (78.2) | 85 (73.2) | 87 (77.6) | 106 (86.1) | 0.100 |
| Post-procedural medication | |||||
| Aspirin | 112 (90.3) | 106 (91.3) | 105 (93.7) | 113 (91.8) | 0.813 |
| Clopidogrel | 101 (81.4) | 104 (89.6) | 102 (91.0) | 110 (89.4) | 0.091 |
| Oral anticoagulants | 5 (4.0) | 1 (0.9) | 6 (5.4) | 5 (4.1) | 0.250 |
| RAS blockers | 71 (57.2) | 75 (64.6) | 70 (62.5) | 80 (65.0) | 0.570 |
| Statins | 110 (89.4) | 109 (97.3) | 103 (88.7) | 100 (80.6) | <0.001 |
| Beta blockers | 62 (50.0) | 53 (45.6) | 56 (50.0) | 58 (47.1) | 0.884 |
| Calcium channel blockers | 40 (32.2) | 38 (32.7) | 34 (30.3) | 38 (30.8) | 0.977 |
| L1 SMI Q1 (n = 124) | L1 SMI Q2 (n = 116) | L1 SMI Q3 (n = 112) | L1 SMI Q4 (n = 123) | Log-Rank p-Value | |
|---|---|---|---|---|---|
| All-cause mortality | 27 (23.2) | 11 (9.9) | 7 (6.6) | 5 (4.4) | <0.001 |
| Cardiac death | 8 (7.4) | 5 (4.6) | 1 (1.0) | 3 (2.6) | 0.081 |
| Non-cardiac death | 19 (17.0) | 6 (5.5) | 6 (5.7) | 2 (1.8) | <0.001 |
| Non-fatal MI | 9 (8.7) | 3 (3.0) | 2 (2.0) | 3 (2.6) | 0.038 |
| STEMI | 5 (4.4) | 1 (1.2) | 1 (1.0) | 2 (1.8) | 0.152 |
| Non-STEMI | 4 (4.5) | 2 (1.8) | 1 (1.0) | 1 (0.8) | 0.301 |
| Repeat revascularization | 20 (24.9) | 15 (15.2) | 7 (7.1) | 4 (3.8) | <0.001 |
| TVR | 16 (20.3) | 11 (11.1) | 6 (6.2) | 3 (2.8) | 0.001 |
| non-TVR | 6 (8.1) | 5 (5.9) | 3 (3.1) | 1 (1.0) | 0.114 |
| MACE | 47 (42.9) | 26 (24.0) | 15 (14.3) | 7 (6.2) | <0.001 |
| 3-Year All-Cause Mortality | 3-Year MACE | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Model 1 a | ||||
| L1 SMI quartiles | <0.001 (for trend) | <0.001 (for trend) | ||
| Quartile 4 | 1.00 (reference) | 1.00 (reference) | ||
| Quartile 3 | 1.52 (0.48–4.81) | 0.468 | 2.41 (0.98–5.91) | 0.054 |
| Quartile 2 | 2.32 (0.80–6.68) | 0.118 | 4.13 (1.79–9.52) | 0.001 |
| Quartile 1 | 6.07 (2.33–15.7) | <0.001 | 8.45 (3.81–18.7) | <0.001 |
| Model 2 b | ||||
| L1 SMI quartiles | 0.007 (for trend) | <0.001 (for trend) | ||
| Quartile 4 | 1.00 (reference) | 1.00 (reference) | ||
| Quartile 3 | 1.71 (0.52–5.56) | 0.370 | 3.17 (1.28–7.86) | 0.013 |
| Quartile 2 | 2.20 (0.72–6.74) | 0.164 | 5.93 (2.47–14.2) | <0.001 |
| Quartile 1 | 5.62 (1.77–17.8) | 0.003 | 15.5 (6.28–38.4) | <0.001 |
| Model 3 c | ||||
| L1 SMI quartiles | 0.030 (for trend) | <0.001 (for trend) | ||
| Quartile 4 | 1.00 (reference) | 1.00 (reference) | ||
| Quartile 3 | 1.76 (0.54–5.71) | 0.342 | 3.09 (1.25–7.65) | 0.014 |
| Quartile 2 | 2.32 (0.76–7.08) | 0.139 | 5.95 (2.49–14.2) | <0.001 |
| Quartile 1 | 4.93 (1.54–15.7) | 0.007 | 12.7 (5.13–31.6) | <0.001 |
| Model 4 d | ||||
| L1 SMI quartiles | 0.032 (for trend) | <0.001 (for trend) | ||
| Quartile 4 | Reference | Reference | ||
| Quartile 3 | 1.83 (0.56–5.97) | 0.315 | 3.23 (1.29–8.07) | 0.012 |
| Quartile 2 | 2.25 (0.74–6.79) | 0.149 | 5.54 (2.31–13.2) | <0.001 |
| Quartile 1 | 4.90 (1.54–15.5) | 0.007 | 12.3 (4.99–30.4) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.J.; Park, S.Y.; Kang, J.; Chu, W.; Kang, D.O. Quantitative Association between Computed-Tomography-Based L1 Skeletal Muscle Indices and Major Adverse Clinical Events Following Percutaneous Coronary Intervention. J. Clin. Med. 2023, 12, 7483. https://doi.org/10.3390/jcm12237483
Park EJ, Park SY, Kang J, Chu W, Kang DO. Quantitative Association between Computed-Tomography-Based L1 Skeletal Muscle Indices and Major Adverse Clinical Events Following Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2023; 12(23):7483. https://doi.org/10.3390/jcm12237483
Chicago/Turabian StylePark, Eun Jin, So Yeon Park, Jaeho Kang, Wonsang Chu, and Dong Oh Kang. 2023. "Quantitative Association between Computed-Tomography-Based L1 Skeletal Muscle Indices and Major Adverse Clinical Events Following Percutaneous Coronary Intervention" Journal of Clinical Medicine 12, no. 23: 7483. https://doi.org/10.3390/jcm12237483
APA StylePark, E. J., Park, S. Y., Kang, J., Chu, W., & Kang, D. O. (2023). Quantitative Association between Computed-Tomography-Based L1 Skeletal Muscle Indices and Major Adverse Clinical Events Following Percutaneous Coronary Intervention. Journal of Clinical Medicine, 12(23), 7483. https://doi.org/10.3390/jcm12237483





