1. Introduction
Glaucoma, a group of neurodegenerative ophthalmic diseases, leads to optic nerve damage, visual field constriction, and vision loss [
1]. Elevated intraocular pressure (IOP) is a significant risk factor for glaucoma, and the only proven treatment involves IOP reduction through medication, laser therapy, or incisional surgery. Minimally Invasive Glaucoma Surgery (MIGS) has emerged as an effective strategy, offering favorable outcomes and enhanced safety [
2]. The Tanito microhook ab interno trabeculotomy (TMH), a MIGS procedure using a metal hook to incise the trabecular meshwork has been noted to effectively reduce IOP and decrease dependency on antiglaucoma medications [
3].
Originally, TMH involved incising both nasal and temporal trabecular meshworks with three types of hooks, resulting in a 240-degree incision. Studies on cadaver eyes suggested that the extent of trabeculotomy incision influences IOP reduction [
4]. While some studies reported varying IOP reduction efficacy based on the width of the surgical extent [
5,
6,
7], others found no such differences [
8,
9,
10,
11,
12,
13]. Assessments of the correlation between surgical extent and IOP reduction efficacy often involved comparing different surgical procedures or groups with varying preoperative IOP or patient backgrounds [
5,
6,
7,
10,
13]. Some studies included only a small number of subjects [
8,
11]. Although the results are not conclusive [
14,
15,
16], cataract removal by goniotomy may favorably impact IOP reduction [
17]. Thus, including both combined cataract and goniotomy and goniotomy alone in efficacy assessments can introduce bias.
In the current study, we comprehensively compared surgical efficacy and safety profiles between 120-degree and 240-degree TMH to evaluate the impact of surgical extent on IOP reduction. To minimize confounding factors, the study included only patients with open-angle glaucoma undergoing combined cataract and TMH surgery performed by a single surgeon. Additionally, patient background factors such as age, sex, preoperative IOP, and glaucoma type were matched between the two groups using propensity score matching before comparison.
2. Subjects and Methods
2.1. Participants
This study adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board (IRB) of Shimane University Hospital (IRB No. 20200517-1, revised protocol issued on 15 June 2023). All participants provided written informed consent for surgery prior to the surgery. The IRB approval did not require individual written informed consent for publication. Instead, the study protocol was publicly posted at the study institutions to inform participants about the study. Only anonymized data were used for statistical analysis.
We retrospectively included participants who met the following criteria: individuals who underwent surgery performed by one surgeon (M.T.) at Shimane University Hospital from April 2018 to August 2021; those who underwent combined TMH and cataract surgery; and those diagnosed with primary open angle glaucoma (POAG) or exfoliation glaucoma (EXG), with no history of previous intraocular or glaucoma surgeries, and were followed up for at least 12 months postoperatively. The exclusion criteria included intraoperative posterior capsular rupture or Zinn’s zonular dialysis. In total, 185 eyes were selected from our institutional database. For comparison between nasal-only incision and bilateral incision procedures in TMH, an equal number of eyes from each group were selected using propensity score matching (caliper coefficient = 0.20), based on age, sex, preoperative IOP, and glaucoma type. Only one eye per subject was chosen for the matching. In our hospital, bilateral incision was predominantly used until June 2019, while nasal incision was primarily chosen from July 2019, with most cases treated with nasal incision after April 2021. This chronological change enabled us to collect data from both nasal and bilateral incisions, resulting in 67 eyes from 67 subjects being selected for each group.
2.2. Measurements
We collected clinical parameters from medical charts, including age, sex, glaucoma type, ocular surgical history, surgical procedure, preoperative and postoperative best corrected visual acuities (BCVA), IOP, number of antiglaucoma medications, anterior chamber (AC) flare (measured using the FM-600 laser flare meter, Kowa, Nagoya, Japan), corneal endothelial cell density (CECD) (measured using the EM-3000 specular microscope, Tomey, Nagoya, Japan), axial length (measured using the OA2000 optical biometer, Tomey), visual field MD (central 30-2 program, Humphrey Visual Field Analyzer, Carl Zeiss Meditec, Dublin, CA, USA), and the duration of postoperative follow-up. Subjects with exfoliation material in only one eye (by slit lamp examination) were defined as unilateral EXG; the other eye was defined as POAG. Decimal BCVA was converted to the logarithm of the minimum angle of resolution VA. Counting fingers, hand motions, light perception, and no light perception were assigned decimal VAs of 0.0025, 0.002, 0.0016, and 0.0013, respectively [
18]. IOP was measured using Goldmann applanation tonometry, except on postoperative day 3 when the iCARE rebound tonometer (M.E. Technica, Tokyo, Japan) was used. The medication score accounted for 1 point per component of topical medication or 1 tablet of oral acetazolamide. Information on the site of trabeculotomy, perioperative complications, interventions for complications, and additional glaucoma surgeries were collected from medical and surgical records. The postoperative follow-up periods were defined as 1–3 days for post-operative day (POD) 3, 1–3 weeks for postoperative week (POW) 2, 2–4 months for post-operative month (POM) 3, 5–7 months for POM 6, 8–10 months for POM 9, and 11–14 months for POM 12.
The Shimane University Postoperative Hyphema Scoring system (SU-RLC) [
19] is utilized to assess the severity of hyphema. This method evaluates hyphema severity through a slit-lamp examination, focusing on three primary elements: red blood cells (RBCs) (R), layering (L), and clot formation (C). The RBCs aspect (R) is rated on a scale from 0 (no visible floating RBCs in the Anterior Chamber [AC]) to 3 (dense floating RBCs obscuring iris patterns). The layering factor (L) is scored from 0 (absence of layer formation) to 3 (layer extending above the lower margin of the pupil). Clot formation (C) is either 0 (no clots in the AC) or 1 (presence of clots). The cumulative total SU-RLC score, therefore, varies from 0 to 7, offering a comprehensive assessment of hyphema severity. The highest total SU-RLC score during postoperative follow-up periods was adopted as the value for each case. In the majority of cases, the highest score was recorded 1–3 days postoperatively.
2.3. Surgical Procedures
Details of the surgical procedures have been previously described [
3,
16,
20]. In brief, TMH was preceded by phacoemulsification cataract surgery through a 2.2 mm wide, clear corneal incision at the 9–10 o’clock position (temporal incision for the right eye, nasal incision for the left eye). A one-piece soft acrylic intraocular lens was inserted into the capsular bag through this incision. After cataract surgery, specifically designed spatula-shaped microhooks (M-2215S, 2215R, and 2215L, Inami, Tokyo, Japan) were used for TMH. Viscoelastic material (1% sodium hyaluronate or Opegan Hi) was injected into the anterior chamber through corneal ports created with a 20-gauge microvitreoretinal knife at the 2–3 and 9–10 o’clock positions. A microhook was inserted into the AC through a corneal port, and the angle opposite the port was observed using a Swan–Jacob gonioprism lens. The microhook tip was inserted into Schlemm’s canal and moved circumferentially to incise the inner wall of Schlemm’s canal and TM for more than 3 clock-hour-width at the nasal or both nasal and temporal positions. After TMH, viscoelastic material was aspirated, and corneal ports were closed by corneal stromal hydration. At the end of surgery, a steroid (2 mg of betamethasone sodium phosphate, Rinderone, Shionogi Pharmaceutical) was injected subconjunctivally, and 0.3% ofloxacin ointment (Tarivid, Santen Pharmaceutical) was applied. Postoperatively, 1.5% levofloxacin (Nipro, Osaka, Japan) and 0.1% betamethasone (Sanbetason, Santen Pharmaceutical) were applied topically four times daily for 3–4 weeks.
2.4. Statistical Analysis
The measurement parameters were compared between the nasal and bilateral incision groups using the Wilcoxon signed rank test for continuous data, Fisher’s exact probability test for categorical data, and the Cochran–Armitage trend test for ordinal data. Kaplan–Meier curves analyzed the estimated survival probability for qualified IOP control. Successful IOP control was evaluated using survival curve analysis, considering cases that were uncensored when IOP exceeded 15 mmHg (criterion A) or 12 mmHg (criterion B) after 3 months postoperatively, when IOP reduction was less than 20% (both definitions), when additional glaucoma surgery was required at any time (both definitions), or when there was a loss of light perception (both definitions). Cases not meeting these criteria were considered censored. The use or non-use of antiglaucoma medication was not factored into the survival curve analysis, as most cases continued medication postoperatively. Log-rank tests assessed the difference in survival rates between surgical groups. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using JMP Pro version 17.1 (SAS Institute, Inc., Cary, NC, USA).
3. Results
The demographic data of the subjects, including age, sex, laterality, types of glaucoma, BCVA, IOP, medication score, axial length, AC flare, CECD, and MD values measured preoperatively, are summarized in
Table 1. In the nasal and bilateral incision groups, the mean ages were 71.0 years and 71.6 years, respectively, with males constituting 51% and 52% of each group, respectively. There were no significant differences in any of the demographic data between the two procedure groups.
Table 2 presents a comparison of IOP between the nasal and bilateral incision groups. Preoperative IOP, which was 18.6 mmHg in both groups, decreased to 13.2 mmHg (29% reduction) in the nasal incision group and to 12.8 mmHg (31% reduction) in the bilateral incision group at 12 months postoperatively. The IOPs were comparable between the groups throughout the study period of up to 12 months postoperatively.
Table 3 shows the comparison of the number of antiglaucoma medications between the nasal and bilateral incision groups. The preoperative medication score of 3.4 in the nasal incision group and 3.1 in the bilateral incision group was reduced to 2.7 (21% reduction) and 2.5 (19% reduction), respectively, at 12 months postoperatively. The medication scores were similar between groups throughout the study period of up to 12 months postoperatively.
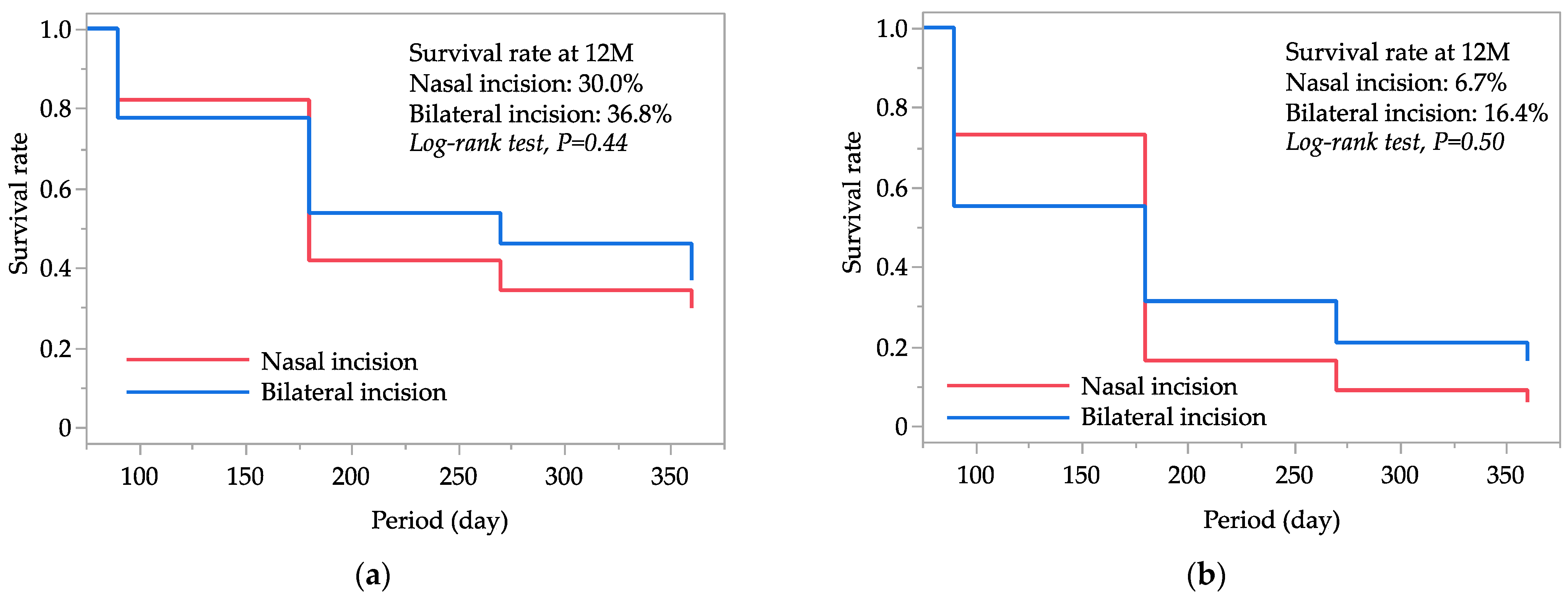
Figure 1 displays the Kaplan–Meier survival curves for successful IOP control in both the nasal and bilateral incision groups. At 12 months postoperatively, the cumulative survival rates for successful IOP control were 30.0% in the nasal incision group and 36.8% in the bilateral incision group based on criterion A, and 6.7% and 16.4%, respectively, based on criterion B. The comparison between the two groups using log-rank statistics yielded
p-values of 0.44 for criterion A and 0.50 for criterion B, indicating no significant differences in survival rates for successful IOP control between the nasal and bilateral incision groups.
Table 4 presents a comparison of postoperative complications, other than hyphema, and interventions between the nasal and bilateral incision groups. The occurrence of any complications and the need for interventions were not significantly different between the groups. All five additional glaucoma surgeries recorded in this dataset were Ahmed Glaucoma Valve implantations.
Table 5 details the comparison of postoperative hyphema scores between the groups. Postoperatively, the frequency of hyphema, including minimal floating RBCs in the AC, was 97% in the nasal incision group and 100% in the bilateral incision group. The mean total RLC scores were 3.1 in the nasal incision group and 3.5 in the bilateral incision group, which was not statistically significant (
p = 0.08); the mode of the score was 2 (24%) in the nasal group, while it was 3 (49%) and 4 (34%) in the bilateral incision group. For each component of the hyphema score, score R was significantly higher in the bilateral incision group than in the nasal incision group (
p < 0.0001), whereas scores L and C were similar between the groups. The incidence of layered hyphema was 33% in the nasal incision group compared to 25% in the bilateral incision group.
Table 6 presents a comparison of BCVAs between the nasal and bilateral incision groups. Preoperative BCVA, which was 0.28 in the nasal incision group and 0.24 in the bilateral incision group, improved to 0.04 in the nasal group and 0.07 in the bilateral group at 12 months postoperatively. The BCVAs were comparable between the groups throughout the study period of up to 12 months.
Table 7 shows the comparison of AC flare between the nasal and bilateral incision groups. The preoperative AC flare was 10.8 pc/ms in both groups, changing to 11.5 pc/ms in the nasal group and 10.6 pc/ms in the bilateral group at 12 months postoperatively. The AC flares were similar between the groups throughout the study period of up to 12 months.
Table 8 details the comparison of CECDs between the nasal and bilateral incision groups. The preoperative CECD was 2468 cells/mm
2 in the nasal group and 2442 cells/mm
2 in the bilateral group, decreasing to 2338 cells/mm
2 (5% reduction) in the nasal group and 2243 cells/mm
2 (8% reduction) in the bilateral group at 12 months postoperatively. The CECDs were equivalent between the groups throughout the study period of up to 12 months.
Table 9 presents a comparison of visual field MDs between the nasal and bilateral incision groups. The preoperative MD was −14.3 dB in the nasal group and −15.2 dB in the bilateral group, improving to −13.6 dB in the nasal group and −13.4 dB in the bilateral group at 12 months postoperatively. The MDs were equivalent between the groups both preoperatively and at 12 months postoperatively.
4. Discussion
In this study of TMH combined with cataract surgery, surgical efficacy indicators such as IOP, medication score, survival rate, BCVA, and visual field MD were comparable between the nasal and bilateral incision groups. Postoperative hyphema was more severe in the bilateral incision group than in the nasal incision group, while other safety profiles, including complications other than hyphema, interventions, AC flare, and CECD, were equivalent between the groups.
A study on perfused autopsy eyes showed that cuts in the trabecular meshwork at width corresponding to 1, 4, and 12 clock hours led to reductions in outflow resistance by 30%, 44%, and 51%, respectively, under a perfusion pressure of 7 mmHg, and by 30%, 56%, and 72%, respectively, under a pressure of 25 mmHg [
4]. This suggests that different extents of trabeculotomy can result in varying levels of IOP reduction. Comparing IOP reductions from angle incisions on either one or both sides and exploring the correlation between the extent of these incisions and IOP reductions following goniotomy/trabeculotomy could be insightful. Previous studies, such as those examining the extent of incision after Trabectome (from 0 to 160 degrees) [
21] or gonioscopy-assisted transluminal trabeculotomy (GATT) (from 150 to 320 degrees) [
8], did not find an association with postoperative IOP. Similarly, a single-center retrospective study observed no significant difference in IOP reduction, medication score, or success rate of IOP reduction between 1-quadrant and 2-quadrants TMH [
11]. More recently, a multicenter study reported no significant differences in IOP reduction, medication number, and cumulative survival probability for complete and qualified success rates among 120-, 240-, and 360-degree standalone goniotomy, as well as when these procedures were combined with cataract surgery [
13]. In light of these findings, along with the results of our current study, a one-quadrant trabeculotomy may be sufficient for treating most cases of open-angle glaucoma. We found no significant difference in IOP reduction effectiveness across all cases, regardless of the width of the trabecular meshwork incision. However, there might be variability in individual cases, suggesting the need for future research to identify specific case characteristics where a wider trabecular meshwork incision could be more beneficial.
Other studies have reported greater IOP reductions with goniotomy using a Kahook Dual Blade (KDB) at 90 degrees compared to the first-generation iStent trabecular bypass implantation with one 120 µm-lumen stent [
22,
23,
24,
25] or the second-generation iStent inject W with two 80 µm-lumen stents [
26,
27]. In our previous studies, fellow-eye comparisons demonstrated a greater IOP reduction with TMH than with both the first- [
28] and second- [
29] generation iStent when combined with cataract surgery. Further, other research has shown similar IOP reduction effectiveness between TMH and KDB [
30,
31]. A recent multicenter study reported comparable IOP reduction effectiveness and safety profiles between one-quadrant TMH and iStent inject W [
32]. These findings suggest that goniotomy with a 90-degree incision may achieve a clinically detectable maximal IOP reduction. However, the minimal incision width required to attain maximal IOP reduction to achieve “minimally invasive” surgery warrants further investigation.
We observed that the presence of RBCs in the anterior chamber, as indicated by the SU-R score, was significantly higher in the bilateral incision group than in the nasal incision group. This increase in RBC presence did not correspond with a higher incidence of layered hyphema or blood clots. Postoperative layered hyphema was more frequently reported with a 360-degree incision compared to a 180-degree incision in GATT procedures [
9]. Similarly, the incidence of hyphema was more common following goniotomy with a 360-degree incision than with a 120-degree incision [
12,
13]. In our previous research, we reported higher hyphema scores in eyes undergoing TMH than in those receiving first- and second-generation iStent implants during the early postoperative period [
19,
29]. These findings suggest that the severity of hyphema correlates with the extent of incision width following angle surgeries. However, the differences in SU-R scores during the early postoperative period did not impact other safety profiles or the final visual function, although they likely influenced the early recovery of vision. Therefore, if the effectiveness of IOP reduction is similar between the two groups, the nasal-incision approach, which may permit a faster recovery of visual function, could be considered clinically more advantageous than the bilateral-incision method.
The study had several limitations, including its retrospective design and lack of randomization. Propensity score matching was utilized to minimize these limitations. The study primarily included cases treated with combined TMH and cataract surgery, predominantly involving an elderly population, which may limit the generalizability of the findings. Lack of information on the use of anticoagulants and antiplatelet agents was also a study limitation. The relatively short follow-up period is another limitation. However, the study also had strengths, such as a comprehensive assessment of patient clinical characteristics and a matched-group analysis to mitigate the influence of background factors.