The Potential of Intertwining Gene Diagnostics and Surgery for Mitral Valve Prolapse
Abstract
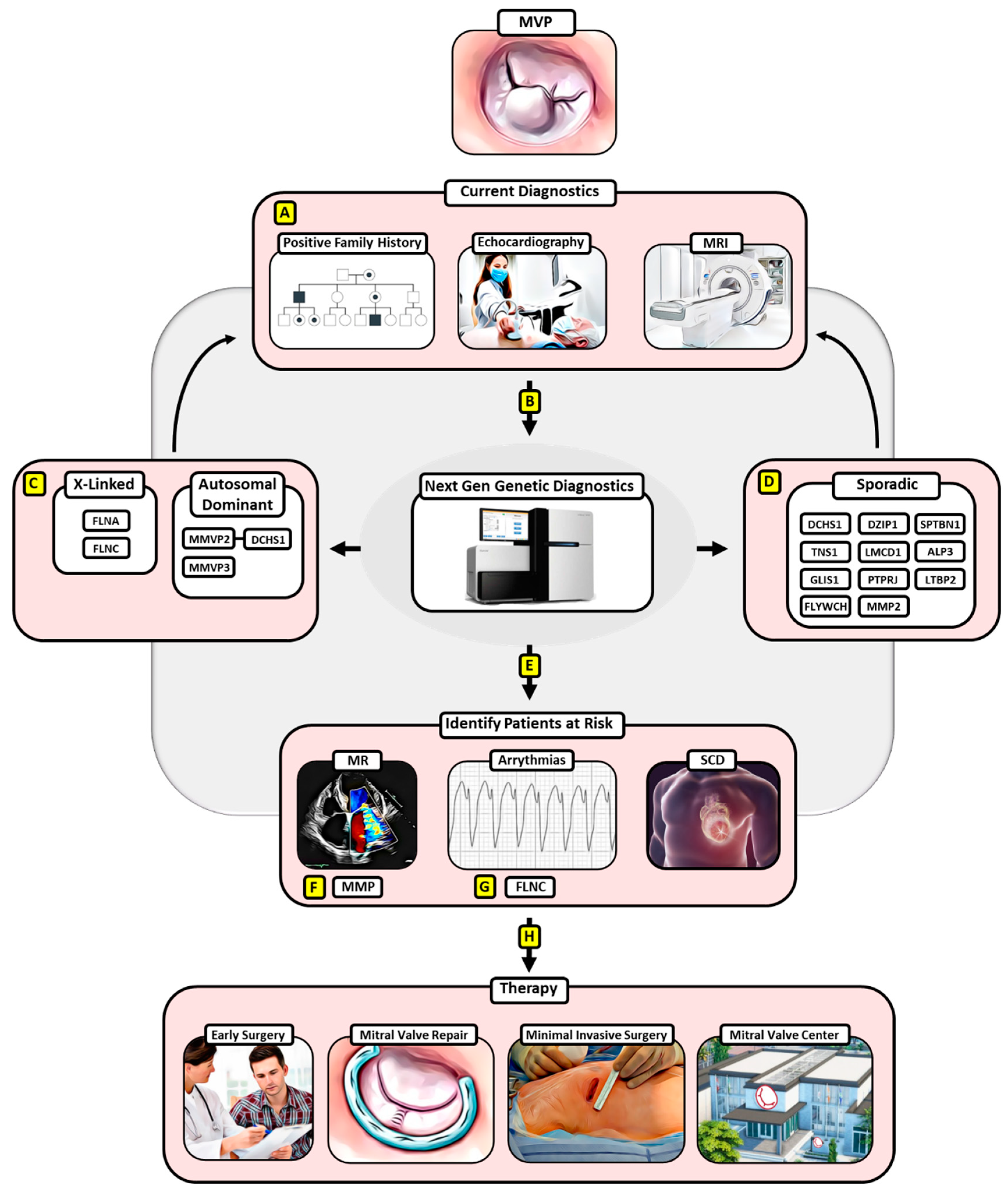
:1. Introduction
2. X-Linked Heritage
3. Autosomal-Dominant Heritage
4. Sporadic MVP
4.1. DZIP1
4.2. TNS1
4.3. LMCD1
4.4. GLIS1
4.5. PTPRJ and FLYWCH
4.6. MMP2
4.7. SPTBN1
4.8. ALPK3
4.9. LTBP2
5. Paving the Way for Screening Patients at Risk
6. Early Surgery for MVP May Provide a Treatment Option for at-Risk MVP Patients
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, P.-Y.; Tsai, K.-Z.; Lin, Y.-P.; Lin, C.-S.; Zeng, H.-C.; Takimoto, E.; Lin, G.-M. Prevalence and characteristics of mitral valve prolapse in military young adults in Taiwan of the CHIEF Heart Study. Sci. Rep. 2021, 11, 2719. [Google Scholar] [CrossRef] [PubMed]
- Zuppiroli, A.; Rinaldi, M.; Kramer-Fox, R.; Favilli, S.; Roman, M.J.; Devereux, R.B. Natural history of mitral valve prolapse. Am. J. Cardiol. 1995, 75, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Waller, B.F.; Morrow, A.G.; Maron, B.J.; Del Negro, A.A.; Kent, K.M.; McGrath, F.J.; Wallace, R.B.; McIntosh, C.L.; Roberts, W.C. Etiology of clinically isolated, severe, chronic, pure mitral regurgitation: Analysis of 97 patients over 30 years of age having mitral valve replacement. Am. Heart J. 1982, 104, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; McGoon, M.D.; Shub, C.; Miller, F.A.; Ilstrup, D.M.; Tajik, A. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N. Engl. J. Med. 1985, 313, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Avierinos, J.-F.; Gersh, B.J.; Melton, L.J.; Bailey, K.R.; Shub, C.; Nishimura, R.A.; Tajik, A.J.; Enriquez-Sarano, M. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002, 106, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Smer, A.; Nanda, N.C.; Akdogan, R.E.; Elmarzouky, Z.M.; Dulal, S. Echocardiographic evaluation of mitral valve regurgitation. Mini-Invasive Surg. 2020, 4, 52. [Google Scholar] [CrossRef]
- Chaliki, H.P.; Nishimura, R.A.; Enriquez-Sarano, M.; Reeder, G.S. A simplified, practical approach to assessment of severity of mitral regurgitation by Doppler color flow imaging with proximal convergence: Validation with concomitant cardiac catheterization. Mayo Clin. Proc. 1998, 73, 929–935. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Biaggi, P.; Jedrzkiewicz, S.; Gruner, C.; Meineri, M.; Karski, J.; Vegas, A.; Tanner, F.C.; Rakowski, H.; Ivanov, J.; David, T.E.; et al. Quantification of mitral valve anatomy by three-dimensional transesophageal echocardiography in mitral valve prolapse predicts surgical anatomy and the complexity of mitral valve repair. J. Am. Soc. Echocardiogr. 2012, 25, 758–765. [Google Scholar] [CrossRef]
- Christiansen, J.P.; Karamitsos, T.D.; Myerson, S.G. Assessment of valvular heart disease by cardiovascular magnetic resonance imaging: A review. Heart Lung Circ. 2011, 20, 73–82. [Google Scholar] [CrossRef]
- Rabkin, E.; Aikawa, M.; Stone, J.R.; Fukumoto, Y.; Libby, P.; Schoen, F.J. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001, 104, 2525–2532. [Google Scholar] [CrossRef]
- van Wijngaarden, A.L.; Kruithof, B.P.T.; Vinella, T.; Barge-Schaapveld, D.; Ajmone Marsan, N. Characterization of Degenerative Mitral Valve Disease: Differences between Fibroelastic Deficiency and Barlow’s Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 23. [Google Scholar] [CrossRef]
- Fornes, P.; Heudes, D.; Fuzellier, J.F.; Tixier, D.; Bruneval, P.; Carpentier, A. Correlation between clinical and histologic patterns of degenerative mitral valve insufficiency: A histomorphometric study of 130 excised segments. Cardiovasc. Pathol. 1999, 8, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, N.A.; De Bonis, M.; Di Resta, C.; Ascione, G.; Alfieri, O.; Maisano, F.; Vergara, P. Genetic background of mitral valve prolapse. Rev. Cardiovasc. Med. 2022, 23, 96. [Google Scholar] [CrossRef] [PubMed]
- Delling, F.N.; Noseworthy, P.A.; Adams, D.H.; Basso, C.; Borger, M.; Bouatia-Naji, N.; Elmariah, S.; Evans, F.; Gerstenfeld, E.; Hung, J.; et al. Research Opportunities in the Treatment of Mitral Valve Prolapse: JACC Expert Panel. J. Am. Coll. Cardiol. 2022, 80, 2331–2347. [Google Scholar] [CrossRef] [PubMed]
- Sriram, C.S.; Syed, F.F.; Ferguson, M.E.; Johnson, J.N.; Enriquez-Sarano, M.; Cetta, F.; Cannon, B.C.; Asirvatham, S.J.; Ackerman, M.J. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2013, 62, 222–230. [Google Scholar] [CrossRef]
- Basso, C.; Marra, M.P.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Badhwar, V.; Chikwe, J.; Gillinov, A.M.; Vemulapalli, S.; O’gara, P.T.; Mehaffey, J.H.; von Ballmoos, M.W.; Bowdish, M.E.; Gray, E.L.; O’brien, S.M.; et al. Risk of Surgical Mitral Valve Repair for Primary Mitral Regurgitation. Ann. Thorac. Surg. 2023, 115, 600–610. [Google Scholar] [CrossRef]
- Suri, R.M.; Vanoverschelde, J.-L.; Grigioni, F.; Schaff, H.V.; Tribouilloy, C.; Avierinos, J.-F.; Barbieri, A.; Pasquet, A.; Huebner, M.; Rusinaru, D.; et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 2013, 310, 609–616. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef] [PubMed]
- Shuhaiber, J.; Anderson, R.J. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur. J. Cardiothorac. Surg. 2007, 31, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, V.; Vemulapalli, S.; Mack, M.A.; Gillinov, A.M.; Chikwe, J.; Dearani, J.A.; Grau-Sepulveda, M.V.; Habib, R.; Rankin, J.S.; Jacobs, J.P.; et al. Volume-Outcome Association of Mitral Valve Surgery in the United States. JAMA Cardiol. 2020, 5, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Sündermann, S.H.; Sromicki, J.; Biefer, H.R.C.; Seifert, B.; Holubec, T.; Falk, V.; Jacobs, S. Mitral valve surgery: Right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2014, 148, 1989–1995.e1984. [Google Scholar] [CrossRef] [PubMed]
- Newbury-Ecob, R.A.; Zuccollo, J.M.; Rutter, N.; Young, I.D. Sex linked valvular dysplasia. J. Med. Genet. 1993, 30, 873–874. [Google Scholar] [CrossRef]
- Kyndt, F.; Gueffet, J.-P.; Probst, V.; Jaafar, P.; Legendre, A.; Le Bouffant, F.; Toquet, C.; Roy, E.; McGregor, L.; Lynch, S.A.; et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation 2007, 115, 40–49. [Google Scholar] [CrossRef]
- Bandaru, S.; Ala, C.; Zhou, A.X.; Akyürek, L.M. Filamin A Regulates Cardiovascular Remodeling. Int. J. Mol. Sci. 2021, 22, 6555. [Google Scholar] [CrossRef]
- Sauls, K.; de Vlaming, A.; Harris, B.S.; Williams, K.; Wessels, A.; Levine, R.A.; Slaugenhaupt, S.A.; Goodwin, R.L.; Pavone, L.M.; Merot, J.; et al. Developmental basis for filamin-A-associated myxomatous mitral valve disease. Cardiovasc. Res. 2012, 96, 109–119. [Google Scholar] [CrossRef]
- Sasaki, A.; Masuda, Y.; Ohta, Y.; Ikeda, K.; Watanabe, K. Filamin associates with Smads and regulates transforming growth factor-beta signaling. J. Biol. Chem. 2001, 276, 17871–17877. [Google Scholar] [CrossRef]
- Galvin, K.M.; Donovan, M.J.; Lynch, C.A.; Meyer, R.I.; Paul, R.J.; Lorenz, J.N.; Fairchild-Huntress, V.; Dixon, K.L.; Dunmore, J.H.; Gimbrone, M.A.; et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat. Genet. 2000, 24, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Aalberts, J.J.; van Tintelen, J.P.; Oomen, T.; Bergman, J.E.; Halley, D.J.; Jongbloed, J.D.; Suurmeijer, A.J.; van den Berg, M.P. Screening of TGFBR1, TGFBR2, and FLNA in familial mitral valve prolapse. Am. J. Med. Genet. A 2014, 164, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Duval, D.; Lardeux, A.; Le Tourneau, T.; Norris, R.; Markwald, R.; Sauzeau, V.; Probst, V.; Le Marec, H.; Levine, R.; Schott, J.; et al. Valvular dystrophy associated filamin A mutations reveal a new role of its first repeats in small-GTPase regulation. Biochim. Biophys. Acta 2014, 1843, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Duval, D.; Labbé, P.; Bureau, L.; Le Tourneau, T.; Norris, R.A.; Markwald, R.R.; Levine, R.; Schott, J.J.; Mérot, J. MVP-Associated Filamin A Mutations Affect FlnA-PTPN12 (PTP-PEST) Interactions. J. Cardiovasc. Dev. Dis. 2015, 2, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Disse, S.; Abergel, E.; Berrebi, A.; Houot, A.-M.; Le Heuzey, J.-Y.; Diebold, B.; Guize, L.; Carpentier, A.; Corvol, P.; Jeunemaitre, X. Mapping of a first locus for autosomal dominant myxomatous mitral-valve prolapse to chromosome 16p11.2-p12.1. Am. J. Hum. Genet. 1999, 65, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.A.; Acierno, J.S.; Dai, D.; Leyne, M.; Marshall, J.E.; Nesta, F.; Levine, R.A.; Slaugenhaupt, S.A. A locus for autosomal dominant mitral valve prolapse on chromosome 11p15.4. Am. J. Hum. Genet. 2003, 72, 1551–1559. [Google Scholar] [CrossRef]
- Nesta, F.; Leyne, M.; Yosefy, C.; Simpson, C.; Dai, D.; Marshall, J.E.; Hung, J.; Slaugenhaupt, S.A.; Levine, R.A. New locus for autosomal dominant mitral valve prolapse on chromosome 13: Clinical insights from genetic studies. Circulation 2005, 112, 2022–2030. [Google Scholar] [CrossRef]
- Durst, R.; Sauls, K.; Peal, D.S.; Devlaming, A.; Toomer, K.; Leyne, M.; Salani, M.; Talkowski, M.E.; Brand, H.; Perrocheau, M.; et al. Mutations in DCHS1 cause mitral valve prolapse. Nature 2015, 525, 109–113. [Google Scholar] [CrossRef]
- Clemenceau, A.; Bérubé, J.-C.; Bélanger, P.; Gaudreault, N.; Lamontagne, M.; Toubal, O.; Clavel, M.-A.; Capoulade, R.; Mathieu, P.; Pibarot, P.; et al. Deleterious variants in DCHS1 are prevalent in sporadic cases of mitral valve prolapse. Mol. Genet. Genom. Med. 2018, 6, 114–120. [Google Scholar] [CrossRef]
- Toomer, K.A.; Yu, M.; Fulmer, D.; Guo, L.; Moore, K.S.; Moore, R.; Drayton, K.D.; Glover, J.; Peterson, N.; Ramos-Ortiz, S.; et al. Primary cilia defects causing mitral valve prolapse. Sci. Transl. Med. 2019, 11, eaax0290. [Google Scholar] [CrossRef]
- Fang, M.; Alfieri, C.M.; Hulin, A.; Conway, S.J.; Yutzey, K.E. Loss of β-catenin promotes chondrogenic differentiation of aortic valve interstitial cells. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2601–2608. [Google Scholar] [CrossRef]
- Hulin, A.; Moore, V.; James, J.M.; Yutzey, K.E. Loss of Axin2 results in impaired heart valve maturation and subsequent myxomatous valve disease. Cardiovasc. Res. 2017, 113, 40–51. [Google Scholar] [CrossRef]
- Guo, L.; Beck, T.; Fulmer, D.; Ramos-Ortiz, S.; Glover, J.; Wang, C.; Moore, K.; Gensemer, C.; Morningstar, J.; Moore, R.; et al. DZIP1 regulates mammalian cardiac valve development through a Cby1-β-catenin mechanism. Dev. Dyn. 2021, 250, 1432–1449. [Google Scholar] [CrossRef] [PubMed]
- Dina, C.; Bouatia-Naji, N.; Tucker, N.; Delling, F.N.; Toomer, K.; Durst, R.; Perrocheau, M.; Fernandez-Friera, L.; Solis, J.; Le Tourneau, T.; et al. Genetic association analyses highlight biological pathways underlying mitral valve prolapse. Nat. Genet. 2015, 47, 1206–1211. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.H.; Daugherty, A.E.; Choi, C.K.; Horwitz, A.F.; Brautigan, D.L. Tensin1 requires protein phosphatase-1alpha in addition to RhoGAP DLC-1 to control cell polarization, migration, and invasion. J. Biol. Chem. 2009, 284, 34713–34722. [Google Scholar] [CrossRef] [PubMed]
- Rath, N.; Wang, Z.; Lu, M.M.; Morrisey, E.E. LMCD1/Dyxin is a novel transcriptional cofactor that restricts GATA6 function by inhibiting DNA binding. Mol. Cell. Biol. 2005, 25, 8864–8873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cui, S.; Chang, S.; Zhang, L.; Wang, J. i-GSEA4GWAS: A web server for identification of pathways/gene sets associated with traits by applying an improved gene set enrichment analysis to genome-wide association study. Nucleic Acids Res. 2010, 38, W90–W95. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Georges, A.; Tucker, N.R.; Kyryachenko, S.; Toomer, K.; Schott, J.J.; Delling, F.N.; Fernandez-Friera, L.; Solis, J.; Ellinor, P.T.; et al. Genome-Wide Association Study-Driven Gene-Set Analyses, Genetic, and Functional Follow-Up Suggest GLIS1 as a Susceptibility Gene for Mitral Valve Prolapse. Circ. Genom. Precis. Med. 2019, 12, e002497. [Google Scholar] [CrossRef]
- Haskell, G.T.; Jensen, B.C.; Skrzynia, C.; Pulikkotil, T.; Tilley, C.R.; Lu, Y.; Marchuk, D.S.; Samsa, L.A.; Wilhelmsen, K.C.; Lange, E.; et al. Genetic Complexity of Mitral Valve Prolapse Revealed by Clinical and Genetic Evaluation of a Large Family. J. Heart Valve Dis. 2017, 26, 569–580. [Google Scholar]
- Takahashi, T.; Takahashi, K.; John, P.L.S.; Fleming, P.A.; Tomemori, T.; Watanabe, T.; Abrahamson, D.R.; Drake, C.J.; Shirasawa, T.; Daniel, T.O. A mutant receptor tyrosine phosphatase, CD148, causes defects in vascular development. Mol. Cell. Biol. 2003, 23, 1817–1831. [Google Scholar] [CrossRef]
- Asl, H.F.; Talukdar, H.A.; Kindt, A.S.; Jain, R.K.; Ermel, R.; Ruusalepp, A.; Nguyen, K.-D.H.; Dobrin, R.; Reilly, D.F.; Schunkert, H.; et al. Expression quantitative trait Loci acting across multiple tissues are enriched in inherited risk for coronary artery disease. Circ. Cardiovasc. Genet. 2015, 8, 305–315. [Google Scholar]
- Garr, R.J.; A Krasuski, R.; Eckart, R.E.; Wang, A.; Pierce, C.; Kisslo, K.B.; Harrison, J.K.; Bashore, T.M. Peripheral blood levels of matrix metalloproteinases in patients referred for percutaneous balloon mitral valve commissurotomy. J. Heart Valve Dis. 2006, 15, 369–374. [Google Scholar]
- Ljungvall, I.; Rajamäki, M.M.; Crosara, S.; Olsen, L.H.; Kvart, C.; Borgarelli, M.; Höglund, K.; Häggström, J. Evaluation of plasma activity of matrix metalloproteinase-2 and -9 in dogs with myxomatous mitral valve disease. Am. J. Vet. Res. 2011, 72, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Aupperle, H.; Thielebein, J.; Kiefer, B.; März, I.; Dinges, G.; Schoon, H.-A.; Schubert, A. Expression of genes encoding matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in normal and diseased canine mitral valves. J. Comp. Pathol. 2009, 140, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Irqsusi, M.; Mansouri, A.L.; Ramaswamy, A.; Rexin, P.; Salman, M.; Mahmood, S.; Mirow, N.; Ghazi, T.; Ramzan, R.; Rastan, A.J.; et al. Role of matrix metalloproteinases in mitral valve regurgitation: Association between the of MMP-1, MMP-9, TIMP-1, and TIMP-2 expression, degree of mitral valve insufficiency, and pathologic etiology. J. Card. Surg. 2022, 37, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Allegra, A.; Crapanzano, F.; Pisano, C.; Triolo, O.F.; Argano, V.; Candore, G.; Lio, D.; Ruvolo, G. Associations of rs3918242 and rs2285053 MMP-9 and MMP-2 polymorphisms with the risk, severity, and short- and long-term complications of degenerative mitral valve diseases: A 4.8-year prospective cohort study. Cardiovasc. Pathol. 2016, 25, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.M.; Pitsis, A.A.; Kelpis, T.G.; Shahin, M.H.; Langaee, T.Y.; Cavallari, L.H.; Theofilogiannakos, E.K.; Boudoulas, H.; Boudoulas, K.D. Matrix Metalloproteinase Polymorphisms in Patients with Floppy Mitral Valve/Mitral Valve Prolapse (FMV/MVP) and FMV/MVP Syndrome. Cardiology 2017, 138, 179–185. [Google Scholar] [CrossRef]
- Roselli, C.; Yu, M.; Nauffal, V.; Georges, A.; Yang, Q.; Love, K.; Weng, L.C.; Delling, F.N.; Maurya, S.R.; Schrölkamp, M.; et al. Genome-wide association study reveals novel genetic loci: A new polygenic risk score for mitral valve prolapse. Eur. Heart J. 2022, 43, 1668–1680. [Google Scholar] [CrossRef]
- Lim, J.A.; Baek, H.J.; Jang, M.S.; Choi, E.K.; Lee, Y.M.; Lee, S.J.; Lim, S.C.; Kim, J.Y.; Kim, T.H.; Kim, H.S.; et al. Loss of β2-spectrin prevents cardiomyocyte differentiation and heart development. Cardiovasc. Res. 2014, 101, 39–47. [Google Scholar] [CrossRef]
- Herkert, J.C.; Verhagen, J.M.; Yotti, R.; Haghighi, A.; Phelan, D.G.; James, P.A.; Brown, N.J.; Stutterd, C.; Macciocca, I.; Leong, K.; et al. Expanding the clinical and genetic spectrum of ALPK3 variants: Phenotypes identified in pediatric cardiomyopathy patients and adults with heterozygous variants. Am. Heart J. 2020, 225, 108–119. [Google Scholar] [CrossRef]
- Levine, R.A.; Hagége, A.A.; Judge, D.P.; Padala, M.; Dal-Bianco, J.P.; Aikawa, E.; Beaudoin, J.; Bischoff, J.; Bouatia-Naji, N.; Bruneval, P.; et al. Mitral valve disease-morphology and mechanisms. Nat. Rev. Cardiol. 2015, 12, 689–710. [Google Scholar] [CrossRef] [PubMed]
- Haji-Seyed-Javadi, R.; Jelodari-Mamaghani, S.; Paylakhi, S.H.; Yazdani, S.; Nilforushan, N.; Fan, J.-B.; Klotzle, B.; Mahmoudi, M.J.; Ebrahimian, M.J.; Chelich, N.; et al. LTBP2 mutations cause Weill-Marchesani and Weill-Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum. Mutat. 2012, 33, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Durst, R. LTBP2 mutation may cause mitral valve prolapse. Eur. Heart J. 2022, 43, ehac544-2878. [Google Scholar] [CrossRef]
- Vohra, J.; Sathe, S.; Warren, R.; Tatoulis, J.; Hunt, D. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin. Electrophysiol. 1993, 16, 387–393. [Google Scholar] [CrossRef]
- Anders, S.; Said, S.; Schulz, F.; Püschel, K. Mitral valve prolapse syndrome as cause of sudden death in young adults. Forensic Sci. Int. 2007, 171, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef]
- Jeresaty, R.M. Sudden death in the mitral valve prolapse-click syndrome. Am. J. Cardiol. 1976, 37, 317–318. [Google Scholar] [CrossRef]
- Corrado, D.; Basso, C.; Nava, A.; Rossi, L.; Thiene, G. Sudden death in young people with apparently isolated mitral valve prolapse. G. Ital. Cardiol. 1997, 27, 1097–1105. [Google Scholar]
- Franchitto, N.; Bounes, V.; Telmon, N.; Rougé, D. Mitral valve prolapse and out-of-hospital sudden death: A case report and literature review. Med. Sci. Law 2010, 50, 164–167. [Google Scholar] [CrossRef]
- Morningstar, J.E.; Gensemer, C.; Moore, R.; Fulmer, D.; Beck, T.C.; Wang, C.; Moore, K.; Guo, L.; Sieg, F.; Nagata, Y.; et al. Mitral Valve Prolapse Induces Regionalized Myocardial Fibrosis. J. Am. Heart Assoc. 2021, 10, e022332. [Google Scholar] [CrossRef]
- James, P.A.; Aftimos, S.; Skinner, J.R. Familial mitral valve prolapse associated with short stature, characteristic face, and sudden death. Am. J. Med. Genet. A 2003, 119, 32–36. [Google Scholar] [CrossRef]
- Freed, L.A.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Levine, R.A. Mitral valve prolapse in the general population: The benign nature of echocardiographic features in the Framingham Heart Study. J. Am. Coll. Cardiol. 2002, 40, 1298–1304. [Google Scholar] [CrossRef]
- Wilcken, D.E.; Hickey, A.J. Lifetime risk for patients with mitral valve prolapse of developing severe valve regurgitation requiring surgery. Circulation 1988, 78, 10–14. [Google Scholar] [CrossRef]
- Avierinos, J.F.; Detaint, D.; Messika-Zeitoun, D.; Mohty, D.; Enriquez-Sarano, M. Risk, determinants, and outcome implications of progression of mitral regurgitation after diagnosis of mitral valve prolapse in a single community. Am. J. Cardiol. 2008, 101, 662–667. [Google Scholar] [CrossRef]
- Détaint, D.; Faivre, L.; Collod-Beroud, G.; Child, A.H.; Loeys, B.L.; Binquet, C.; Gautier, E.; Arbustini, E.; Mayer, K.; Arslan-Kirchner, M.; et al. Cardiovascular manifestations in men and women carrying a FBN1 mutation. Eur. Heart J. 2010, 31, 2223–2229. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Maleszewski, J.J.; Tester, D.J.; Ackerman, M.J. Prevalence and potential genetic determinants of young sudden unexplained death victims with suspected arrhythmogenic mitral valve prolapse syndrome. Heart Rhythm O2 2021, 2, 431–438. [Google Scholar] [CrossRef]
- Bains, S.; Tester, D.J.; Asirvatham, S.J.; Noseworthy, P.A.; Ackerman, M.J.; Giudicessi, J.R. A Novel Truncating Variant in FLNC-Encoded Filamin C May Serve as a Proarrhythmic Genetic Substrate for Arrhythmogenic Bileaflet Mitral Valve Prolapse Syndrome. Mayo Clin. Proc. 2019, 94, 906–913. [Google Scholar] [CrossRef]
- Dabbagh, G.S.; Obeng-Gyimah, E.; Perry, R.; Tran, S.; Malik, A.; Chahal, A. P303: Genetics bridge the link between mitral valve prolapse, arrhythmia and sudden death! Genet. Med. Open 2023, 1, 100331. [Google Scholar] [CrossRef]
- Levy, S.; Dabbagh, G.S.; Giudicessi, J.R.; Haqqani, H.; Khanji, M.Y.; Obeng-Gyimah, E.; Betts, M.N.; Ricci, F.; Asatryan, B.; Bouatia-Naji, N.; et al. Genetic mechanisms underlying arrhythmogenic mitral valve prolapse: Current and future perspectives. Heart Rhythm O2 2023, 4, 581–591. [Google Scholar] [CrossRef]
- Tetreault, M.; Bareke, E.; Nadaf, J.; Alirezaie, N.; Majewski, J. Whole-exome sequencing as a diagnostic tool: Current challenges and future opportunities. Expert. Rev. Mol. Diagn. 2015, 15, 749–760. [Google Scholar] [CrossRef]
- De Cario, R.; Kura, A.; Suraci, S.; Magi, A.; Volta, A.; Marcucci, R.; Gori, A.M.; Pepe, G.; Giusti, B.; Sticchi, E. Sanger Validation of High-Throughput Sequencing in Genetic Diagnosis: Still the Best Practice? Front. Genet. 2020, 11, 592588. [Google Scholar] [CrossRef]
- Scordo, K.A. Medication use and symptoms in individuals with mitral valve prolapse syndrome. Clin. Nurs. Res. 2007, 16, 58–71. [Google Scholar] [CrossRef]
- Harb, S.C.; Griffin, B.P. Mitral Valve Disease: A Comprehensive Review. Curr. Cardiol. Rep. 2017, 19, 73. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2438–2488. [Google Scholar] [CrossRef]
- Coutinho, G.F.; Antunes, M.J. Current status of the treatment of degenerative mitral valve regurgitation. Rev. Port. Cardiol. (Engl. Ed.) 2021, 40, 293–304. [Google Scholar] [CrossRef]
- Kang, D.-H.; Kim, J.H.; Rim, J.H.; Kim, M.-J.; Yun, S.-C.; Song, J.-M.; Song, H.; Choi, K.-J.; Song, J.-K.; Lee, J.-W.; et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation 2009, 119, 797–804. [Google Scholar] [CrossRef]
- Chenot, F.; Montant, P.; Vancraeynest, D.; Pasquet, A.; Gerber, B.; Noirhomme, P.H.; El Khoury, G.; Vanoverschelde, J.-L. Long-term clinical outcome of mitral valve repair in asymptomatic severe mitral regurgitation. Eur. J. Cardiothorac. Surg. 2009, 36, 539–545. [Google Scholar] [CrossRef]
- Coutinho, G.F.; Garcia, A.L.; Correia, P.M.; Branco, C.; Antunes, M.J. Long-term follow-up of asymptomatic or mildly symptomatic patients with severe degenerative mitral regurgitation and preserved left ventricular function. J. Thorac. Cardiovasc. Surg. 2014, 148, 2795–2801. [Google Scholar] [CrossRef]
- Can, T.; Kirov, H.; Caldonazo, T.; Mukharyamov, M.; Färber, G.; Doenst, T. Surgical mitral valve repair technique considerations based on the available evidence. Turk. Gogus Kalp Damar Cerrahisi Derg. 2022, 30, 302–316. [Google Scholar] [CrossRef]
- Akmaz, B.; van Kuijk, S.M.J.; Sardari Nia, P. Association between individual surgeon volume and outcome in mitral valve surgery: A systematic review. J. Thorac. Dis. 2021, 13, 4500–4510. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.G.; Anyanwu, A.C.; Fuster, V.; Adams, D.H. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: Implications for future guidelines. J. Thorac. Cardiovasc. Surg. 2012, 144, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.B.; Prendergast, B.; Iung, B.; Rosenhek, R.; Zamorano, J.L.; Piérard, L.A.; Modine, T.; Falk, V.; Kappetein, A.P.; Pibarot, P.; et al. Standards defining a ‘Heart Valve Centre’: ESC Working Group on Valvular Heart Disease and European Association for Cardiothoracic Surgery Viewpoint. Eur. Heart J. 2017, 38, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iske, J.; Roesel, M.J.; Cesarovic, N.; Pitts, L.; Steiner, A.; Knoedler, L.; Nazari-Shafti, T.Z.; Akansel, S.; Jacobs, S.; Falk, V.; et al. The Potential of Intertwining Gene Diagnostics and Surgery for Mitral Valve Prolapse. J. Clin. Med. 2023, 12, 7441. https://doi.org/10.3390/jcm12237441
Iske J, Roesel MJ, Cesarovic N, Pitts L, Steiner A, Knoedler L, Nazari-Shafti TZ, Akansel S, Jacobs S, Falk V, et al. The Potential of Intertwining Gene Diagnostics and Surgery for Mitral Valve Prolapse. Journal of Clinical Medicine. 2023; 12(23):7441. https://doi.org/10.3390/jcm12237441
Chicago/Turabian StyleIske, Jasper, Maximilian J. Roesel, Nikola Cesarovic, Leonard Pitts, Annabel Steiner, Leonard Knoedler, Timo Z. Nazari-Shafti, Serdar Akansel, Stephan Jacobs, Volkmar Falk, and et al. 2023. "The Potential of Intertwining Gene Diagnostics and Surgery for Mitral Valve Prolapse" Journal of Clinical Medicine 12, no. 23: 7441. https://doi.org/10.3390/jcm12237441
APA StyleIske, J., Roesel, M. J., Cesarovic, N., Pitts, L., Steiner, A., Knoedler, L., Nazari-Shafti, T. Z., Akansel, S., Jacobs, S., Falk, V., Kempfert, J., & Kofler, M. (2023). The Potential of Intertwining Gene Diagnostics and Surgery for Mitral Valve Prolapse. Journal of Clinical Medicine, 12(23), 7441. https://doi.org/10.3390/jcm12237441





