REM Sleep Behavior Disorder and Cognitive Functions in Parkinson’s Patients: A Systematic Review
Abstract
:1. Introduction
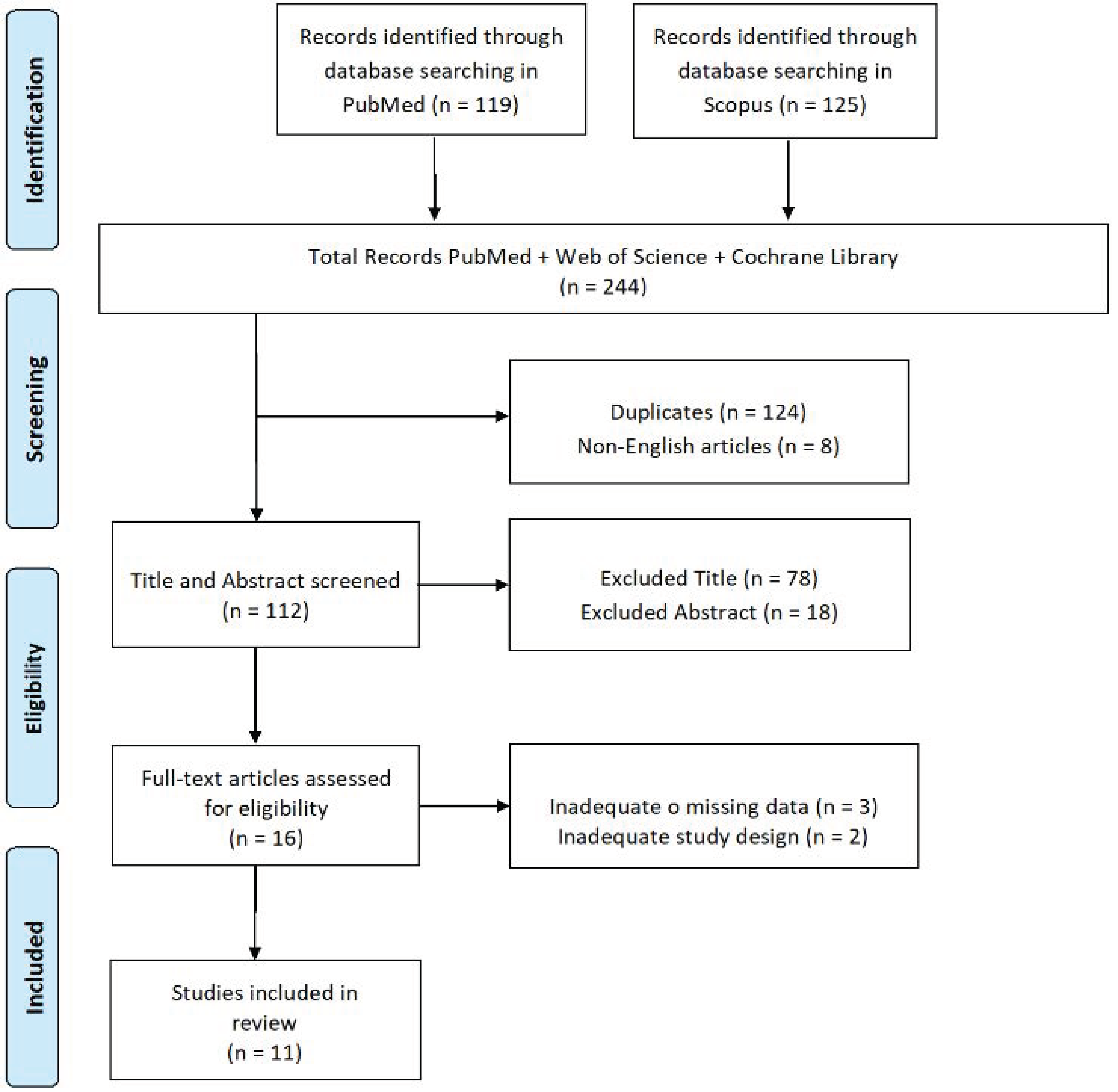
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
3. Results
| Study | Sleep Quality Tools | Cognitive Deterioration Tools | Subjects | Outcomes |
|---|---|---|---|---|
| Bjørnarå KA, et al. [1] | RBDSQ PDSS MD-UPDRS | MD-UPDRS | 107 patients with PD without dementia, of which 62 use MAO-B concomitantly with other drugs and 52 use a combination of antagonists and levodopa | The presence of cognitive decline as well as EDS is evident in patients with PD with and without pRBD. |
| Postuma RB, et al. [11] | RBD-HK | UPDRS MMSE MoCA MDS | 61 of which 27 had basal RBD and 15 had no | The authors suggest that RBD in PD is associated with MCI. |
| Hermann W, et al. [16] | ESS PSQI TAP | PANDA | 26 patients with PD with sleep disorders | The authors found significant differences in cognitive performance between patients with and without RBD, all associated with sleep quality disturbances and respiratory disturbances. |
| Gagnon JF, et al. [20] | ESS | DIGIT SPAN TMT- B RAVLT | 32 patients with idiopathic RBD, 22 patients with PD with RBD, 18 patients with PD without RBD and 40 healthy control subjects | PD patients with RBD had more MCI than PD patients without RBD. |
| Zhang JR, et al. [44] | RBDSQ | DIGIT SPAN TMT-A TMT-B SCWT SVFT AVLT UPDRS | 42 patients with PD without RBD, 32 with PD with RBD, 15 with iRBD, 36 healthy | Patients demonstrated clear associations between RBD symptoms and different PD-MCI domains. |
| Kamble N, et al. [45] | RBDSQ MSQ | MMSE MoCA FAB AVLT UPDRS | 25 PD patients with RBD and 25 PD patients without RBD | In this study, the authors found many differences between PD patients with and without RBD, with different variations in sleep efficiency and cognitive decline being more rapid in PD patients with RBD. |
| Liu H, et al. [47] | RBDSQ PDSS-2 | UPDRS MoCA | 158 patients with PD of which 31 reported RBD | PD patients with RBD have high scores with respect to sleep and a large effect was also found on cognitive decline. |
| Bugalho P, et al. [48] | RBDSQ | MMSE UPDRS | 75 patients with PD with RBD including 58 under dopaminergic treatment | The authors found high level of male PD patients with RBD, but found no significant differences in the scores obtained. |
| Nomura T, et al. [49] | RBDSQ | MMSE MoCA | 70 patients with PD of which 19 with pRBD | PD patients with pRBD had higher levels of dementia than those without pRBD. |
| Yan YY, et al. [50] | RBD-HK | MMSE MoCA | 89 patients with PD of which 46 with PD-RBD and 43 PD-npRBD | PD-pRBD patients demonstrate increased cognitive decline compared to PD-npRBD patients. |
| Suzuki K, et al. [51] | RBDSQ-J PDSS ESS PSQI | UPDRS PDQ-39 | 93 patients with PD and 93 control subjects with neurological or psychiatric diseases | In this study the authors found significant differences in PD patients with RBD showing high scores compared to the control group in the cognitive dimension which in turn affects sleep quality and vice versa. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bjørnarå, K.A.; Dietrichs, E.; Toft, M. Clinical features associated with sleep disturbances in Parkinson’s disease. Clin. Neurol. Neurosurg. 2014, 124, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.E. Biology of Parkinson’s disease: Pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin. Neurosci. 2004, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Lo Buono, V.; Corallo, F.; Foti, M.; Di Lorenzo, G.; Bramanti, P.; Marino, S. Nonmotor symptoms in Parkinson disease: A descriptive review on social cognition ability. J. Geriatr. Psychiatry Neurol. 2017, 30, 109–121. [Google Scholar] [CrossRef]
- Hustad, E.; Aasly, J.O. Clinical and imaging markers of prodromal Parkinson’s disease. Front. Neurol. 2020, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Loddo, G.; Calandra-Buonaura, G.; Sambati, L.; Giannini, G.; Cecere, A.; Cortelli, P.; Provini, F. The treatment of sleep disorders in Parkinson’s disease: From research to clinical practice. Front. Neurol. 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Abad, V.C.; Guilleminault, C. Diagnosis and treatment of sleep disorders: A brief review for clinicians. Dialogues Clin. Neurosci. 2003, 5, 371. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Lo Buono, V.; Bonanno, L.; Sorbera, C.; Cimino, V.; Bramanti, P.; Di Lorenzo, G.; Marino, S. Potential predictors of quality of life in Parkinson’s Disease: Sleep and mood disorders. J. Clin. Neurosci. 2019, 70, 113–117. [Google Scholar] [CrossRef]
- Schenck, C.; Hurwitz, T.; Mahowald, M. REM sleep behaviour disorder: An update on a series of 96 patients and a review of the world literature. J. Sleep Res. 1993, 2, 224–231. [Google Scholar] [CrossRef]
- Iranzo, A.; Tolosa, E.; Gelpi, E.; Molinuevo, J.L.; Valldeoriola, F.; Serradell, M.; Sanchez-Valle, R.; Vilaseca, I.; Lomeña, F.; Santamaria, J.; et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol. 2013, 12, 443–453. [Google Scholar] [CrossRef]
- Tekriwal, A.; Kern, D.S.; Tsai, J.; Ince, N.F.; Wu, J.; Thompson, J.A.; Abosch, A. REM sleep behaviour disorder: Prodromal and mechanistic insights for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2017, 88, 445–451. [Google Scholar] [CrossRef]
- Postuma, R.B.; Bertrand, J.A.; Montplaisir, J.; Desjardins, C.; Vendette, M.; Rios Romenets, S.; Panisset, M.; Gagnon, J.F. Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: A prospective study. Mov. Disord. 2012, 27, 720–726. [Google Scholar] [CrossRef]
- Barone, D.A.; Henchcliffe, C. Rapid eye movement sleep behavior disorder and the link to alpha-synucleinopathies. Clin. Neurophysiol. 2018, 129, 1551–1564. [Google Scholar] [CrossRef]
- Solla, P.; Wang, Q.; Frau, C.; Floris, V.; Loy, F.; Sechi, L.A.; Masala, C. Olfactory Impairment Is the Main Predictor of Higher Scores at REM Sleep Behavior Disorder (RBD) Screening Questionnaire in Parkinson’s Disease Patients. Brain Sci. 2023, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Huang, X.; Yu, J.; Chen, L.; Huang, Y.; Tang, B.; Guo, J. Association Between REM Sleep Behavior Disorder and Cognitive Dysfunctions in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Observational Studies. Front. Neurol. 2020, 11, 577874. [Google Scholar] [CrossRef]
- Arnaldi, D.; Morbelli, S.; Brugnolo, A.; Girtler, N.; Picco, A.; Ferrara, M.; Accardo, J.; Buschiazzo, A.; de Carli, F.; Nobili, F.; et al. Functional neuroimaging and clinical features of drug naive patients with de novo Parkinson’s disease and probable RBD. Park. Relat. Disord. 2016, 29, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hermann, W.; Schmitz-Peiffer, H.; Kasper, E.; Fauser, M.; Franke, C.; Wienecke, M.; Otto, K.; Löhle, M.; Brandt, M.D.; Storch, A.; et al. Sleep disturbances and sleep disordered breathing impair cognitive performance in Parkinson’s disease. Front. Neurosci. 2020, 14, 689. [Google Scholar] [CrossRef] [PubMed]
- Trὂster, A.I. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson’s disease with dementia: Differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychol. Rev. 2008, 18, 103–119. [Google Scholar] [CrossRef]
- Goldman, J.G.; Aggarwal, N.T.; Schroeder, C.D. Mild cognitive impairment: An update in Parkinson’s disease and lessons learned from Alzheimer’s disease. Neurodegener. Dis. Manag. 2015, 5, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef]
- Gagnon, J.F.; Vendette, M.; Postuma, R.B.; Desjardins, C.; Massicotte-Marquez, J.; Panisset, M.; Montplaisir, J. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann. Neurol. 2009, 66, 39–47. [Google Scholar] [CrossRef]
- Gagnon, J.F.; Bédard, M.A.; Fantini, M.L.; Petit, D.; Panisset, M.; Rompre, S.; Montplaisir, J. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology 2002, 59, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, N.; Postuma, R.B.; Montplaisir, J.; Latreille, V.; Panisset, M.; Chouinard, S.; Bourgouin, P.A.; Gagnon, J.F. REM sleep behavior disorder and cognitive impairment in Parkinson’s disease. Sleep 2017, 40, zsx101. [Google Scholar] [CrossRef] [PubMed]
- Schrag, A.; Siddiqui, U.F.; Anastasiou, Z.; Weintraub, D.; Schott, J.M. Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: A cohort study. Lancet Neurol. 2017, 16, 66–75. [Google Scholar] [CrossRef]
- Maggi, G.; Trojano, L.; Barone, P.; Santangelo, G. Sleep disorders and cognitive dysfunctions in Parkinson’s disease: A meta-analytic study. Neuropsychol. Rev. 2021, 31, 643–682. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Glaesmer, H.; Brähler, E.; Löffler, M.; Engel, C.; Enzenbach, C.; Hegerl, U.; Sander, C. Sleep quality in the general population: Psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Med. 2017, 30, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Stiasny-Kolster, K.; Mayer, G.; Schäfer, S.; Möller, J.C.; Heinzel-Gutenbrunner, M.; Oertel, W.H. The REM sleep behavior disorder screening questionnaire—A new diagnostic instrument. Mov. Disord. 2007, 22, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Wing, Y.K.; Lam, S.P.; Zhang, J.; Yu, M.W.; Ho, C.K.; Tsoh, J.; Mok, V. Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK). Sleep Med. 2010, 11, 43–48. [Google Scholar] [CrossRef]
- Catale, C.; Marique, P.; Closset, A.; Meulemans, T. Attentional and executive functioning following mild traumatic brain injury in children using the Test for Attentional Performance (TAP) battery. J. Clin. Exp. Neuropsychol. 2009, 31, 331–338. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; LaPelle, N.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Kohnen, R.; Högl, B.; Metta, V.; Sixel-Döring, F.; Frauscher, B.; Hülsmann, J.; Martinez-Martin, P.; Chaudhuri, K.R. Parkinson’s disease sleep scale—Validation of the revised version PDSS-2. Mov. Disord. 2011, 26, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Kalbe, E.; Calabrese, P.; Kohn, N.; Hilker, R.; Riedel, O.; Wittchen, H.U.; Dodel, R.; Otto, J.; Ebersbach, G.; Kessler, J. PArkinson Neuropsychometric Dementia Assessment PANDA©. Park. Relat. Disord. 2008, 14, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kurlowicz, L.; Wallace, M. The mini-mental state examination (MMSE). J. Gerontol. Nurs. 1999, 25, 8–9. [Google Scholar] [CrossRef]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The unified Parkinson’s disease rating scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Ivnik, R.J.; Malec, J.F.; Tangalos, E.G.; Petersen, R.C.; Kokmen, E.; Kurland, L.T. The Auditory-Verbal Learning Test (AVLT): Norms for ages 55 years and older. Psychol. Assess. A J. Consult. Clin. Psychol. 1990, 2, 304. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B.F.A.B. The FAB: A frontal assessment battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef]
- Schmidt, M. Rey Auditory Verbal Learning Test: A Handbook; Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Ostrosky-Solís, F.; Lozano, A. Digit span: Effect of education and culture. Int. J. Psychol. 2006, 41, 333–341. [Google Scholar] [CrossRef]
- Lamberty, G.J.; Putnam, S.H.; Chatel, D.M.; Bieliauskas, L.A. Derived Trail Making Test indices: A preliminary report. Neuropsychiatry Neuropsychol. Behav. Neurol. 1994, 7, 230–234. [Google Scholar]
- Jenkinson, C.; Fitzpatrick, R.A.Y.; Peto, V.I.V.; Greenhall, R.; Hyman, N. The Parkinson’s Disease Questionnaire (PDQ-39): Development and validation of a Parkinson’s disease summary index score. Age Ageing 1997, 26, 353–357. [Google Scholar] [CrossRef]
- Carnero, C.; Lendínez, A.; Maestre, J.; Zunzunegui, M.V. Semantic verbal fluency in neurological patients without dementia with a low educational level. Rev. Neurol. 1999, 28, 858–862. [Google Scholar] [PubMed]
- Scarpina, F.; Tagini, S. The stroop color and word test. Front. Psychol. 2017, 8, 557. [Google Scholar] [CrossRef]
- Zhang, J.R.; Chen, J.; Yang, Z.J.; Zhang, H.J.; Fu, Y.T.; Shen, Y.; He, P.C.; Mao, C.J.; Liu, C.F. Rapid eye movement sleep behavior disorder symptoms correlate with domains of cognitive impairment in Parkinson’s disease. Chin. Med. J. 2016, 129, 379. [Google Scholar] [CrossRef] [PubMed]
- Kamble, N.; Yadav, R.; Lenka, A.; Kumar, K.; Nagaraju, B.C.; Pal, P.K. Impaired sleep quality and cognition in patients of Parkinson’s disease with REM sleep behavior disorder: A comparative study. Sleep Med. 2019, 62, 1–5. [Google Scholar] [CrossRef]
- Boeve, B.F.; Silber, M.H.; Saper, C.B.; Ferman, T.J.; Dickson, D.W.; Parisi, J.E.; Benarroch, E.E.; Ahlskog, J.E.; Smith, G.E.; Braak, H.; et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007, 130 Pt 11, 2770–2788. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ou, R.; Wei, Q.; Hou, Y.; Cao, B.; Zhao, B.; Shang, H. Rapid eye movement behavior disorder in drug-naïve patients with Parkinson’s disease. J. Clin. Neurosci. 2019, 59, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Bugalho, P.; da Silva, J.A.; Neto, B. Clinical features associated with REM sleep behavior disorder symptoms in the early stages of Parkinson’s disease. J. Neurol. 2011, 258, 50–55. [Google Scholar] [CrossRef]
- Nomura, T.; Tanaka, K.; Tajiri, Y.; Kishi, M.; Nakashima, K. Screening tools for clinical characteristics of probable REM sleep behavior disorder in patients with Parkinson’s disease. Eneurologicalsci 2016, 4, 22–24. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Lei, K.; Li, Y.Y.; Liu, X.F.; Chang, Y. The correlation between possible RBD and cognitive function in Parkinson’s disease patients in China. Ann. Clin. Transl. Neurol. 2019, 6, 848–853. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, T.; Miyamoto, M.; Watanabe, Y.; Suzuki, S.; Tatsumoto, M.; Iwanami, M.; Sada, T.; Kadowaki, T.; Hirata, K.; et al. Probable rapid eye movement sleep behavior disorder, nocturnal disturbances and quality of life in patients with Parkinson’s disease: A case-controlled study using the rapid eye movement sleep behavior disorder screening questionnaire. BMC Neurol. 2013, 13, 18. [Google Scholar] [CrossRef]
- Miller, M.A. The role of sleep and sleep disorders in the development, diagnosis, and management of neurocognitive disorders. Front. Neurol. 2015, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, X.P.; Yang, G.Y. Sleep Disorders in Stroke: An Update on Management. Aging Dis. 2021, 12, 570–585. [Google Scholar] [CrossRef]
- Van Laar, A.D.; Jain, S. Non-motor symptoms of Parkinson disease: Update on the diagnosis and treatment. Neurologist 2004, 10, 185. [Google Scholar]
- Schulte, E.C.; Winkelmann, J. When Parkinson’s disease patients go to sleep: Specific sleep disturbances related to Parkinson’s disease. J. Neurol. 2011, 258, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Tandberg, E.; Larsen, J.P.; Karlsen, K. Excessive daytime sleepiness and sleep benefit in Parkinson’s disease: A community-based study. Mov. Disord. 1999, 14, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.; Antonini, A.; Colosimo, C.; Marconi, R.; Morgante, L.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, G.; Dotto, P.D.; et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2009, 24, 1641–1649. [Google Scholar] [CrossRef]
- Pagano, G.; De Micco, R.; Yousaf, T.; Wilson, H.; Chandra, A.; Politis, M. REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology 2018, 91, e894–e905. [Google Scholar] [CrossRef]
- Schenck, C.H.; Boeve, B.F.; Mahowald, M.W. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: A 16-year update on a previously reported series. Sleep Med. 2013, 14, 744–748. [Google Scholar] [CrossRef]
- Schenck, C.H.; Bundlie, S.R.; Mahowald, M.W. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 1996, 46, 388–393. [Google Scholar] [CrossRef]
- Gagnon, J.F.; Fantini, M.L.; Bédard, M.A.; Petit, D.; Carrier, J.; Rompré, S.; Panisset, M.; Montplaisir, J. Association between waking EEG slowing and REM sleep behavior disorder in PD without dementia. Neurology 2004, 62, 401–406. [Google Scholar] [CrossRef]
- Ford, A.H.; Duncan, G.W.; Firbank, M.J.; Yarnall, A.J.; Khoo, T.K.; Burn, D.J.; O’Brien, J.T. Rapid eye movement sleep behavior disorder in Parkinson’s disease: Magnetic resonance imaging study. Mov. Disord. 2013, 28, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Boucetta, S.; Salimi, A.; Dadar, M.; Jones, B.E.; Collins, D.L.; Dang-Vu, T.T. Structural brain alterations associated with rapid eye movement sleep behavior disorder in Parkinson’s disease. Sci. Rep. 2016, 6, 26782. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Q.; Chen, S.D. RBD: A red flag for cognitive impairment in Parkinson’s disease? Sleep Med. 2018, 44, 38–44. [Google Scholar] [CrossRef]
- Stavitsky, K.; Neargarder, S.; Bogdanova, Y.; McNamara, P.; Cronin-Golomb, A. The impact of sleep quality on cognitive functioning in Parkinson’s disease. J. Int. Neuropsychol. Soc. JINS 2012, 18, 108. [Google Scholar] [CrossRef]
- Sasai, T.; Matsuura, M.; Inoue, Y. Electroencephalographic findings related with mild cognitive impairment in idiopathic rapid eye movement sleep behavior disorder. Sleep 2013, 36, 1893–1899. [Google Scholar] [CrossRef]
- Aarsland, D.; Bronnick, K.; Williams-Gray, C.; Weintraub, D.; Marder, K.; Kulisevsky, J.; Burn, D.; Barone, P.; Pagonabarraga, J.; Allcock, L.; et al. Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 2010, 75, 1062–1069. [Google Scholar] [CrossRef]
- Diaconu, Ș.; Falup-Pecurariu, O.; Țînț, D.; Falup-Pecurariu, C. REM sleep behaviour disorder in Parkinson’s disease. Exp. Ther. Med. 2021, 22, 1–5. [Google Scholar] [CrossRef]
- Beyer, M.K.; Janvin, C.C.; Larsen, J.P.; Aarsland, D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J. Neurol. Neurosurg. Psychiatry 2007, 78, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Oswal, A.; Gratwicke, J.; Akram, H.; Jahanshahi, M.; Zaborszky, L.; Brown, P.; Hariz, M.; Zrinzo, L.; Foltynie, T.; Litvak, V. Cortical connectivity of the nucleus basalis of Meynert in Parkinson’s disease and Lewy body dementias. Brain 2021, 144, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Williams-Gray, C.H.; Evans, J.R.; Goris, A.; Foltynie, T.; Ban, M.; Robbins, T.W.; Brayne, C.; Kolachana, B.S.; Weinberger, D.R.; Barker, R.A.; et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain 2009, 132, 2958–2969. [Google Scholar] [CrossRef] [PubMed]
- Taximaimaiti, R.; Luo, X.; Wang, X.P. Pharmacological and Non-pharmacological Treatments of Sleep Disorders in Parkinson’s Disease. Curr. Neuropharmacol. 2021, 19, 2233–2249. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marafioti, G.; Corallo, F.; Cardile, D.; Di Lorenzo, G.; Quartarone, A.; Lo Buono, V. REM Sleep Behavior Disorder and Cognitive Functions in Parkinson’s Patients: A Systematic Review. J. Clin. Med. 2023, 12, 7397. https://doi.org/10.3390/jcm12237397
Marafioti G, Corallo F, Cardile D, Di Lorenzo G, Quartarone A, Lo Buono V. REM Sleep Behavior Disorder and Cognitive Functions in Parkinson’s Patients: A Systematic Review. Journal of Clinical Medicine. 2023; 12(23):7397. https://doi.org/10.3390/jcm12237397
Chicago/Turabian StyleMarafioti, Giulia, Francesco Corallo, Davide Cardile, Giuseppe Di Lorenzo, Angelo Quartarone, and Viviana Lo Buono. 2023. "REM Sleep Behavior Disorder and Cognitive Functions in Parkinson’s Patients: A Systematic Review" Journal of Clinical Medicine 12, no. 23: 7397. https://doi.org/10.3390/jcm12237397
APA StyleMarafioti, G., Corallo, F., Cardile, D., Di Lorenzo, G., Quartarone, A., & Lo Buono, V. (2023). REM Sleep Behavior Disorder and Cognitive Functions in Parkinson’s Patients: A Systematic Review. Journal of Clinical Medicine, 12(23), 7397. https://doi.org/10.3390/jcm12237397







