Artificial Intelligence in Scoliosis: Current Applications and Future Directions
Abstract
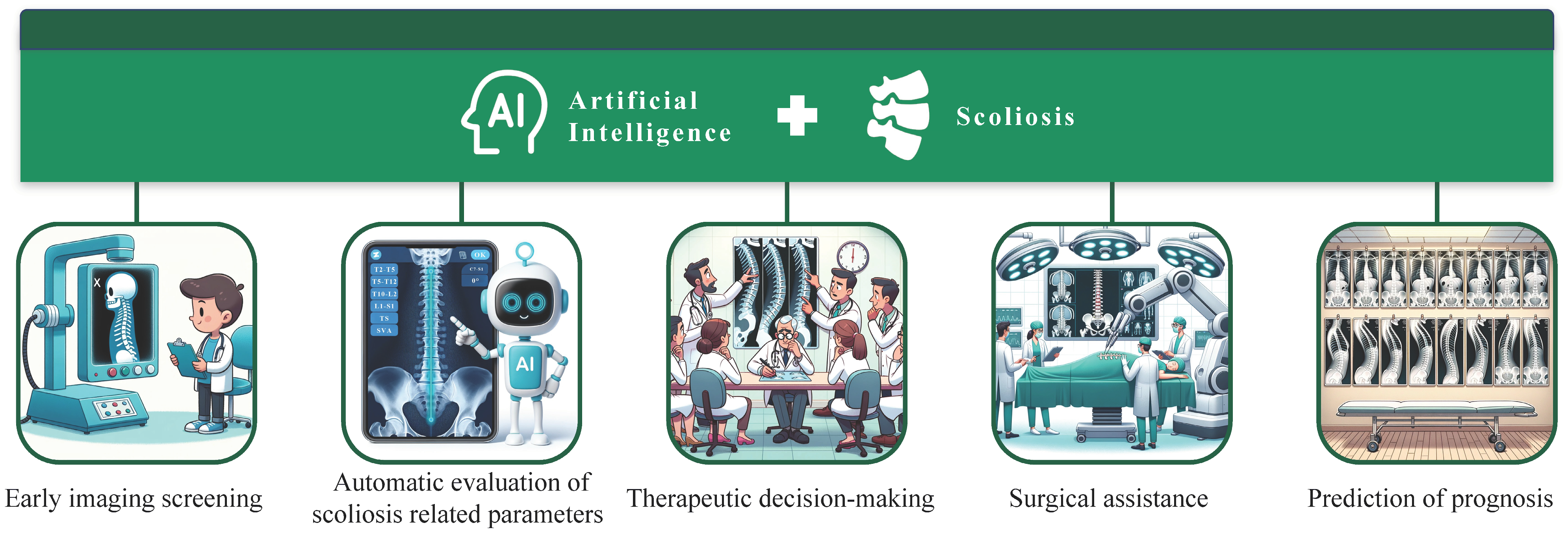
1. Introduction
2. Early Imaging Screening
3. Automatic Evaluation of Scoliosis-Related Parameters
4. Therapeutic Decision-Making
5. Surgical Assistance
5.1. Insertion of Pedicle Screw
5.2. Simulation of Deformity Correction and Intraoperative Monitoring
5.3. Robotics in Scoliosis Surgery
6. Prediction of Prognosis
7. Future Directions of AI in Scoliosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horne, J.P.; Flannery, R.; Usman, S. Adolescent idiopathic scoliosis: Diagnosis and management. Am. Fam. Physician 2014, 89, 193–198. [Google Scholar] [PubMed]
- Mesiti, B.L. Scoliosis: An Overview. Radiol. Technol. 2021, 93, 55–72. [Google Scholar] [PubMed]
- Negrini, S.; Donzelli, S.; Aulisa, A.G.; Czaprowski, D.; Schreiber, S.; de Mauroy, J.C.; Diers, H.; Grivas, T.B.; Knott, P.; Kotwicki, T.; et al. 2016 SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Shakil, H.; Iqbal, Z.A.; Al-Ghadir, A.H. Scoliosis: Review of types of curves, etiological theories and conservative treatment. J. Back Musculoskelet. Rehabil. 2014, 27, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour-Feizi, H.; Soleimanpour, J.; Sales, J.G.; Arzroumchilar, A. Lenke and King classification systems for adolescent idiopathic scoliosis: Interobserver agreement and postoperative results. Int. J. Gen. Med. 2011, 4, 821–825. [Google Scholar] [CrossRef][Green Version]
- Lenke, L.G.; Betz, R.R.; Harms, J.; Bridwell, K.H.; Clements, D.H.; Lowe, T.G.; Blanke, K. Adolescent idiopathic scoliosis: A new classification to determine extent of spinal arthrodesis. J. Bone Jt. Surg. Am. 2001, 83, 1169–1181. [Google Scholar] [CrossRef]
- Zhuang, Q.; Qiu, G.; Li, Q.; Zhang, J.; Shen, J.; Wang, Y.; Zhao, H.; Zhao, Y.; Li, S.; Yu, B.; et al. Modified PUMC classification for adolescent idiopathic scoliosis. Spine J. 2019, 19, 1518–1528. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, S.R.; Qiu, G.X.; Zhang, J.G.; Zhuang, Q.Y. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin. Med. J. 2020, 133, 483–493. [Google Scholar] [CrossRef]
- Weinstein, S.L.; Zavala, D.C.; Ponseti, I.V. Idiopathic scoliosis: Long-term follow-up and prognosis in untreated patients. J. Bone Jt. Surg. Am. 1981, 63, 702–712. [Google Scholar] [CrossRef]
- Kuznia, A.L.; Hernandez, A.K.; Lee, L.U. Adolescent Idiopathic Scoliosis: Common Questions and Answers. Am. Fam. Physician 2020, 101, 19–23. [Google Scholar]
- Yu, K.H.; Beam, A.L.; Kohane, I.S. Artificial intelligence in healthcare. Nat. Biomed. Eng. 2018, 2, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.S.; Lau, D.; Ames, C.P. Artificial Intelligence and the Future of Spine Surgery. Neurospine 2019, 16, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, S.; Mateen, B.A.; Bohner, G.; Király, F.J.; Ghani, R.; Jonsson, P.; Cumbers, S.; Jonas, A.; McAllister, K.S.L.; Myles, P.; et al. Machine learning and artificial intelligence research for patient benefit: 20 critical questions on transparency, replicability, ethics, and effectiveness. BMJ 2020, 368, l6927. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Coyner, A.S.; Kalpathy-Cramer, J.; Chiang, M.F.; Campbell, J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020, 9, 14. [Google Scholar] [PubMed]
- Chen, K.; Zhai, X.; Sun, K.; Wang, H.; Yang, C.; Li, M. A narrative review of machine learning as promising revolution in clinical practice of scoliosis. Ann. Transl. Med. 2021, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, M.; Gendreau, J.; Feng, A.; Kim, L.H.; Ho, A.L.; Veeravagu, A. Robotic-Assisted Spine Surgery: History, Efficacy, Cost, And Future Trends. Robot Surg. 2019, 6, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Henrikson, N.B.; Morrison, C.C.; Blasi, P.R.; Nguyen, M.; Lin, J.S. Screening for Adolescent Idiopathic Scoliosis: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 173–187. [Google Scholar] [CrossRef]
- Jaremko, J.; Delorme, S.; Dansereau, J.; Labelle, H.; Ronsky, J.; Poncet, P.; Harder, J.; Dewar, R.; Zernicke, R.F. Use of Neural Networks to Correlate Spine and Rib Deformity in Scoliosis. Comput. Methods Biomech. Biomed. Eng. 2000, 3, 203–213. [Google Scholar] [CrossRef]
- Ramirez, L.; Durdle, N.G.; Raso, V.J.; Hill, D.L. A support vector machines classifier to assess the severity of idiopathic scoliosis from surface topography. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 84–91. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, K.; Fan, H.; Huang, Z.; Xiang, Y.; Yang, J.; He, L.; Zhang, L.; Yang, Y.; Li, R.; et al. Development and validation of deep learning algorithms for scoliosis screening using back images. Commun. Biol. 2019, 2, 390. [Google Scholar] [CrossRef]
- Watanabe, K.; Aoki, Y.; Matsumoto, M. An Application of Artificial Intelligence to Diagnostic Imaging of Spine Disease: Estimating Spinal Alignment From Moiré Images. Neurospine 2019, 16, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Greer, H.; Gerber, S.; Niethammer, M.; Kwitt, R.; McCormick, M.; Chittajallu, D.; Siekierski, N.; Oetgen, M.; Cleary, K.; Aylward, S. Scoliosis Screening and Monitoring Using Self Contained Ultrasound and Neural Networks. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; pp. 1500–1503. [Google Scholar] [CrossRef]
- Wei, J.; Tay, Y.; Bommasani, R.; Raffel, C.; Zoph, B.; Borgeaud, S.; Yogatama, D.; Bosma, M.; Zhou, D.; Metzler, D. Emergent abilities of large language models. arXiv 2022, arXiv:2206.07682. [Google Scholar]
- Thirunavukarasu, A.J.; Ting, D.S.J.; Elangovan, K.; Gutierrez, L.; Tan, T.F.; Ting, D.S.W. Large language models in medicine. Nat. Med. 2023, 29, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.; Kim, J.W.; Hallacy, C.; Ramesh, A.; Goh, G.; Agarwal, S.; Sastry, G.; Askell, A.; Mishkin, P.; Clark, J. Learning transferable visual models from natural language supervision. In Proceedings of the International conference on machine learning, in virtual, 18–24 July 2021; pp. 8748–8763. [Google Scholar]
- Fabijan, A.; Fabijan, R.; Zawadzka-Fabijan, A.; Nowosławska, E.; Zakrzewski, K.; Polis, B. Evaluating Scoliosis Severity Based on Posturographic X-ray Images Using a Contrastive Language-Image Pretraining Model. Diagnostics 2023, 13, 2142. [Google Scholar] [CrossRef] [PubMed]
- Aubin, C.E.; Bellefleur, C.; Joncas, J.; de Lanauze, D.; Kadoury, S.; Blanke, K.; Parent, S.; Labelle, H. Reliability and accuracy analysis of a new semiautomatic radiographic measurement software in adult scoliosis. Spine 2011, 36, E780–E790. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, S.; Yang, G.; Li, E.; Liang, Z. A Review of the Methods on Cobb Angle Measurements for Spinal Curvature. Sensors 2022, 22, 3258. [Google Scholar] [CrossRef]
- Pruijs, J.E.; Hageman, M.A.; Keessen, W.; van der Meer, R.; van Wieringen, J.C. Variation in Cobb angle measurements in scoliosis. Skelet. Radiol. 1994, 23, 517–520. [Google Scholar] [CrossRef]
- Weng, C.H.; Wang, C.L.; Huang, Y.J.; Yeh, Y.C.; Fu, C.J.; Yeh, C.Y.; Tsai, T.T. Artificial Intelligence for Automatic Measurement of Sagittal Vertical Axis Using ResUNet Framework. J. Clin. Med. 2019, 8, 1826. [Google Scholar] [CrossRef]
- Vo, D.M.; Le, T.P.; Nguyen, D.M.; Lee, S.W. BoostNet: A Boosted Convolutional Neural Network for Image Blind Denoising. IEEE Access 2021, 9, 115145–115164. [Google Scholar] [CrossRef]
- Wu, H.; Bailey, C.; Rasoulinejad, P.; Li, S. Automated comprehensive Adolescent Idiopathic Scoliosis assessment using MVC-Net. Med. Image Anal. 2018, 48, 1–11. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Q.; Leung, S.; Chung, J.; Chen, B.; Li, S. Accurate automated Cobb angles estimation using multi-view extrapolation net. Med. Image Anal. 2019, 58, 101542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, N.; Guo, C.; Wu, J. MPF-net: An effective framework for automated cobb angle estimation. Med. Image Anal. 2022, 75, 102277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lou, E.; Hill, D.L.; Raso, J.V.; Wang, Y.; Le, L.H.; Shi, X. Computer-aided assessment of scoliosis on posteroanterior radiographs. Med. Biol. Eng. Comput. 2010, 48, 185–195. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, E.; Le, L.H.; Hill, D.L.; Raso, J.V.; Wang, Y. Automatic Cobb measurement of scoliosis based on fuzzy Hough Transform with vertebral shape prior. J. Digit. Imaging 2009, 22, 463–472. [Google Scholar] [CrossRef] [PubMed]
- H, A.; Prabhu, G.K. Automatic quantification of spinal curvature in scoliotic radiograph using image processing. J. Med. Syst. 2012, 36, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Sardjono, T.A.; Wilkinson, M.H.; Veldhuizen, A.G.; van Ooijen, P.M.; Purnama, K.E.; Verkerke, G.J. Automatic Cobb angle determination from radiographic images. Spine 2013, 38, E1256–E1262. [Google Scholar] [CrossRef]
- Horng, M.H.; Kuok, C.P.; Fu, M.J.; Lin, C.J.; Sun, Y.N. Cobb Angle Measurement of Spine from X-Ray Images Using Convolutional Neural Network. Comput. Math. Methods Med. 2019, 2019, 6357171. [Google Scholar] [CrossRef]
- Wang, L.; Xie, C.; Lin, Y.; Zhou, H.Y.; Chen, K.; Cheng, D.; Dubost, F.; Collery, B.; Khanal, B.; Khanal, B.; et al. Evaluation and comparison of accurate automated spinal curvature estimation algorithms with spinal anterior-posterior X-Ray images: The AASCE2019 challenge. Med. Image Anal. 2021, 72, 102115. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Li, H.; Gu, X.; Li, Z.; Zhang, S. Automatic Cobb angle measurement method based on vertebra segmentation by deep learning. Med. Biol. Eng. Comput. 2022, 60, 2257–2269. [Google Scholar] [CrossRef]
- Hoashi, J.S.; Cahill, P.J.; Bennett, J.T.; Samdani, A.F. Adolescent scoliosis classification and treatment. Neurosurg. Clin. N. Am. 2013, 24, 173–183. [Google Scholar] [CrossRef]
- Phan, P.; Mezghani, N.; Nault, M.L.; Aubin, C.E.; Parent, S.; de Guise, J.; Labelle, H. A decision tree can increase accuracy when assessing curve types according to Lenke classification of adolescent idiopathic scoliosis. Spine 2010, 35, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhou, Z.; Yu, H.; Chen, K.; Yang, Y. Computerized-Assisted Scoliosis Diagnosis Based on Faster R-CNN and ResNet for the Classification of Spine X-Ray Images. Comput. Math. Methods Med. 2022, 2022, 3796202. [Google Scholar] [CrossRef]
- Yahara, Y.; Tamura, M.; Seki, S.; Kondo, Y.; Makino, H.; Watanabe, K.; Kamei, K.; Futakawa, H.; Kawaguchi, Y. A deep convolutional neural network to predict the curve progression of adolescent idiopathic scoliosis: A pilot study. BMC Musculoskelet. Disord. 2022, 23, 610. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, T.; Cheung, K.M.; Shea, G.K. Application of deep learning upon spinal radiographs to predict progression in adolescent idiopathic scoliosis at first clinic visit. EClinicalMedicine 2021, 42, 101220. [Google Scholar] [CrossRef] [PubMed]
- Mezghani, N.; Phan, P.; Mitiche, A.; Labelle, H.; de Guise, J.A. A Kohonen neural network description of scoliosis fused regions and their corresponding Lenke classification. Int. J. Comput. Assist. Radiol. Surg. 2012, 7, 257–264. [Google Scholar] [CrossRef]
- Phan, P.; Mezghani, N.; Wai, E.K.; de Guise, J.; Labelle, H. Artificial neural networks assessing adolescent idiopathic scoliosis: Comparison with Lenke classification. Spine J. 2013, 13, 1527–1533. [Google Scholar] [CrossRef]
- Pasha, S.; Flynn, J. Data-driven Classification of the 3D Spinal Curve in Adolescent Idiopathic Scoliosis with an Applications in Surgical Outcome Prediction. Sci. Rep. 2018, 8, 16296. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.; Shah, S.; Yaszay, B.; Newton, P. Discovering the association between the pre- and post-operative 3D spinal curve patterns in adolescent idiopathic scoliosis. Spine Deform. 2021, 9, 1053–1062. [Google Scholar] [CrossRef]
- Koller, H.; Hitzl, W.; Marks, M.C.; Newton, P.O. Accurate prediction of spontaneous lumbar curve correction following posterior selective thoracic fusion in adolescent idiopathic scoliosis using logistic regression models and clinical rationale. Eur. Spine J. 2019, 28, 1987–1997. [Google Scholar] [CrossRef]
- Bertoncelli, C.M.; Bertoncelli, D.; Elbaum, L.; Latalski, M.; Altamura, P.; Musoff, C.; Rampal, V.; Solla, F. Validation of a Clinical Prediction Model for the Development of Neuromuscular Scoliosis: A Multinational Study. Pediatr. Neurol. 2018, 79, 14–20. [Google Scholar] [CrossRef]
- García-Cano, E.; Arámbula Cosío, F.; Duong, L.; Bellefleur, C.; Roy-Beaudry, M.; Joncas, J.; Parent, S.; Labelle, H. Prediction of spinal curve progression in Adolescent Idiopathic Scoliosis using Random Forest regression. Comput. Biol. Med. 2018, 103, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, H.; Newell, R.; Anglin, C.; Street, J.; Hodgson, A.J. A deep learning framework for segmentation and pose estimation of pedicle screw implants based on C-arm fluoroscopy. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Burström, G.; Buerger, C.; Hoppenbrouwers, J.; Nachabe, R.; Lorenz, C.; Babic, D.; Homan, R.; Racadio, J.M.; Grass, M.; Persson, O.; et al. Machine learning for automated 3-dimensional segmentation of the spine and suggested placement of pedicle screws based on intraoperative cone-beam computer tomography. J. Neurosurg. Spine 2019, 31, 147–154. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Zhao, C.; Yuan, Z.; Cai, J. Surgical application of pedicle drill template navigation technology for complicated scoliosis. Zhonghua Yi Xue Za Zhi 2014, 94, 840–843. [Google Scholar]
- Zhang, X.; Uneri, A.; Huang, Y.; Jones, C.K.; Witham, T.F.; Helm, P.A.; Siewerdsen, J.H. Deformable 3D-2D image registration and analysis of global spinal alignment in long-length intraoperative spine imaging. Med. Phys. 2022, 49, 5715–5727. [Google Scholar] [CrossRef]
- Elmi-Terander, A.; Burström, G.; Nachabé, R.; Fagerlund, M.; Ståhl, F.; Charalampidis, A.; Edström, E.; Gerdhem, P. Augmented reality navigation with intraoperative 3D imaging vs fluoroscopy-assisted free-hand surgery for spine fixation surgery: A matched-control study comparing accuracy. Sci. Rep. 2020, 10, 707. [Google Scholar] [CrossRef]
- Amaritsakul, Y.; Chao, C.K.; Lin, J. Multiobjective optimization design of spinal pedicle screws using neural networks and genetic algorithm: Mathematical models and mechanical validation. Comput. Math. Methods Med. 2013, 2013, 462875. [Google Scholar] [CrossRef]
- Solla, F.; Clément, J.L.; Cunin, V.; Bertoncelli, C.M.; Fière, V.; Rampal, V. Patient-specific rods for thoracic kyphosis correction in adolescent idiopathic scoliosis surgery: Preliminary results. Orthop. Traumatol. Surg. Res. 2020, 106, 159–165. [Google Scholar] [CrossRef]
- Tachi, H.; Kato, K.; Abe, Y.; Kokabu, T.; Yamada, K.; Iwasaki, N.; Sudo, H. Surgical Outcome Prediction Using a Four-Dimensional Planning Simulation System With Finite Element Analysis Incorporating Pre-bent Rods in Adolescent Idiopathic Scoliosis: Simulation for Spatiotemporal Anatomical Correction Technique. Front. Bioeng. Biotechnol. 2021, 9, 746902. [Google Scholar] [CrossRef]
- Fei, N.; Li, R.; Cui, H.; Hu, Y. A Prediction Model for Normal Variation of Somatosensory Evoked Potential During Scoliosis Surgery. Int. J. Neural Syst. 2023, 33, 2350005. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Yu, L.; Li, Q.; Wang, B.; Yang, L.; Cheng, M.; Wang, F.; Zhang, L.; Chen, L.; Li, K.; et al. Development and Clinical Trial of a New Orthopedic Surgical Robot for Positioning and Navigation. J. Clin. Med. 2022, 11, 7091. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Liu, Y.J.; Liu, B.; He, D.; Wu, J.Y.; Han, X.G.; Zhao, J.W.; Fan, M.X. Guideline for Thoracolumbar Pedicle Screw Placement Assisted by Orthopaedic Surgical Robot. Orthop. Surg. 2019, 11, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhu, X.; Dong, L.; Liu, T. Study on robot-assisted pedicle screw implantation in adolescent idiopathic scoliosis surgery. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2021, 35, 1457–1462. [Google Scholar] [CrossRef]
- Hou, C.; Yang, H.; Chen, Y.; Yang, Y.; Zhang, B.; Chen, K.; Li, M.; Yang, M.; Chen, K. Comparison of robot versus fluoroscopy-assisted pedicle screw instrumentation in adolescent idiopathic scoliosis surgery: A retrospective study. Front. Surg. 2022, 9, 1085580. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, T.; Torii, Y.; Ueno, J.; Umehara, T.; Iinuma, M.; Yoshida, A.; Tomochika, K.; Ohtori, S.; Niki, H. Accuracy of computer-assisted pedicle screw placement for adolescent idiopathic scoliosis: A comparison between robotics and navigation. Eur. Spine J. 2023, 32, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.K.; Smith, J.S.; Schwab, F.; Lafage, V.; Shaffrey, C.I.; Bess, S.; Daniels, A.H.; Hart, R.A.; Protopsaltis, T.S.; Mundis, G.M., Jr.; et al. Development of a preoperative predictive model for major complications following adult spinal deformity surgery. J. Neurosurg. Spine 2017, 26, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Lan, L.; Xiu, P.; Zhang, G.; Hu, B.; Yang, X.; Song, Y.; Yang, X.; Gu, Y.; Yang, R.; et al. Prediction of Proximal Junctional Kyphosis After Posterior Scoliosis Surgery With Machine Learning in the Lenke 5 Adolescent Idiopathic Scoliosis Patient. Front. Bioeng. Biotechnol. 2020, 8, 559387. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Hosogane, N.; Fujita, N.; Okada, E.; Tsuji, O.; Nagoshi, N.; Asazuma, T.; Tsuji, T.; Nakamura, M.; Matsumoto, M.; et al. Predictive model for major complications 2 years after corrective spine surgery for adult spinal deformity. Eur. Spine J. 2019, 28, 180–187. [Google Scholar] [CrossRef]
- Pellisé, F.; Serra-Burriel, M.; Smith, J.S.; Haddad, S.; Kelly, M.P.; Vila-Casademunt, A.; Sánchez Pérez-Grueso, F.J.; Bess, S.; Gum, J.L.; Burton, D.C.; et al. Development and validation of risk stratification models for adult spinal deformity surgery. J. Neurosurg. Spine 2019, 31, 587–599. [Google Scholar] [CrossRef]
- Ames, C.P.; Smith, J.S.; Pellisé, F.; Kelly, M.P.; Gum, J.L.; Alanay, A.; Acaroğlu, E.; Pérez-Grueso, F.J.S.; Kleinstück, F.S.; Obeid, I.; et al. Development of Deployable Predictive Models for Minimal Clinically Important Difference Achievement Across the Commonly Used Health-related Quality of Life Instruments in Adult Spinal Deformity Surgery. Spine 2019, 44, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Ames, C.P.; Smith, J.S.; Pellisé, F.; Kelly, M.; Gum, J.L.; Alanay, A.; Acaroğlu, E.; Pérez-Grueso, F.J.S.; Kleinstück, F.S.; Obeid, I.; et al. Development of predictive models for all individual questions of SRS-22R after adult spinal deformity surgery: A step toward individualized medicine. Eur. Spine J. 2019, 28, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Farhud, D.D.; Zokaei, S. Ethical Issues of Artificial Intelligence in Medicine and Healthcare. Iran J. Public Health 2021, 50, i–v. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Prakash, S.; Janapa Reddi, V.; Kim, D.; Rajpurkar, P. A framework for integrating artificial intelligence for clinical care with continuous therapeutic monitoring. Nat. Biomed. Eng. 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Huang, C.; Wang, D.; Li, K.; Han, X.; Chen, X.; Li, Z. Artificial Intelligence in Scoliosis: Current Applications and Future Directions. J. Clin. Med. 2023, 12, 7382. https://doi.org/10.3390/jcm12237382
Zhang H, Huang C, Wang D, Li K, Han X, Chen X, Li Z. Artificial Intelligence in Scoliosis: Current Applications and Future Directions. Journal of Clinical Medicine. 2023; 12(23):7382. https://doi.org/10.3390/jcm12237382
Chicago/Turabian StyleZhang, Haozhi, Changfa Huang, Daoyun Wang, Kuan Li, Xiao Han, Xin Chen, and Zheng Li. 2023. "Artificial Intelligence in Scoliosis: Current Applications and Future Directions" Journal of Clinical Medicine 12, no. 23: 7382. https://doi.org/10.3390/jcm12237382
APA StyleZhang, H., Huang, C., Wang, D., Li, K., Han, X., Chen, X., & Li, Z. (2023). Artificial Intelligence in Scoliosis: Current Applications and Future Directions. Journal of Clinical Medicine, 12(23), 7382. https://doi.org/10.3390/jcm12237382








