How Cardiac Fibrosis Assessed via T1 Mapping Is Associated with Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Protocol
2.3. Statistical Analysis
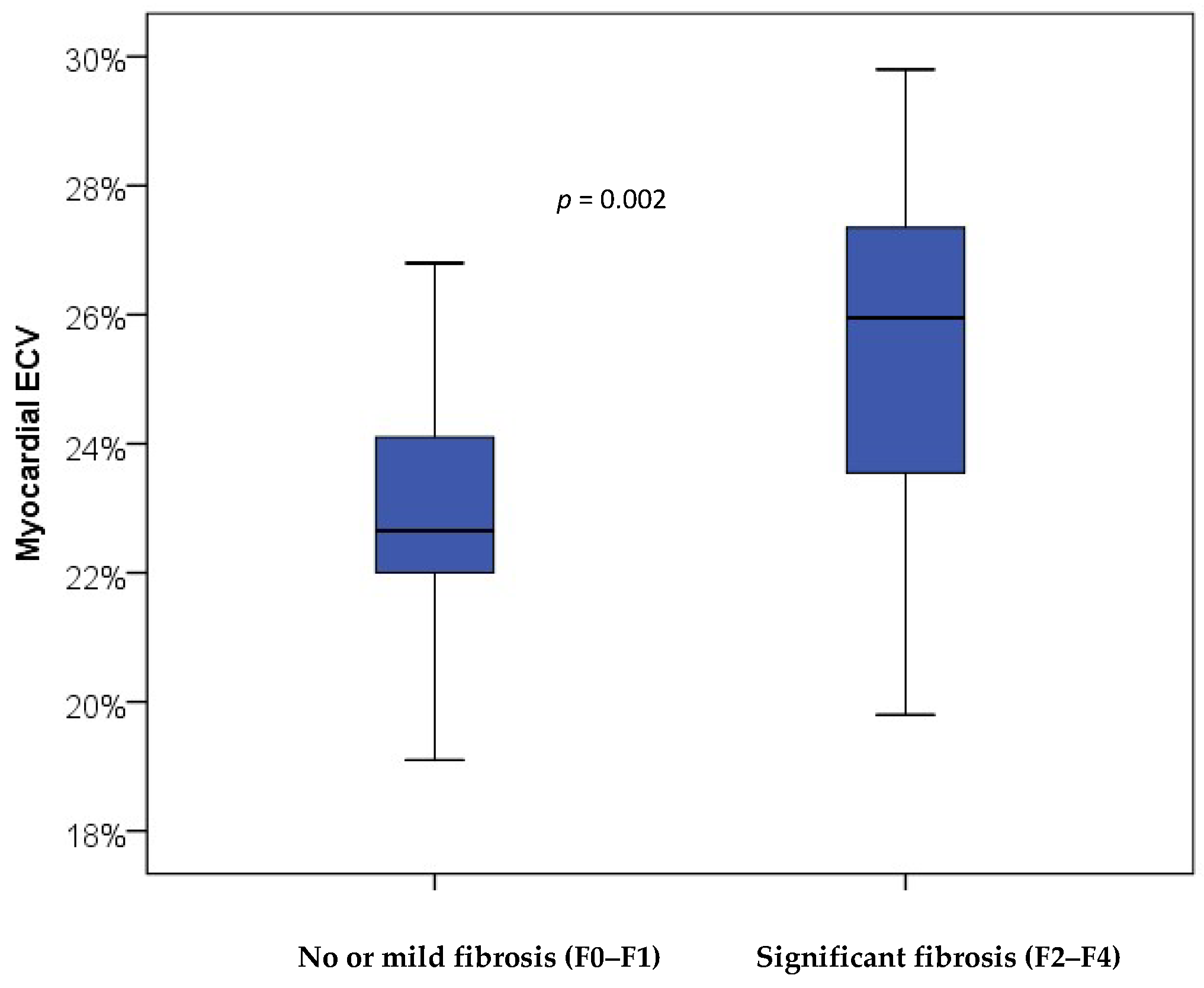
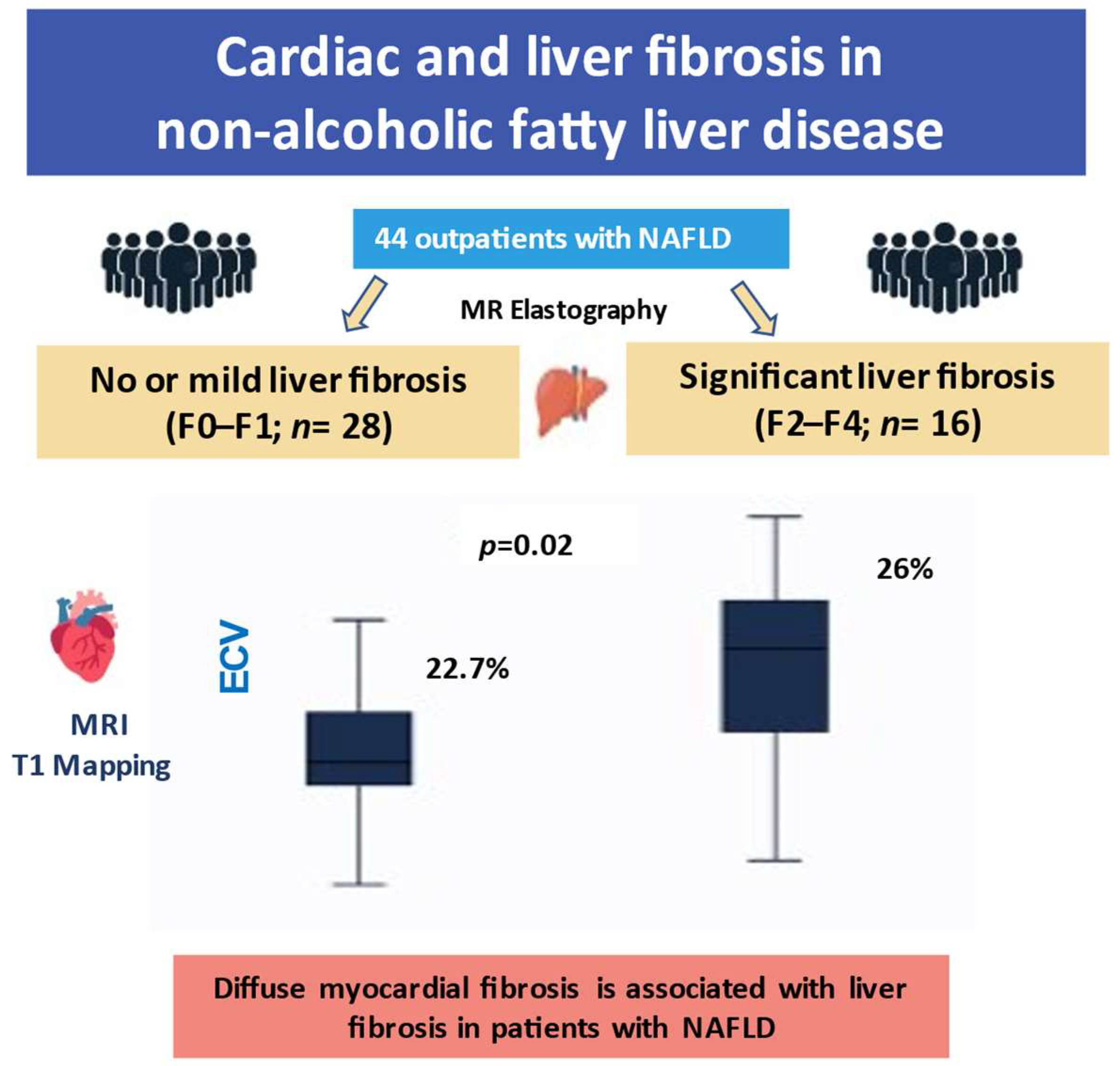
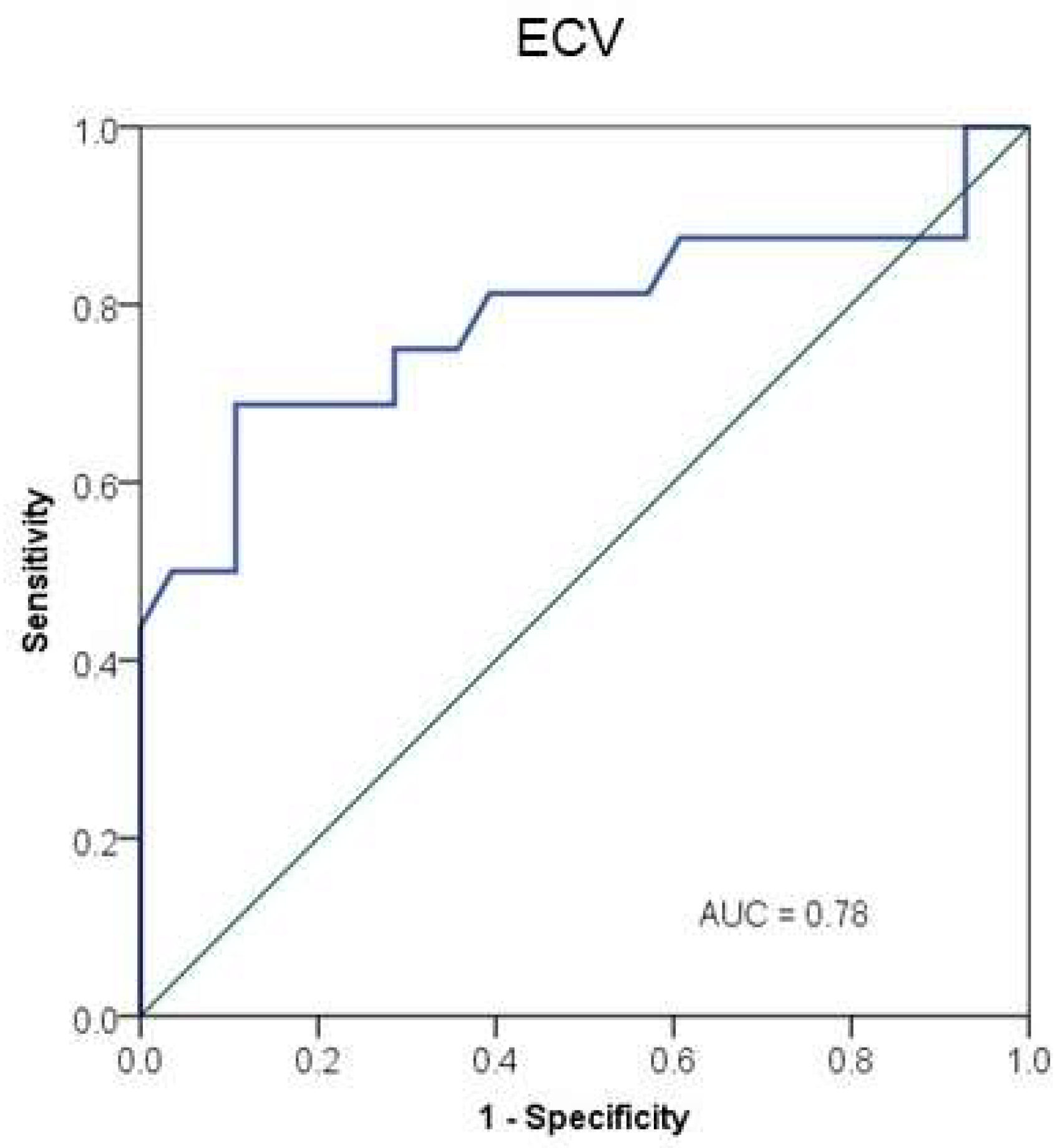
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, Y.; Kim, K.J.; Yoo, M.e.; Kim, G.; Yoon, H.; Jo, K.; Youn, J.-C.; Yun, M.; Park, J.Y.; Shim, C.Y.; et al. Association of Non-Alcoholic Steatohepatitis with Subclinical Myocardial Dysfunction in Non-Cirrhotic Patients. J. Hepatol. 2018, 68, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Pernigo, M.; Bergamini, C.; Bonapace, S.; Lipari, P.; Pichiri, I.; Bertolini, L.; Valbusa, F.; Barbieri, E.; Zoppini, G.; et al. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS ONE 2015, 10, e0135329. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Non-Alcoholic Fatty Liver Disease Is Strongly Associated with Carotid Atherosclerosis: A Systematic Review. J. Hepatol. 2008, 49, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Lonardo, A.; Bonapace, S.; Byrne, C.D.; Loria, P.; Targher, G. Risk of Cardiovascular, Cardiac and Arrhythmic Complications in Patients with Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 1724–1745. [Google Scholar] [CrossRef] [PubMed]
- Welle, C.L.; Olson, M.C.; Reeder, S.B.; Venkatesh, S.K. Magnetic Resonance Imaging of Liver Fibrosis, Fat, and Iron. Radiol. Clin. N. Am. 2022, 60, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Dzyubak, B.; Yin, M.; Schlein, A.; Henderson, W.C.; Hooker, J.C.; Delgado, T.I.; Middleton, M.S.; Zheng, L.; Wolfson, T.; et al. MR Elastography in Nonalcoholic Fatty Liver Disease: Inter-Center and Inter-Analysis-Method Measurement Reproducibility and Accuracy at 3T. Eur. Radiol. 2022, 32, 2937–2948. [Google Scholar] [CrossRef]
- Briasoulis, A.; Mallikethi-Reddy, S.; Palla, M.; Alesh, I.; Afonso, L. Myocardial Fibrosis on Cardiac Magnetic Resonance and Cardiac Outcomes in Hypertrophic Cardiomyopathy: A Meta-Analysis. Heart Br. Cardiovasc. Soc. 2015, 101, 1406–1411. [Google Scholar] [CrossRef]
- Ambale-Venkatesh, B.; Lima, J.A.C. Cardiac MRI: A Central Prognostic Tool in Myocardial Fibrosis. Nat. Rev. Cardiol. 2015, 12, 18–29. [Google Scholar] [CrossRef]
- Pinheiro, M.V.T.; Moll-Bernardes, R.J.; Camargo, G.C.; Siqueira, F.P.; de Azevedo, C.F.; de Holanda, M.T.; Mendes, F.d.S.N.S.; Sangenis, L.H.C.; Mediano, M.F.F.; de Sousa, A.S. Associations between Cardiac Magnetic Resonance T1 Mapping Parameters and Ventricular Arrhythmia in Patients with Chagas Disease. Am. J. Trop. Med. Hyg. 2020, 103, 745–751. [Google Scholar] [CrossRef]
- Schelbert, E.B.; Piehler, K.M.; Zareba, K.M.; Moon, J.C.; Ugander, M.; Messroghli, D.R.; Valeti, U.S.; Chang, C.-C.H.; Shroff, S.G.; Diez, J.; et al. Myocardial Fibrosis Quantified by Extracellular Volume Is Associated with Subsequent Hospitalization for Heart Failure, Death, or Both Across the Spectrum of Ejection Fraction and Heart Failure Stage. J. Am. Heart Assoc. 2015, 4, e002613. [Google Scholar] [CrossRef]
- Long, M.T.; Benjamin, E.J. Prevalent Cardiovascular Disease Events and T1 Mapping Defined Hepatic Fibrosis. Circ. Cardiovasc. Imaging 2018, 11, e007553. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, H.-K.; Lee, J.-H.; Lee, Y.B.; Park, E.-A.; Park, J.-B.; Lee, S.-P.; Kim, Y.J.; Kim, Y.-J.; Yoon, J.-H.; et al. Myocardial Structural and Functional Changes in Patients with Liver Cirrhosis Awaiting Liver Transplantation: A Comprehensive Cardiovascular Magnetic Resonance and Echocardiographic Study. J. Cardiovasc. Magn. Reson. 2020, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Ostovaneh, M.R.; Ambale-Venkatesh, B.; Fuji, T.; Bakhshi, H.; Shah, R.; Murthy, V.L.; Tracy, R.P.; Guallar, E.; Wu, C.O.; Bluemke, D.A.; et al. Association of Liver Fibrosis with Cardiovascular Diseases in the General Population: The Multi-Ethnic Study of Atherosclerosis (MESA). Circ. Cardiovasc. Imaging 2018, 11, e007241. [Google Scholar] [CrossRef] [PubMed]
- General Cardiovascular Risk Profile for Use in Primary Care. Available online: https://www.ahajournals.org/doi/epub/10.1161/CIRCULATIONAHA.107.699579 (accessed on 18 July 2023).
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Claus, P.; Omar, A.M.S.; Pedrizzetti, G.; Sengupta, P.P.; Nagel, E. Tissue Tracking Technology for Assessing Cardiac Mechanics. JACC Cardiovasc. Imaging 2015, 8, 1444–1460. [Google Scholar] [CrossRef]
- Negishi, K.; Negishi, T.; Haluska, B.A.; Hare, J.L.; Plana, J.C.; Marwick, T.H. Use of Speckle Strain to Assess Left Ventricular Responses to Cardiotoxic Chemotherapy and Cardioprotection. Eur. Heart J.—Cardiovasc. Imaging 2014, 15, 324–331. [Google Scholar] [CrossRef]
- Lumens, J.; Prinzen, F.W.; Delhaas, T. Longitudinal Strain: “Think Globally, Track Locally”. JACC Cardiovasc. Imaging 2015, 8, 1360–1363. [Google Scholar] [CrossRef]
- Voigt, J.-U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of Coronary Artery Calcium Using Ultrafast Computed Tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Park, S.H. Radiologic Evaluation of Nonalcoholic Fatty Liver Disease. World J. Gastroenterol. 2014, 20, 7392–7402. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa Babu, A.; Wells, M.L.; Teytelboym, O.M.; Mackey, J.E.; Miller, F.H.; Yeh, B.M.; Ehman, R.L.; Venkatesh, S.K. Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions. Radiographics 2016, 36, 1987–2006. [Google Scholar] [CrossRef] [PubMed]
- Rettmann, D.W.; Saranathan, M.; Wu, K.C.; Azevedo, C.F.; Bluemke, D.A.; Foo, T.K.F. High Temporal Resolution Breathheld 3D FIESTA CINE Imaging: Validation of Ventricular Function in Patients with Chronic Myocardial Infarction. J. Magn. Reson. Imaging 2007, 25, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Torreão, J.A.; Ianni, B.M.; Mady, C.; Naia, E.; Rassi, C.H.; Nomura, C.; Parga, J.R.; Avila, L.F.; Ramires, J.A.F.; Kalil-Filho, R.; et al. Myocardial Tissue Characterization in Chagas’ Heart Disease by Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2015, 17, 97. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Plein, S.; Higgins, D.M.; Walters, K.; Jones, T.R.; Ridgway, J.P.; Sivananthan, M.U. Human Myocardium: Single-Breath-Hold MR T1 Mapping with High Spatial Resolution—Reproducibility Study. Radiology 2006, 238, 1004–1012. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Radjenovic, A.; Kozerke, S.; Higgins, D.M.; Sivananthan, M.U.; Ridgway, J.P. Modified Look-Locker Inversion Recovery (MOLLI) for High-Resolution T1 Mapping of the Heart. Magn. Reson. Med. 2004, 52, 141–146. [Google Scholar] [CrossRef]
- Lorenz, C.H.; Walker, E.S.; Morgan, V.L.; Klein, S.S.; Graham, T.P. Normal Human Right and Left Ventricular Mass, Systolic Function, and Gender Differences by Cine Magnetic Resonance Imaging. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 1999, 1, 7–21. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in Clinical Practice: A Comprehensive Review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Oni, E.T.; Agatston, A.S.; Blaha, M.J.; Fialkow, J.; Cury, R.; Sposito, A.; Erbel, R.; Blankstein, R.; Feldman, T.; Al-Mallah, M.H.; et al. A Systematic Review: Burden and Severity of Subclinical Cardiovascular Disease among Those with Nonalcoholic Fatty Liver; Should We Care? Atherosclerosis 2013, 230, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Fox, C.S. The Association between Non-Invasive Hepatic Fibrosis Markers and Cardiometabolic Risk Factors in the Framingham Heart Study. PLoS ONE 2016, 11, e0157517. [Google Scholar] [CrossRef] [PubMed]
- Treeprasertsuk, S.; Leverage, S.; Adams, L.A.; Lindor, K.D.; Sauver, J.; Angulo, P. The Framingham Risk Score and Heart Disease in Nonalcoholic Fatty Liver Disease. Liver Int. 2012, 32, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Zoppini, G.; Zenari, L.; Cigolini, M.; Falezza, G.; Arcaro, G. Relations between Carotid Artery Wall Thickness and Liver Histology in Subjects with Nonalcoholic Fatty Liver Disease. Diabetes Care 2006, 29, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Al Rifai, M.; Silverman, M.G.; Nasir, K.; Budoff, M.J.; Blankstein, R.; Szklo, M.; Katz, R.; Blumentha, R.S.; Blaha, M.J. The Association of Nonalcoholic Fatty Liver Disease, Obesity, and Metabolic Syndrome, with Systemic Inflammation and Subclinical Atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015, 239, 629–633. [Google Scholar] [CrossRef]
- Mellinger, J.L.; Pencina, K.M.; Massaro, J.M.; Hoffmann, U.; Seshadri, S.; Fox, C.S.; O’Donnell, C.J.; Speliotes, E.K. Hepatic Steatosis and Cardiovascular Disease Outcomes: An Analysis of the Framingham Heart Study. J. Hepatol. 2015, 63, 470–476. [Google Scholar] [CrossRef]
- Powell, E.E.; Cooksley, W.G.E.; Hanson, R.; Searle, J.; Halliday, J.W.; Powell, W. The Natural History of Nonalcoholic Steatohepatitis: A Follow-up Study of Forty-Two Patients for up to 21 Years. Hepatology 1990, 11, 74–80. [Google Scholar] [CrossRef]
- Caldwell, S.H.; Crespo, D.M. The Spectrum Expanded: Cryptogenic Cirrhosis and the Natural History of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2004, 40, 578–584. [Google Scholar] [CrossRef]
- Mantovani, A.; Ballestri, S.; Lonardo, A.; Targher, G. Cardiovascular Disease and Myocardial Abnormalities in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1246–1267. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Wilcox, J.E.; Colangelo, L.A.; Lloyd-Jones, D.M.; Carr, J.J.; Lima, J.A.; Lewis, C.E.; Rinella, M.E.; Shah, S.J. Association of Nonalcoholic Fatty Liver Disease with Subclinical Myocardial Remodeling and Dysfunction: A Population-Based Study. Hepatology 2015, 62, 773–783. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, Y.; Song, J.; Dong, N.; Kong, X.; Wang, J.; Yuan, Y.; Zhu, X.; Yan, X.; Greiser, A.; et al. Association between Myocardial Extracellular Volume and Strain Analysis through Cardiovascular Magnetic Resonance with Histological Myocardial Fibrosis in Patients Awaiting Heart Transplantation. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2018, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.Y.; Ghosn, M.G.; Khan, M.A.; Gramze, N.L.; Brunner, G.; Nabi, F.; Nambi, V.; Nagueh, S.F.; Nguyen, D.T.; Graviss, E.A.; et al. MYOCARDIAL EXTRACELLULAR VOLUME FRACTION ADDS PROGNOSTIC INFORMATION BEYOND MYOCARDIAL REPLACEMENT FIBROSIS. Circ. Cardiovasc. Imaging 2019, 12, e009535. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Fuchs, F.; Goncalves, A.; Kaiser-Albers, C.; Ali, Z.A.; Bengel, F.M.; Dimmeler, S.; Fayad, Z.A.; Mebazaa, A.; Meder, B.; et al. Non-Invasive Imaging as the Cornerstone of Cardiovascular Precision Medicine. Eur. Heart J.—Cardiovasc. Imaging 2022, 23, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kammerlander, A.A.; Marzluf, B.A.; Zotter-Tufaro, C.; Aschauer, S.; Duca, F.; Bachmann, A.; Knechtelsdorfer, K.; Wiesinger, M.; Pfaffenberger, S.; Greiser, A.; et al. T1 Mapping by CMR Imaging: From Histological Validation to Clinical Implication. JACC Cardiovasc. Imaging 2016, 9, 14–23. [Google Scholar] [CrossRef]


| Absent or Mild Liver Fibrosis (n = 28) | Significant Liver Fibrosis (n = 16) | p Value * | |
|---|---|---|---|
| Age, mean (±SD), years | 55.6 | 60.1 | 0.256 |
| Male, n (%) | 13 (46.4) | 5 (31.3) | 0.361 |
| Hypertension, n (%) | 21 (75) | 12 (75) | 1.000 |
| Diabetes, n (%) | 16 (57.1) | 12 (75) | 0.333 |
| Dyslipidemia, n (%) | 24 (85.7) | 10 (62.5) | 0.133 |
| CAD, n (%) | 0 | 1 (6) | 0.364 |
| Stroke, n (%) | 0 | 2 (12.5) | 0.127 |
| PVD, n (%) | 1 (3.6) | 2 (12.5) | 0.543 |
| Smoking, n (%) | 3 (10.7) | 2 (12.5) | 1.000 |
| Obesity 1, n (%) | 18 (64) | 9 (56) | 0.749 |
| Family history of CAD, n (%) | 12 (43) | 8 (50) | 0.757 |
| High Framingham Risk Score 2, n (%) | 19 (68) | 15 (94) | 0.067 |
| Clinical signs of cirrhosis, n (%) | 0 | 11 (68.7) | 1.000 |
| ALT, mean (±SD), U/L | 36.9 | 46.5 | 0.280 |
| AST, mean (±SD), U/L | 26.8 | 50.5 | 0.001 |
| GGT, mean (±SD), U/L | 48.3 | 129.8 | 0.007 |
| Absent or Mild Liver Fibrosis (n = 28) | Significant Liver Fibrosis (n = 16) | p Value * | |
|---|---|---|---|
| Echocardiogram | |||
| LV EF (Simpson) %, mean (SD) | 59.2 (4.5) | 60.5 (4.7) | 0.28 |
| E/e’ medium, mean (SD) | 8.7 (1.95) | 10.0 (2.1) | 0.04 |
| LA indexed volume, mL/m2, mean (SD) | 29 (6.7) | 33 (7.9) | 0.15 |
| GLS, %, mean (SD) | −18.2 (1.7) | −19.9 (3.1) | 0.02 |
| Carotid Doppler | |||
| Plaque and/or increased IMT, n (%) | 17 (60.7) | 13 (81) | 0.20 |
| CT scan | |||
| Calcium score, median [IQR] | 11.0 [129.5] | 124.5 [453.8] | 0.05 |
| Cardiac and liver MRI | |||
| LV EDVI, mL/m2, mean (SD) | 66.5 (±13.9) | 67.1 (±20.5) | 0.634 |
| LV indexed mass, g/m2, mean (SD) | 53.2 (10.9) | 50.0 (8.4) | 0.311 |
| LA indexed volume, mL/m2, mean (SD) | 24.9 (5.9) | 26.5 (11.0) | 0.912 |
| LV EF, %, mean (SD) | 68.1 (5.8) | 72 (5.6) | 0.032 |
| RV EDVI, mL/m2, mean (SD) | 63.1 (15.2) | 61.4 (16.6) | 0.608 |
| RV EF, %, mean (SD) | 58.1 (6.1) | 58.1 (6.1) | 0.971 |
| T1, ms, mean (SD) | 1207.8 (32.7) | 1264 (66.2) | 0.086 |
| Cardiac ECV, %, mean (SD) | 22.9 (1.9) | 25.5(3.0) | 0.001 |
| Myocardial LGE, n (%) | 3 (10.7) | 2 (12.5) | 1.00 |
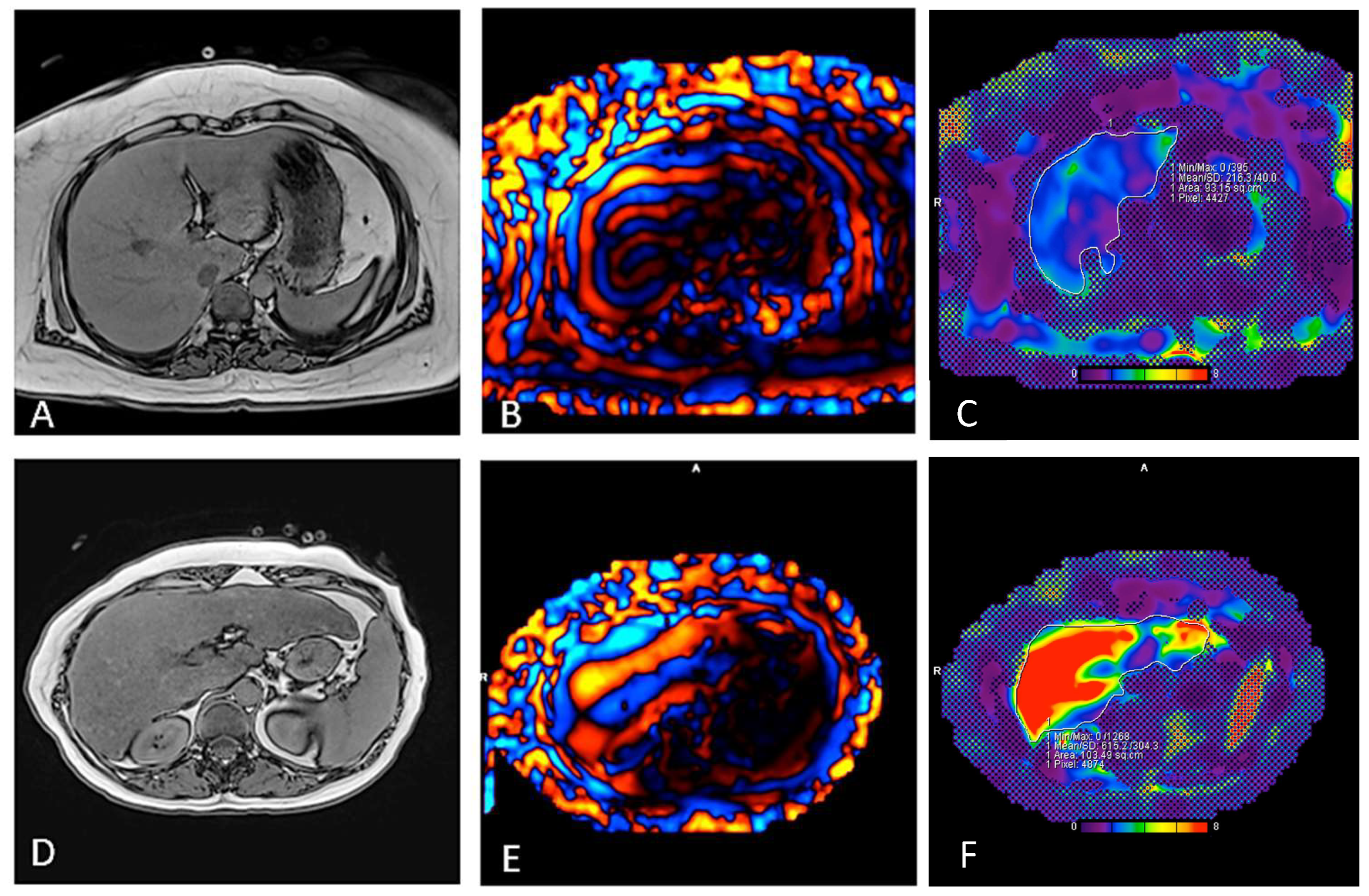
| Liver stiffness MRE, mean (SD) | 2.15 (0.41) | 4.81 (1.08) | < 0.001 |
| Liver fat fraction 1, %, mean (SD) | 16.0 (11.7) | 7.3 (5.9) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terzi, F.V.d.O.; Camargo, G.C.; Parente, D.B.; Pittella, A.M.; Silva-Junior, G.; de Novaes, G.G.; Oliveira Neto, J.A.; Barroso, J.M.; Pinheiro, M.V.T.; Xavier de Brito, A.S.; et al. How Cardiac Fibrosis Assessed via T1 Mapping Is Associated with Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2023, 12, 7381. https://doi.org/10.3390/jcm12237381
Terzi FVdO, Camargo GC, Parente DB, Pittella AM, Silva-Junior G, de Novaes GG, Oliveira Neto JA, Barroso JM, Pinheiro MVT, Xavier de Brito AS, et al. How Cardiac Fibrosis Assessed via T1 Mapping Is Associated with Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Journal of Clinical Medicine. 2023; 12(23):7381. https://doi.org/10.3390/jcm12237381
Chicago/Turabian StyleTerzi, Flavia Vernin de Oliveira, Gabriel Cordeiro Camargo, Daniella Braz Parente, Ana Maria Pittella, Gilberto Silva-Junior, Gabrielle Gonçalves de Novaes, Jaime Araújo Oliveira Neto, Julia Machado Barroso, Martha Valéria Tavares Pinheiro, Adriana Soares Xavier de Brito, and et al. 2023. "How Cardiac Fibrosis Assessed via T1 Mapping Is Associated with Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease" Journal of Clinical Medicine 12, no. 23: 7381. https://doi.org/10.3390/jcm12237381
APA StyleTerzi, F. V. d. O., Camargo, G. C., Parente, D. B., Pittella, A. M., Silva-Junior, G., de Novaes, G. G., Oliveira Neto, J. A., Barroso, J. M., Pinheiro, M. V. T., Xavier de Brito, A. S., de Oliveira, R. S., Rodrigues, R. S., de Mello Perez, R., de Sousa, A. S., & Moll-Bernardes, R. J. (2023). How Cardiac Fibrosis Assessed via T1 Mapping Is Associated with Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Journal of Clinical Medicine, 12(23), 7381. https://doi.org/10.3390/jcm12237381






