Relationships between CD34-, CD105- and bcl-2-Expression Levels and Contrast-Enhanced Ultrasound-Based Differential Diagnosis of Adnexal Tumours
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CEUS
2.3. Immunohistochemistry
3. Results
3.1. S Parameter
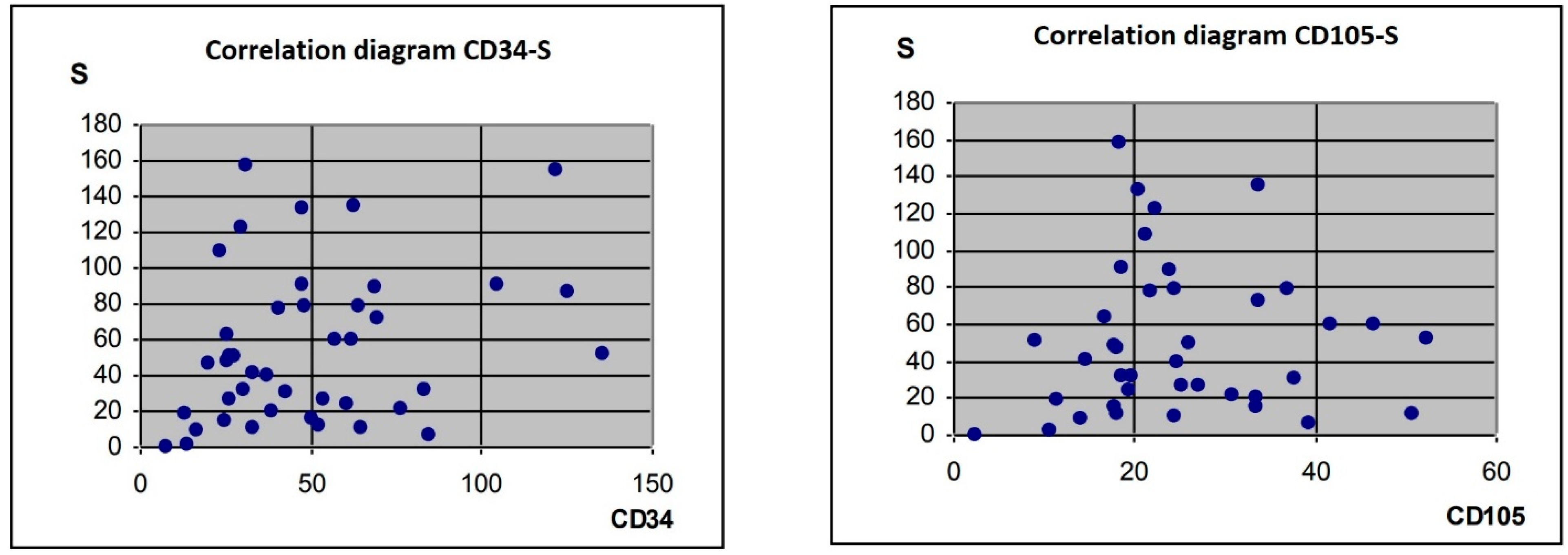
3.2. Correlation of the S Parameter with CD34-, CD105- and BCL2-Expression Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.; Tinker, A.V.; Friedlander, M. “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecol. Oncol. 2014, 133, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Pasipanodya, T.; Dwivedi, R. The management of suspected ovarian masses in premenopausal women in a DGH setting. BJOG 2013, 120, 372. [Google Scholar]
- Dora, S.K.; Dandapat, A.B.; Pande, B.; Hota, J.P. A prospective study to evaluate the risk malignancy index and its diagnostic implication in patients with suspected ovarian mass. J. Ovarian Res. 2017, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, A.P.; Pratapkumar; Sujatha, K.; Vani, R. Comparison of three risk of malignancy indices in evaluation of pelvic masses. Gynecol. Oncol. 2001, 81, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Huang, Y.-J.; Mao, M.-Y.; Li, T.; Wang, S.-J.; He, D.-N.; Liu, W.-F.; Li, M.-X.; Zhu, X.-M.; Chen, X.-Y.; et al. Contrast-enhanced US to improve diagnostic performance of O-RADS US risk stratification system for malignancy. Radiology 2023, 308, e223003. [Google Scholar] [CrossRef]
- Abbas, A.M.; Shalabi, M.G.; Elsiddig, S.A.; Eltahir, Z.; Babker, A.M.A.; Ahmed, H.G. Evaluation of angiogenesis by using CD105 and CD34 in Sudanese breast cancer patients. Pak. J. Biol. Sci. 2021, 24, 1144–1151. [Google Scholar] [CrossRef]
- Huret, J.L.; Ahmad, M.; Arsaban, M.; Bernheim, A.; Cigna, J.; Desangles, F.; Guignard, J.C.; Jacquemot-Perbal, M.C.; Labarussias, M.; Leberre, V.; et al. Atlas of genetics and cytogenetics in oncology and haematology in 2013. Nucleic Acids Res. 2013, 41, D920–D924. [Google Scholar] [CrossRef]
- Lam, L.T.; Zhang, H.; Chyla, B. Biomarkers of therapeutic response to BCL2 antagonists in cancer. Mol. Diagn. Ther. 2012, 16, 347–356. [Google Scholar] [CrossRef]
- Marret, H. Doppler ultrasonography in the diagnosis of ovarian cysts: Indications, pertinence and diagnostic criteria. J. Gynecol. Obstet. Biol. Reprod. 2001, 30, S20–S33. [Google Scholar]
- Kaijser, J.; Vandecaveye, V.; Deroose, C.M.; Rockall, A.; Thomassin-Naggara, I.; Bourne, T.; Timmerman, D. Imaging techniques for the pre-surgical diagnosis of adnexal tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 683–695. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists ACOG. Practice Bulletin. Management of adnexal masses. Obstet. Gynecol. 2007, 110, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.E.; Covens, A.L.; Lacchetti, C.; Elit, L.M.; Le, T.; Devries-Aboud, M.; Fung-Kee-Fung, M.; Gynecology Cancer Disease Site Group. Management of a suspicious adnexal mass: A clinical practice guideline. Curr. Oncol. 2012, 19, e244–e257. [Google Scholar] [CrossRef] [PubMed]
- D’arcy, T.J.; Jayaram, V.; Lynch, M.; Soutter, W.P.; Cosgrove, D.O.; Harvey, C.J.; Patel, N. Ovarian cancer detected non-invasively by contrast-enhanced power Doppler ultrasound. BJOG 2004, 111, 619–622. [Google Scholar] [CrossRef]
- Cohen, L.S.; Escobar, P.F.; Scharm, C.; Glimco, B.; Fishman, D.A. Three-dimensional power Doppler ultrasound improves the diagnostic accuracy for ovarian cancer prediction. Gynecol. Oncol. 2001, 82, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.C.; Wojcicki, W.E.; Donnelly, E.F.; Pickens, D.R.; Thirsk, G.; Thurman, G.B.; Hellerqvist, C.G. Quantified color Doppler sonography of tumor vascularity in an animal model. J. Ultrasound Med. 1999, 18, 547–551. [Google Scholar] [CrossRef]
- Stewart, L.; Advanced Ovarian Cancer Trialists Group. Chemotherapy for advanced ovarian cancer. Cochrane Database Syst. Rev. 2000, CD001418. [Google Scholar] [CrossRef]
- Engel, J.; Eckel, R.; Schubert-Fritschle, G.; Kerr, J.; Kuhn, W.; Diebold, J.; Kimmig, R.; Rehbock, J.; Hölzel, D. Moderate progress for ovarian cancer in the last 20 years: Prolongation of survival, but no improvement in the cure rate. Eur. J. Cancer 2002, 38, 2435–2445. [Google Scholar] [CrossRef]
- Ordén, M.-R.; Jurvelin, J.S.; Kirkinen, P.P. Kinetics of a US contrast agent in benign and malignant adnexal tumors. Radiology 2003, 226, 405–410. [Google Scholar] [CrossRef]
- Marret, H.; Sauget, S.; Giraudeau, B.; Brewer, M.; Ranger-Moore, J.; Body, G.; Tranquart, F. Contrast-enhanced sonography helps in discrimination of benign from malignant adnexal masses. J Ultrasound Med. 2004, 23, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.C.; Ferrandina, G.; Fruscella, E.; Van Holsbeke, C.; Ferrazzi, E.; Leone, F.P.G.; Arduini, D.; Exacoustos, C.; Bokor, D.; Scambia, G.; et al. The use of contrasted transvaginal sonography in the diagnosis of gynecologic diseases: A preliminary study. J. Ultrasound Med. 2005, 24, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, A.C.; Lyshchik, A.; Andreotti, R.F.; Hwang, M.; Jones, H.W.; Fishman, D.A. Advances in sonographic detection of ovarian cancer: Depiction of tumor neovascularity with microbubbles. AJR Am. J. Roentgenol. 2010, 194, 343–348. [Google Scholar] [CrossRef]
- Testa, A.C.; Timmerman, D.; Van Belle, V.; Fruscella, E.; Van Holsbeke, C.; Savelli, L.; Ferrazzi, E.; Leone, F.P.G.; Marret, H.; Tranquart, F.; et al. Intravenous contrast ultrasound examination using contrast-tuned imaging (CnTI) and the contrast medium SonoVue for discrimination between benign and malignant adnexal masses with solid components. Ultrasound Obstet. Gynecol. 2009, 34, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sconfienza, L.M.; Perrone, N.; Delnevo, A.; Lacelli, F.; Murolo, C.; Gandolfo, N.; Serafini, G. Diagnostic value of contrast-enhanced ultrasonography in the characterization of ovarian tumors. J. Ultrasound 2010, 13, 9–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qiu, L.; Yang, F.; Luo, H. A Preliminary Study: The sequential use of the risk malignancy index and contrast-enhanced ultrasonography in differential diagnosis of adnexal masses. Medicine 2018, 97, e11536. [Google Scholar] [CrossRef]
- Liu, P.; Sun, Y.-L.; Du, J.; Hou, X.-S.; Meng, H. CD105/Ki67 coexpression correlates with tumor progression and poor prognosis in epithelial ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Szubert, S.; Moszynski, R.; Michalak, S.; Nowicki, M.; Sajdak, S.; Szpurek, D. The associations between serum VEGF, bFGF and endoglin levels with microvessel density and expression of proangiogenic factors in malignant and benign ovarian tumors. Microvasc. Res. 2016, 107, 91–96. [Google Scholar] [CrossRef]
- Taskiran, C.; Erdem, O.; Onan, A.; Arisoy, O.; Acar, A.; Vural, C.; Erdem, M.; Ataoglu, O.; Guner, H. The prognostic value of endoglin (CD105) expression in ovarian carcinoma. Int. J. Gynecol. Cancer 2006, 16, 1789–1793. [Google Scholar] [CrossRef]
- Yuan, J.; Lan, H.; Jiang, X.; Zeng, D.; Xiao, S. Bcl-2 family: Novel insight into individualized therapy for ovarian cancer (Review). Int. J. Mol. Med. 2020, 46, 1255–1265. [Google Scholar] [CrossRef]
- Munjishvili, V.; Barabadze, E.; Musashvili, T.; Gachechiladze, M.; Burkadze, G. Morphophenotypic characteristics of ovarian serous borderline tumors. Georgian Med. News 2019, 20–25. [Google Scholar]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.S.; Bermudez, Y.; Badgwell, D.; Chen, R.; Nicosia, S.V.; Bast, R.C.; Kruk, P.A. Urinary levels of Bcl-2 are elevated in ovarian cancer patients. Gynecol. Oncol. 2009, 112, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Szubert, S.; Michalak, S.; Szpurek, D.; Moszynski, R.; Krygowska-Zielinska, J.; Sajdak, S. Anti-ovarian antibodies in sera of patients with ovarian tumors. Immunol. Lett. 2012, 148, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, L.; Xin, Z. Combination of serum CA19-9 and CA125 levels and contrast-enhanced ultrasound parametric data facilitates to differentiate ovarian serous carcinoma from ovarian malignant epithelial cancer. Medicine 2018, 97, e0358. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Jin, C.; Hu, Y.; Zhang, Q.; Yan, X.; Zhu, F.; Lin, F. High CA-125 and CA19-9 levels in spontaneous ruptured ovarian endometriomas. Clin. Chim. Acta 2015, 450, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.-J.; Yu, J.; Yu, Z.; Li, N.; Song, C.; Li, M. Contrast-enhanced ultrasonography in differential diagnosis of benign and malignant ovarian tumors. PLoS ONE 2015, 10, e0118872. [Google Scholar] [CrossRef]
- Yang, F.; Yang, T.-Z.; Tian, T.; Wang, J.-X.; Luo, H. Perfusion imaging of ovarian masses with contrast-enhanced ultrasonography. Sichuan Da Xue Xue Bao Yi Xue Ban 2018, 49, 587–593. [Google Scholar]
- Dietrich, C.F.; Averkiou, M.A.; Correas, J.-M.; Lassau, N.; Leen, E.; Piscaglia, F. An EFSUMB introduction into dynamic contrast-enhanced ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012, 33, 344–351. [Google Scholar] [CrossRef]
- Xu, A.; Nie, F.; Liu, T.; Dong, T.; Bu, L.; Yang, D. Adnexal masses: Diagnostic performance of contrast-enhanced ultrasound using the simple rules from the International Ovarian Tumor Analysis Group. Int. J. Gynaecol. Obstet. 2022, 157, 568–573. [Google Scholar] [CrossRef]
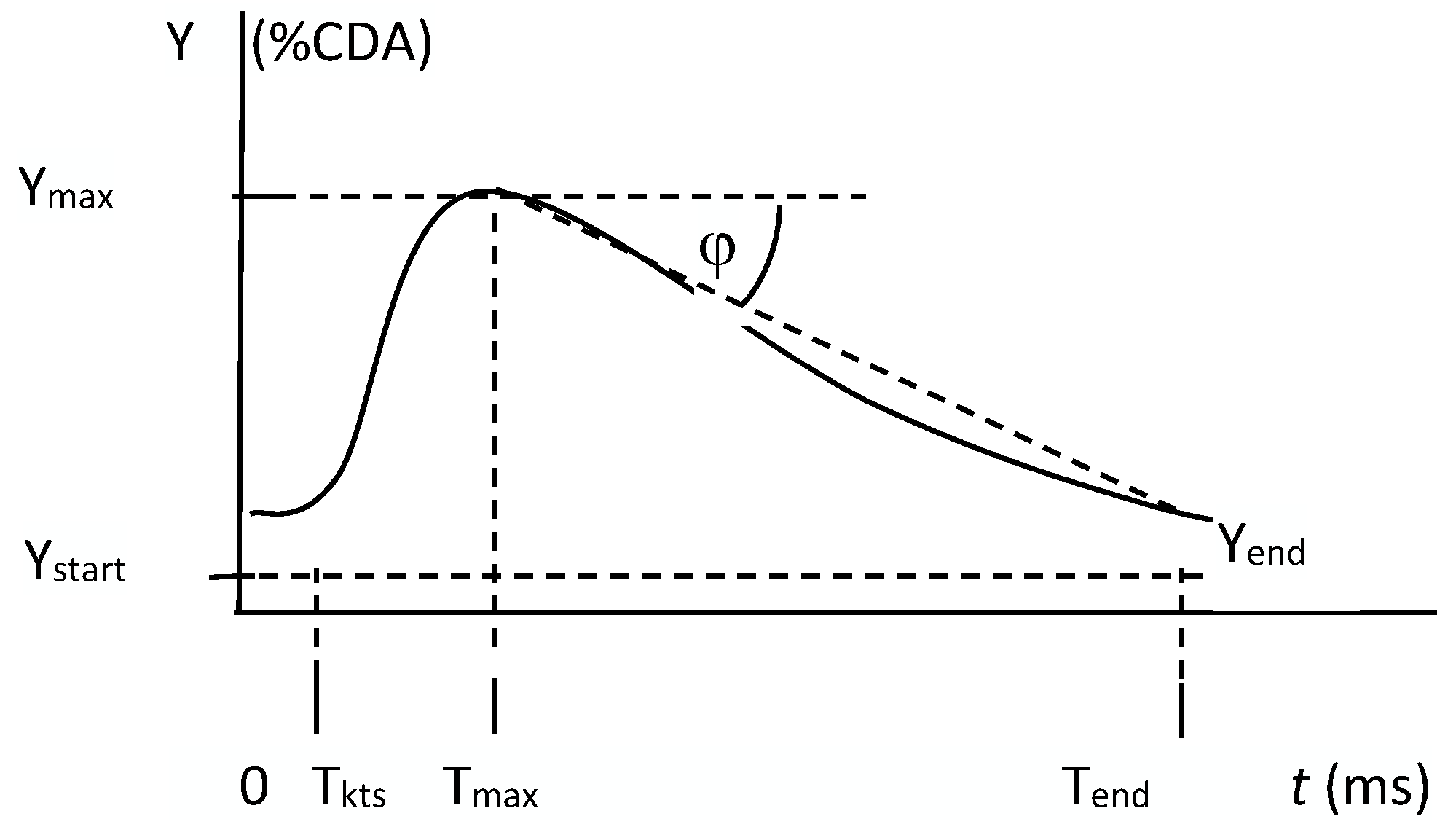
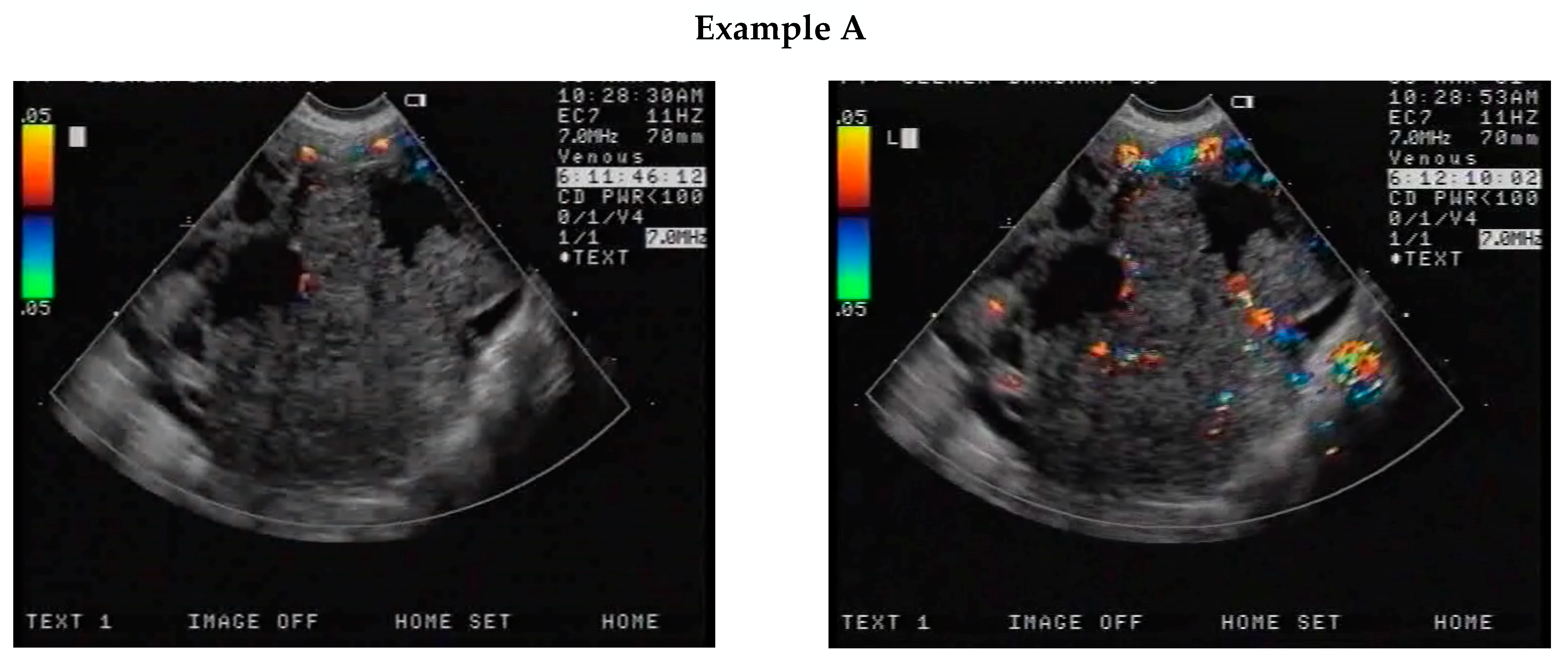
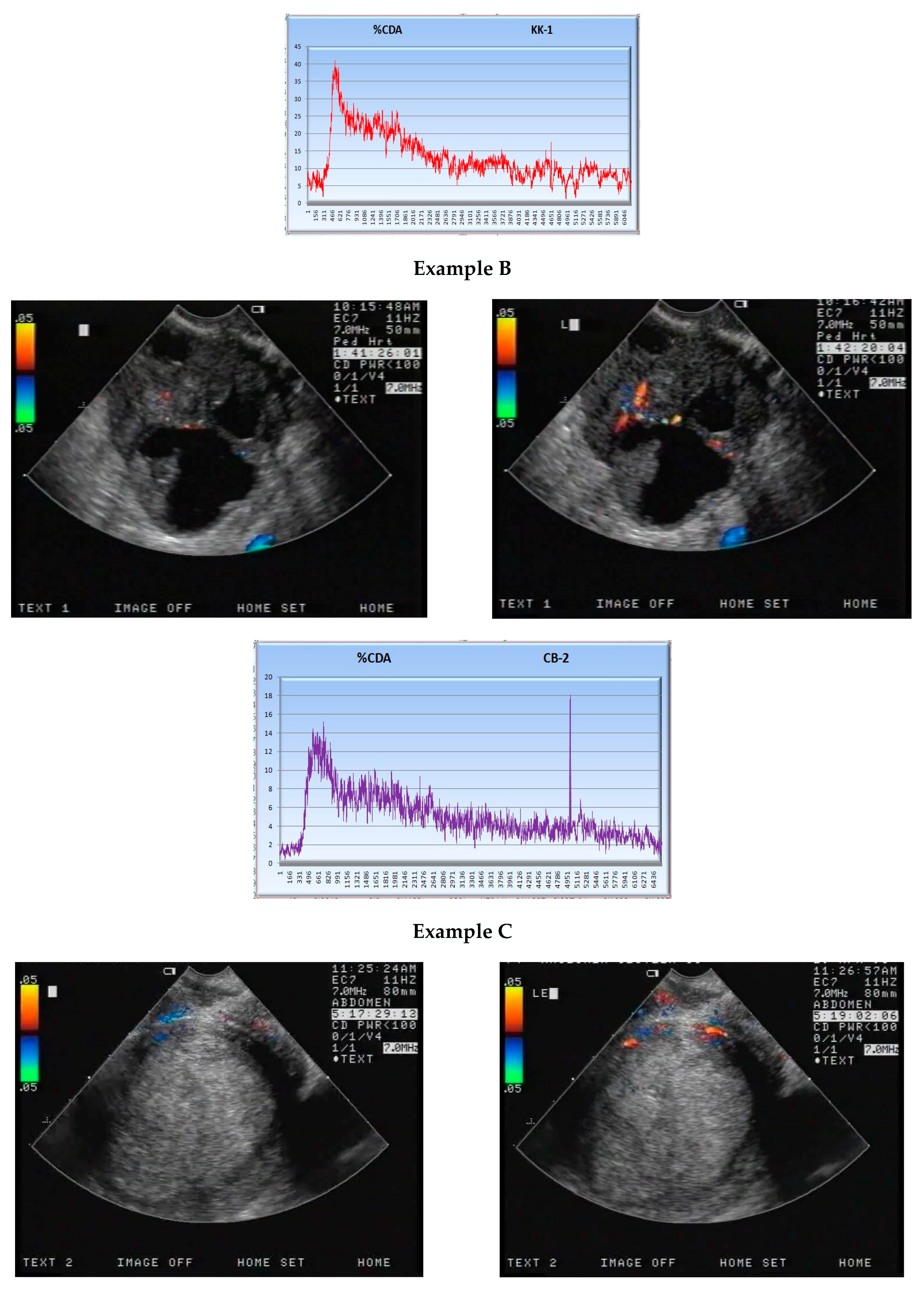
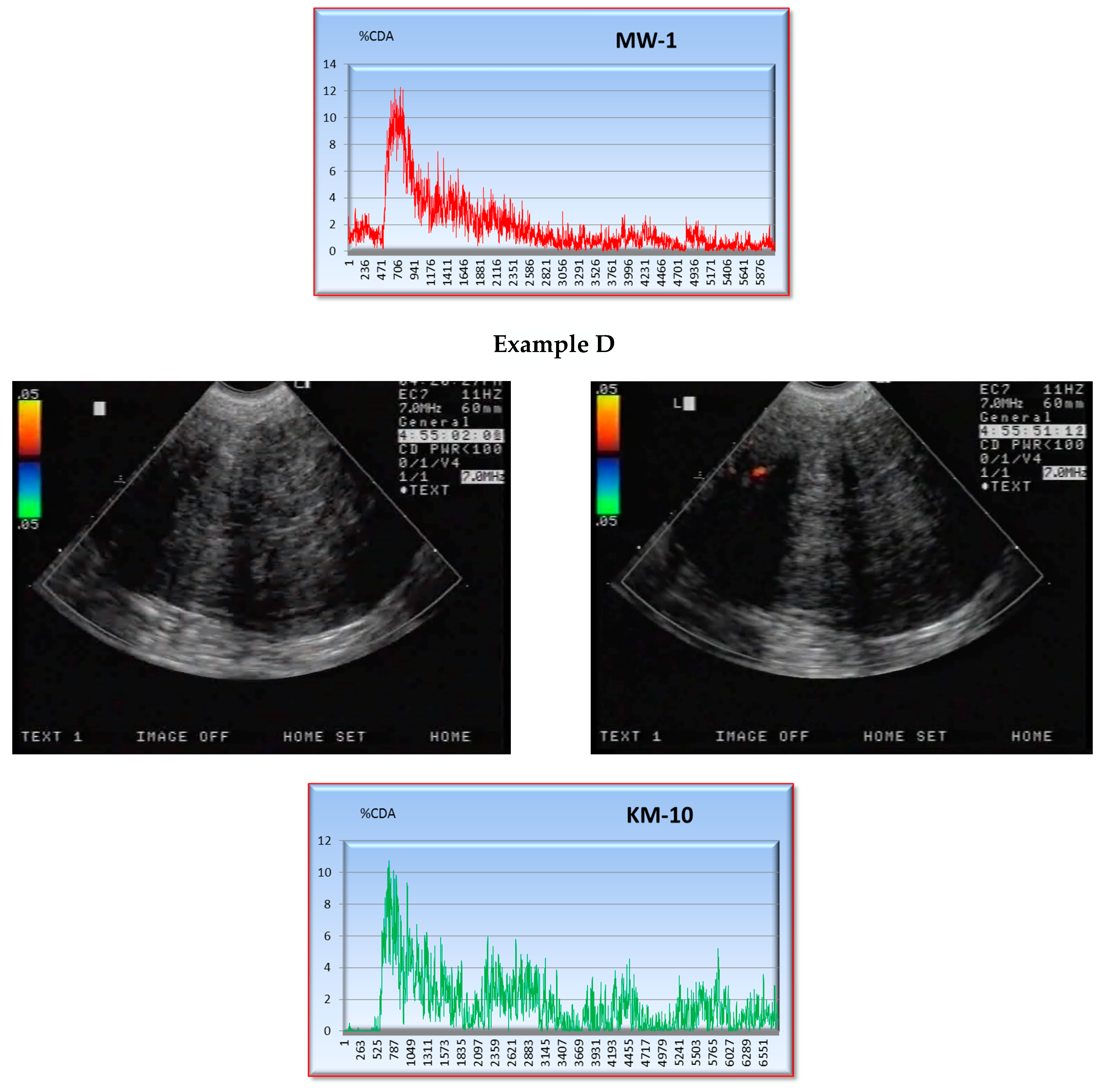


| Ystart | Ymax | S × 10−3 | ||
|---|---|---|---|---|
| Malignant tumours (n = 33) | Mean | 10.91 | 31.30 | 80.5 |
| SD | 8.39 | 16.91 | 50.6 | |
| Coefficient of variation | 76.9% | 54.0% | 62.9% | |
| Benign tumours (n = 18) | Mean | 4.27 | 11.29 | 25.00 |
| SD | 5.30 | 8.78 | 22.67 | |
| Coefficient of variation | 124.1% | 77.8% | 90.7% | |
| Cochran–Cox test Ckr = 2.06 | C | – | 5.56 | – |
| p | – | <0.0001 | – | |
| Mann–Whitney U test Ukr = 1.96 | U | 3.47 | – | 4.26 |
| p | 0.0005 | – | <0.0001 |
| S (Me = 46.9) | ||||
|---|---|---|---|---|
| £ Me | >Me | Sum | ||
| bcl-2-intens | 0 | 7 | 10 | 17 |
| 1 | 5 | 6 | 11 | |
| 2 | 6 | 2 | 8 | |
| 3 | 2 | 3 | 5 | |
| Sum | 20 | 21 | 41 | |
| Chi-square test | c2 = 2.80 | |||
| P = 0.42 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymanski, M.; Florczyk, I.; Janicki, R.; Bernard, P.; Domaracki, P.; Brycht, L.; Szyca, R.; Szymanska, A.; Paniutycz, J. Relationships between CD34-, CD105- and bcl-2-Expression Levels and Contrast-Enhanced Ultrasound-Based Differential Diagnosis of Adnexal Tumours. J. Clin. Med. 2023, 12, 7372. https://doi.org/10.3390/jcm12237372
Szymanski M, Florczyk I, Janicki R, Bernard P, Domaracki P, Brycht L, Szyca R, Szymanska A, Paniutycz J. Relationships between CD34-, CD105- and bcl-2-Expression Levels and Contrast-Enhanced Ultrasound-Based Differential Diagnosis of Adnexal Tumours. Journal of Clinical Medicine. 2023; 12(23):7372. https://doi.org/10.3390/jcm12237372
Chicago/Turabian StyleSzymanski, Marek, Iwona Florczyk, Radoslaw Janicki, Piotr Bernard, Piotr Domaracki, Lukasz Brycht, Robert Szyca, Angelika Szymanska, and Julia Paniutycz. 2023. "Relationships between CD34-, CD105- and bcl-2-Expression Levels and Contrast-Enhanced Ultrasound-Based Differential Diagnosis of Adnexal Tumours" Journal of Clinical Medicine 12, no. 23: 7372. https://doi.org/10.3390/jcm12237372
APA StyleSzymanski, M., Florczyk, I., Janicki, R., Bernard, P., Domaracki, P., Brycht, L., Szyca, R., Szymanska, A., & Paniutycz, J. (2023). Relationships between CD34-, CD105- and bcl-2-Expression Levels and Contrast-Enhanced Ultrasound-Based Differential Diagnosis of Adnexal Tumours. Journal of Clinical Medicine, 12(23), 7372. https://doi.org/10.3390/jcm12237372






