Remimazolam Pilot for Office-Based Dental Sedation: Adverse Events, Awareness and Outcomes
Abstract
:1. Introduction
2. Materials and Methods
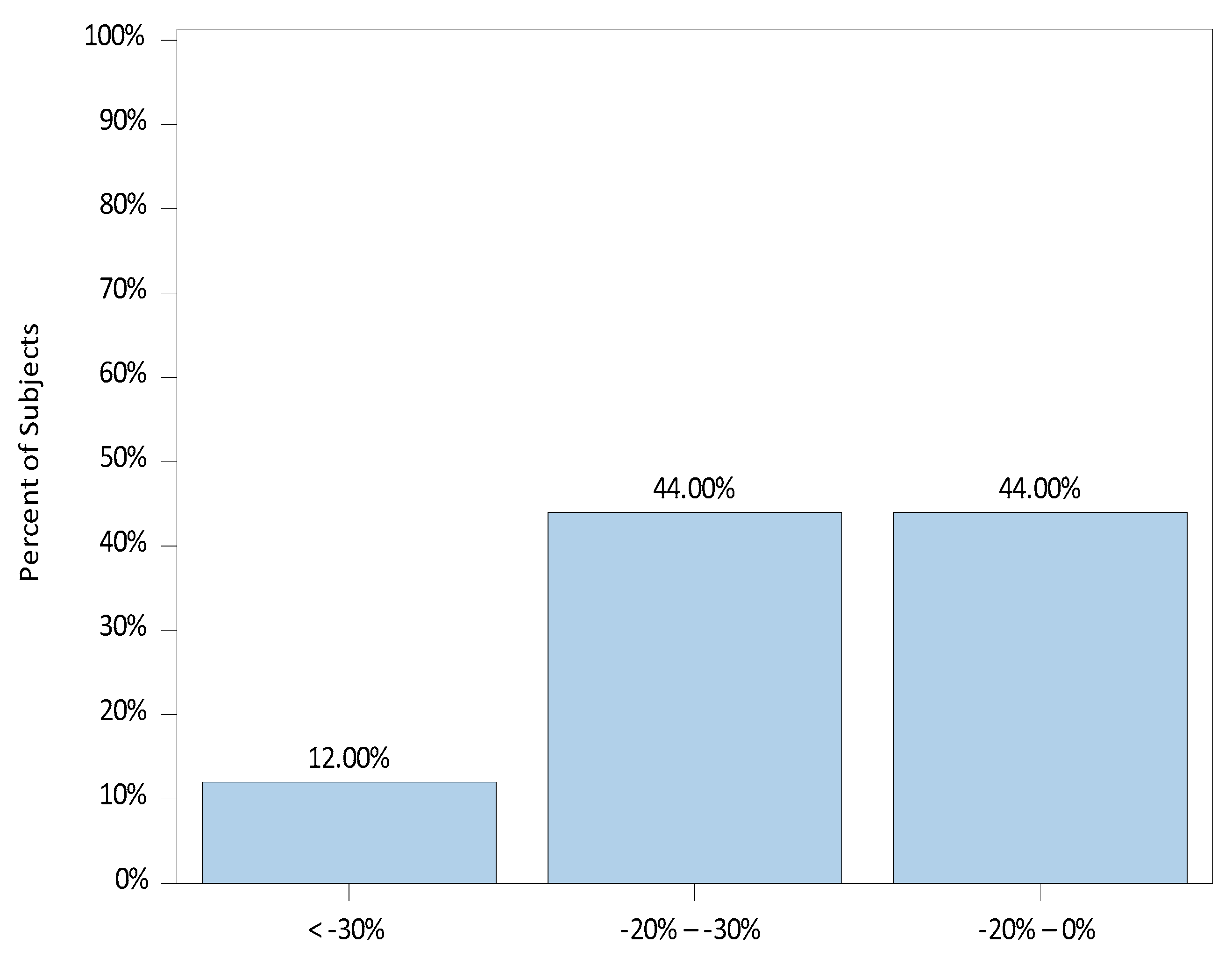
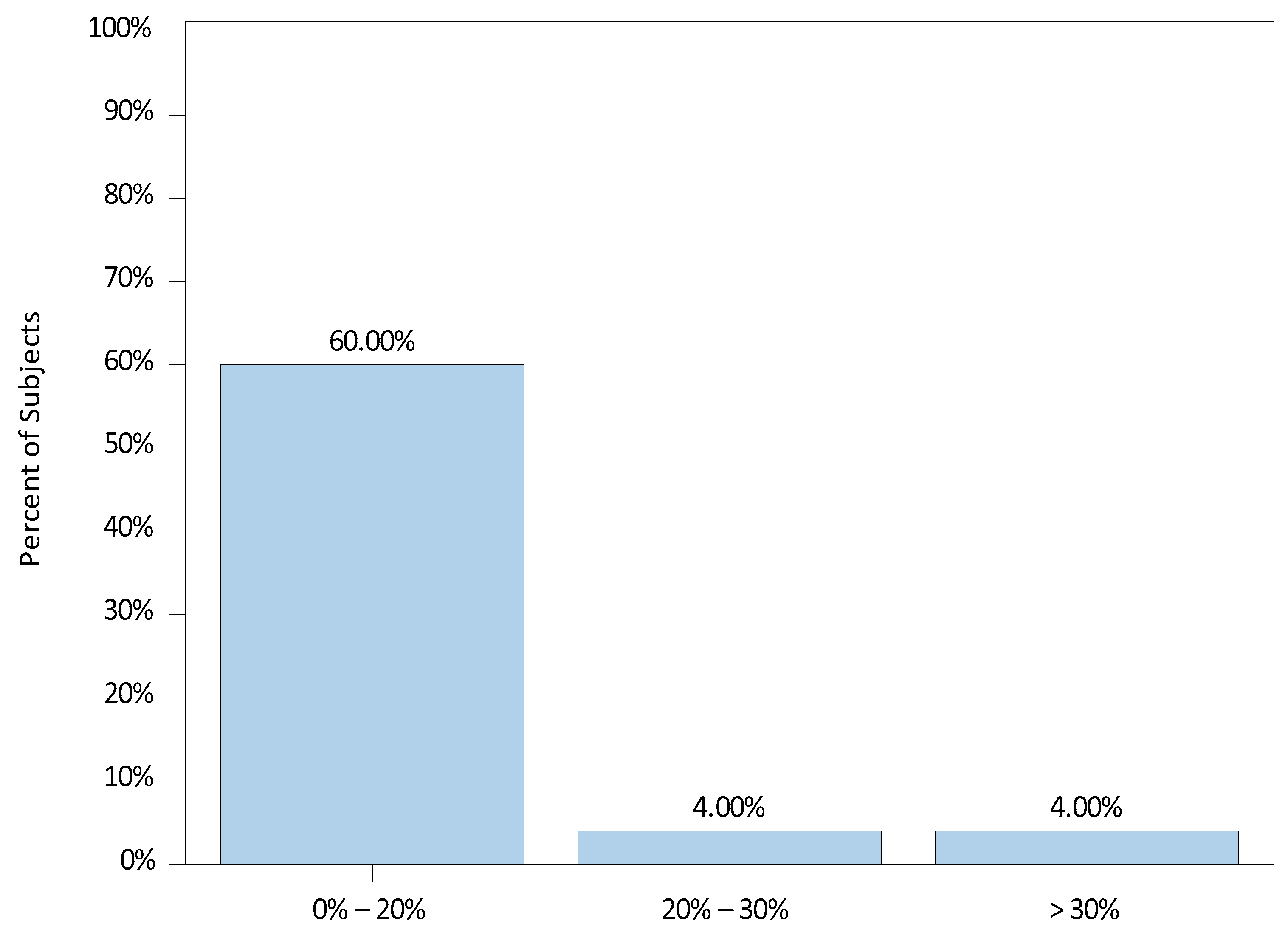
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kilpatrick, G.J. Remimazolam: Non-Clinical and Clinical Profile of a New Sedative/Anesthetic Agent. Front. Pharmacol. 2021, 12, 690875. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Cheng, Z.; Li, H.; Zhang, X.; Luan, H.; Zhao, Z.; Zhu, P. Efficacy and Safety of Different Doses of Remimazolam Tosilate Applied in Upper Gastrointestinal Endoscopy: A Prospective Randomized Controlled Double-Blind Trial. Drug Des. Dev. Ther. 2023, 17, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, S.; Lee, D.H.; Park, J.S. Finding the ideal sedative: A non-inferiority study of remimazolam vs. propofol in endoscopic retrograde cholangiopancreatography. J. Gastroenterol. Hepatol. 2023. [Google Scholar] [CrossRef]
- Zhao, M.J.; Hu, H.F.; Li, X.L.; Li, X.M.; Wang, D.C.; Kuang, M.J. The safety and efficacy between remimazolam and propofol in intravenous anaesthesia of endoscopy operation: A systematic review and meta-analysis. Int. J. Surg. 2023, 109, 3566–3577. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Guan, J.; Liu, L.; Fu, B.; Chen, L.; Zheng, X. Discharge readiness after remimazolam versus propofol for colonoscopy: A randomised, double-blind trial. Eur. J. Anaesthesiol. 2022, 39, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Bhandari, R.; Desta, T.; DeMicco, M.P.; Schaeffer, C.; Etzkorn, K.; Barish, C.F.; Pruitt, R.; Cash, B.D.; Quirk, D.; et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest. Endosc. 2018, 88, 427–437.e6. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Steinfath, M.; Schulz, M. Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil. An update. Clin. Pharmacokinet. 1996, 31, 275–292. [Google Scholar] [CrossRef]
- Xin, Y.; Chu, T.; Wang, J.; Xu, A. Sedative effect of remimazolam combined with alfentanil in colonoscopic polypectomy: A prospective, randomized, controlled clinical trial. BMC Anesthesiol. 2022, 22, 262. [Google Scholar] [CrossRef]
- Zhao, N.; Zeng, J.; Fan, L.; Wang, J.; Zhang, C.; Zou, S.; Zhang, B.; Li, K.; Yu, C. Moderate sedation by total intravenous remimazolam-alfentanil vs. propofol-alfentanil for third molar extraction: A prospective randomized controlled trial. Front. Med. 2022, 9, 950564. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, X.; Wang, L.; Liu, Y.; Yang, X. Can Remimazolam Be a New Sedative Option for Outpatients Undergoing Ambulatory Oral and Maxillofacial Surgery? J. Oral Maxillofac. Surg. 2023, 81, 8–16. [Google Scholar] [CrossRef]
- Li, X.; Tian, M.; Deng, Y.; She, T.; Li, K. Advantages of Sedation with Remimazolam Compared to Midazolam for the Removal of Impacted Tooth in Patients with Dental Anxiety. J. Oral Maxillofac. Surg. 2023, 81, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Roback, M.G.; Green, S.M.; Andolfatto, G.; Leroy, P.L.; Mason, K.P. Tracking and Reporting Outcomes of Procedural Sedation (TROOPS): Standardized Quality Improvement and Research Tools from the International Committee for the Advancement of Procedural Sedation. Br. J. Anaesth. 2018, 120, 164–172. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, Office for Human Research Protections, Regulations, 45 CFR 46. Available online: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html (accessed on 4 October 2023).
- National Health Service, Health Research Authority, Medical Research Council, Decision Tool. Available online: https://www.hra.nhs.uk/ (accessed on 4 October 2023).
- Royal College of Surgeons of England, Faculty of Dental Surgery, Standards for Conscious Sedation in the Provision of Dental Care and Accreditation, April 2015, Revised March 2020. Available online: https://www.rcseng.ac.uk/dental-faculties/fds/publications-guidelines/standards-for-conscious-sedation-in-the-provision-of-dental-care-and-accreditation/ (accessed on 4 October 2023).
- Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018: A Report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology 2018, 128, 437–479.
- Intercollegiate Advisory Committee for Sedation in Dentistry The Intercollegiate Advisory Committee for Sedation in Dentistry Is an Intercollegiate Committee of the Following: The Faculty of Dental Surgery of the Royal College of Surgeons of England (FDS RCS Eng), The Faculty of General Dental Practice (UK) (FGDP (UK)), The Faculty of Dental Surgery of the Royal College of Surgeons of Edinburgh (FDS RCS Edin), The Dental Faculty of the Royal College of Physicians and Surgeons of Glasgow (FDS RCPSG) The Royal College of Anaesthetists (RCoA) Remimazolam for Intravenous Conscious Sedation for Dental Procedures IACSD Standard on Clinical Use and Training. 9 January 2023. Available online: https://www.saad.org.uk/index.php/component/users/?view=remind&Itemid=435 (accessed on 4 October 2023).
- European Medicines Agency, Science Medicine Health, Medicines, Human Byfavo|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/byfavo (accessed on 4 October 2023).
- Ramsay, M.A.; Savege, T.M.; Simpson, B.R.; Goodwin, R. Controlled sedation with alphaxalone-alphadolone. Br. Med. J. 1974, 2, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Brice, D.D.; Hetherington, R.R.; Utting, J.E. A simple study of awareness and dreaming during anaesthesia. Br. J. Anaesth. 1970, 42, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Nordström, O.; Engström, A.M.; Persson, S.; Sandin, R. Incidence of awareness in total i.v. anaesthesia based on propofol, alfentanil and neuromuscular blockade. Acta Anaesthesiol. Scand. 1997, 41, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Vargo, J.; Howard, K.; Petrillo, J.; Scott, J.; Revicki, D.A. Development and validation of the patient and clinician sedation satisfaction index for colonoscopy and upper endoscopy. Clin. Gastroenterol. Hepatol. 2009, 7, 156–162. [Google Scholar] [CrossRef]
- Antonik, L.J.; Goldwater, D.R.; Kilpatrick, G.J.; Tilbrook, G.S.; Borkett, K.M. A placebo- and midazolam-controlled phase I Single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth. Analg. 2012, 115, 274–283. [Google Scholar] [CrossRef]
- Gao, Y.-Q.; Ihmsen, H.; Hu, Z.-Y.; Sun, W.; Fang, Y.-B.; Wang, Z.; Schüttler, J.; Jeleazcov, C.; Liu, H.-C. Pharmacokinetics of remimazolam after intravenous infusion in anaesthetised children. Br. J. Anaesth. 2023, 131, 914–920. [Google Scholar] [CrossRef]
- Schüttler, J.; Eisenried, A.; Lerch, M.; Fechner, J.; Jeleazcov, C.; Ihmsen, H. Pharmacokinetics and Pharmacodynamics of Remimazolam (CNS 7056) after Continuous Infusion in Healthy Male Volunteers: Part I. Pharmacokinetics and Clinical Pharmaco-dynamics. Anesthesiology 2020, 132, 636–651. [Google Scholar] [CrossRef]
- Eisenried, A.; Schüttler, J.; Lerch, M.; Ihmsen, H.; Jeleazcov, C. Pharmacokinetics and Pharmacodynamics of Remimazolam (CNS 7056) after Continuous Infusion in Healthy Male Volunteers: Part II. Pharmacodynamics of Electroencephalogram Effects. Anesthesiology 2020, 132, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yuan, T.; Zhang, J.; Bai, H.; Tian, M.; Pan, C.; Bao, H.; Jin, X.; Ji, F.; Zhong, T.; et al. Remimazolam tosilate in upper gastrointestinal endoscopy: A multicenter, randomized, non-inferiority, phase III trial. J. Gastroenterol. Hepatol. 2021, 36, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, L.; Xu, L.; Wang, J.-F.; Li, J.-Y.; Chen, Z.-J. Effectiveness of remimazolam besylate combined with alfentanil for fiberoptic bronchoscopy with preserved spontaneous breathing: A prospective, randomized, controlled clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6071–6080. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Huang, Y.T.; Chi, K.Y.; Huang, Y.T. Remimazolam versus propofol for procedural sedation: A meta-analysis of randomized controlled trials. PeerJ 2023, 11, e15495. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Sun, M.; Wan, J.; Zhang, Z.; Huang, J.; Zhang, J.; Xiong, W.; Xia, L.; Xu, P.; Miao, C.; et al. Efficacy and safety of remimazolam tosilate versus propofol in patients undergoing day surgery: A prospective randomized controlled trial. BMC Anesthesiol. 2023, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Zhong, N.; Ye, C.; Zhu, X.; Wei, F. Propofol Versus Remimazolam on Cognitive Function, Hemodynamics, and Oxygenation during One-Lung Ventilation in Older Patients Undergoing Pulmonary Lobectomy: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2023, 37, 1996–2005. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Q.; Min, J.; Wu, Z.X.; Hu, Z. Comparison of the effects of remimazolam and dexmedetomidine on early postoperative cognitive function in elderly patients with gastric cancer. Front. Aging Neurosci. 2023, 15, 1123089. [Google Scholar] [CrossRef]
- Matsuo, M.; Okada, K.; Onuki, Y.; Yamazaki, M. Incompatibility of remimazolam besylate with Ringer’s acetate infusion resulting in total occlusion of an intravenous catheter. BMJ Case Rep. 2021, 14, e241622. [Google Scholar] [CrossRef]
- Sasaki, H.; Hoshijima, H.; Mizuta, K. Ringer’s acetate solution-induced precipitation of remimazolam. Br. J. Anaesth. 2021, 126, e87–e89. [Google Scholar] [CrossRef]
- Cinotti, R. An update on remimazolam and anaphylaxis. Eur. J. Anaesthesiol. 2023, 40, 153–154. [Google Scholar] [CrossRef]
- Masui, K. Caution!! Reappearance of remimazolam effect after a flumazenil bolus: A larger bolus of flumazenil and a lower total remimazolam clearance are higher risks. J. Anesth. 2022, 37, 1–5. [Google Scholar] [CrossRef]
- Stöhr, T.; Colin, P.J.; Ossig, J.; Pesic, M.; Borkett, K.; Winkle, P.; Struys, M.M.; Schippers, F. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br. J. Anaesth. 2021, 127, 415–423. [Google Scholar] [CrossRef]
- Nobukuni, K.; Shirozu, K.; Maeda, A.; Funakoshi, K.; Higashi, M.; Yamaura, K. Recovery of memory retention after anesthesia with remimazolam: An exploratory, randomized, open, propofol-controlled, single-center clinical trial. JA Clin. Rep. 2023, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sang, N.; Song, K.; Zhong, W.; Wang, H.; Jiang, J.; Huang, Y.; Hu, P. Psychomotor Recovery Following Remimazolam-induced Sedation and the Effectiveness of Flumazenil as an Antidote. Clin. Ther. 2020, 42, 614–624. [Google Scholar] [CrossRef]
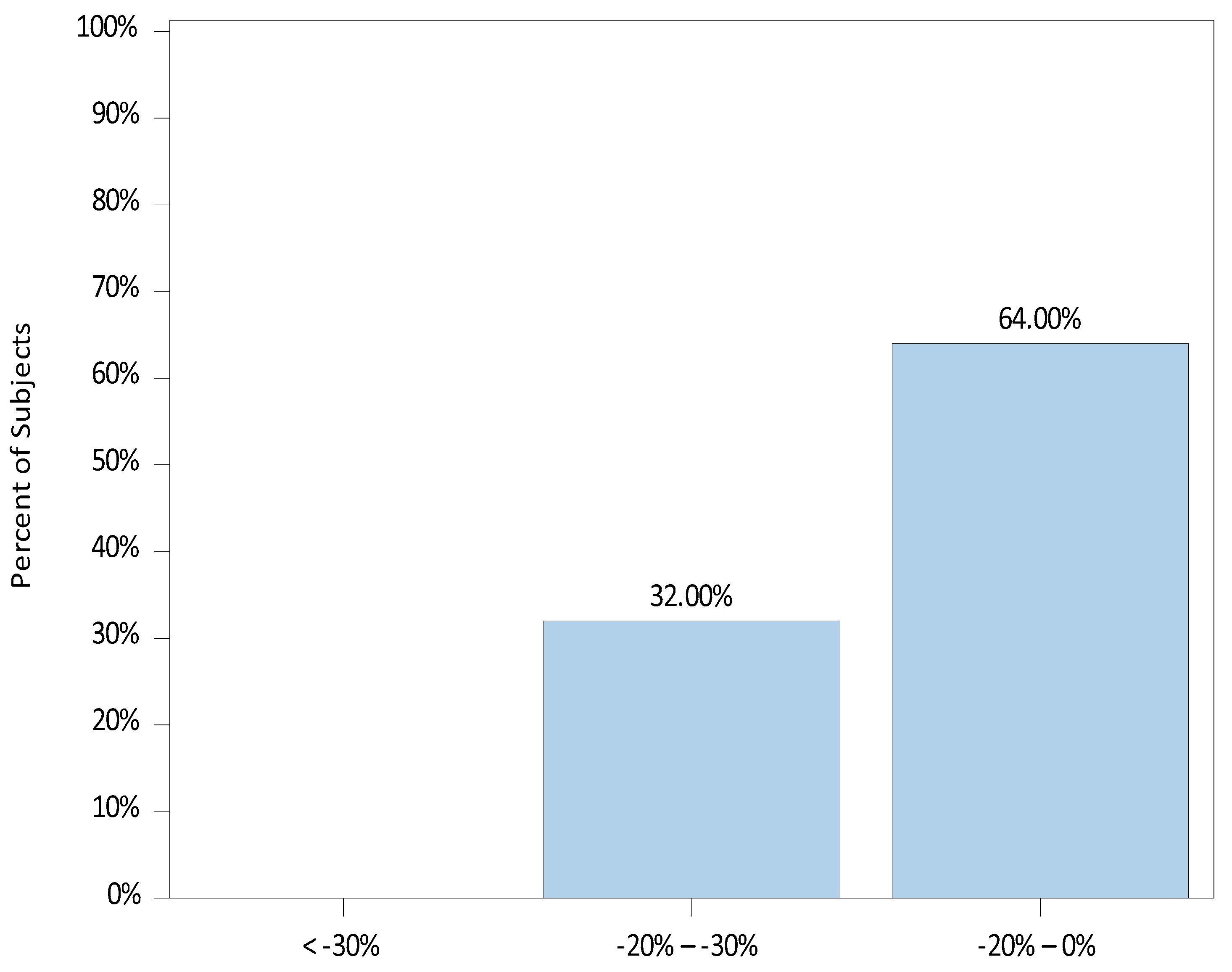
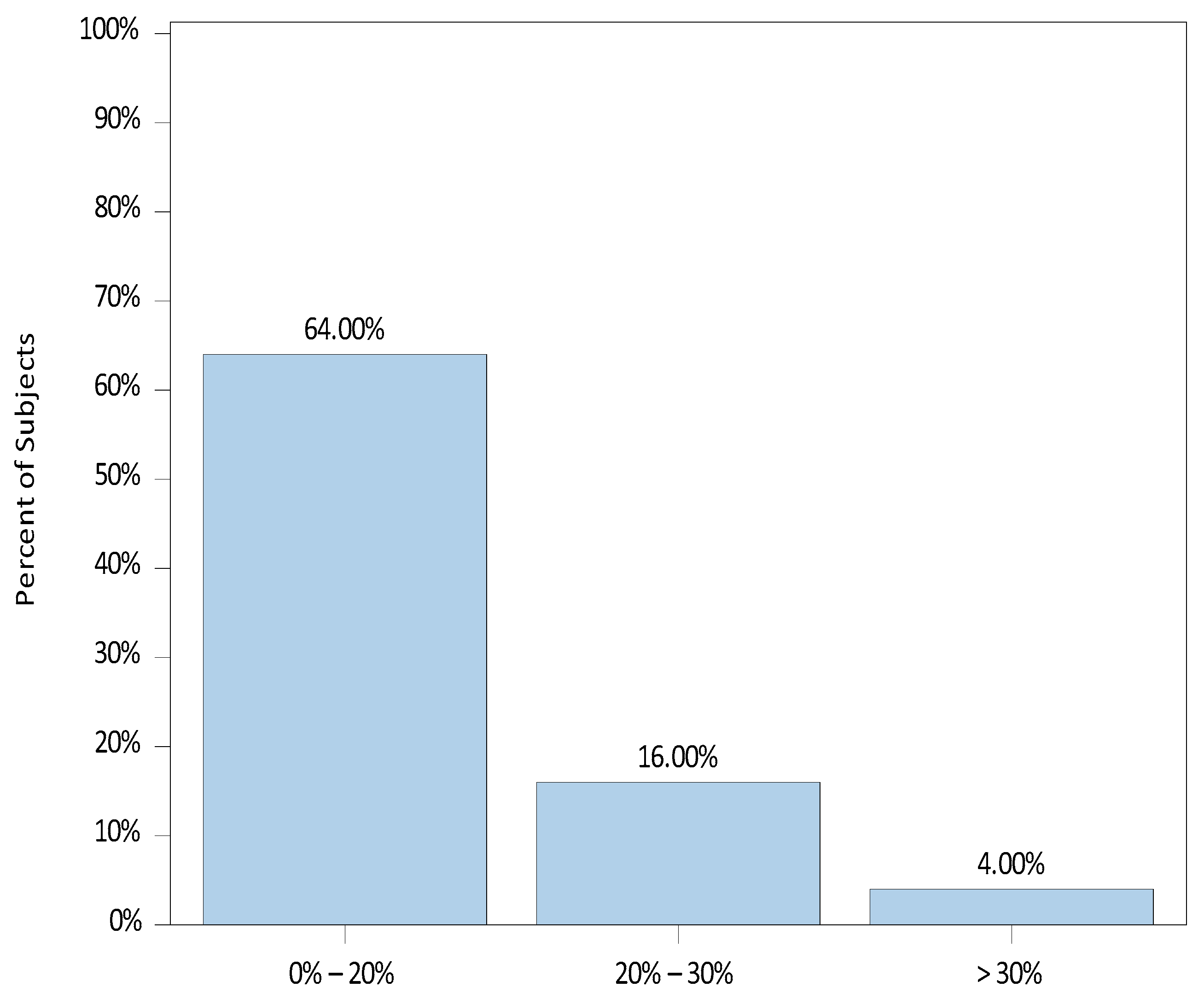
| Variable | N (%) or Mean (SD) | Min, Max |
|---|---|---|
| Gender | ||
| Female | 17 (68.0) | |
| Male | 8 (32.0) | |
| Weight (kg) | 76.9 (19.56) | 38.0, 123.0 |
| Age (years) | 55.7 (18.48) | 21.0, 82.0 |
| Height (cm) | 169.7 (8.80) | 152.0, 182.0 |
| BMI (kg/m2) | 27.6 (6.49) | 15.0, 40.0 |
| ASA | ||
| 1 | 11 (44.0) | |
| 2 | 14 (56.0) | |
| Adverse Events | ||
| No adverse events | 25 (100.0) | |
| Remimazolam total dose (mg) | 30.9 (14.12) | 12.5, 60.0 |
| Remimazolam administered (mg/kg/duration in minutes) | 0.0083557 (0.0045309) | 0.0036134, 0.0263158 |
| Remimazolam duration administered (min) | 61.8 (44.01) | 15.0, 205.0 |
| Alfentanil total dose (microgram) | 1276.0 (1104.26) | 200.0, 4400.0 |
| Alfentanyl total dose (microgram/kg) | 16.3 (13.31) | 2.4, 62.9 |
| Duration of procedure (min) | 67.4 (42.82) | 15.0, 209.0 |
| Intraprocedural Modified Ramsay sedation scale | 3.2 (0.47) | 2.0, 4.0 |
| Aldrete Discharge Score | 9.8 (0.52) | 8.0, 10.0 |
| Time to discharge after treatment (min) | 35.4 (17.91) | 15.0, 100.0 |
| Discharge after last dose of remimazolam (min) | 41.4 (20.59) | 10.0, 120.0 |
| Start of procedure after initial dose (min) | 4.9 (2.35) | 2.0, 10.0 |
| Question | Response n (%) | |
|---|---|---|
| What is the last thing you remember before going to sleep? | A. Time before initial dose administered | 9 (36.0) |
| B. Time initial dose delivered | 5 (20.0) | |
| C. Time after initial dose before LA | 9 (36.0) | |
| D. Time LA given | 1 (4.0) | |
| F. Time procedure started | 1 (4.0) | |
| What is the first thing you remember waking up? | H. Immediately after the procedure | 12 (48.0) |
| I. More than 5 min after the procedure | 13 (52.0) | |
| Do you remember anything between going to sleep and waking up? | No discomfort only instructions/talking | 9 (36.0) |
| Nothing | 13 (52.0) | |
| Some events unrelated to the procedure | 3 (12.0) | |
| Did you dream during the procedure? | Nil | 2 (8.0) |
| No | 16 (64.0) | |
| Not sure | 1 (4.0) | |
| Yes | 1 (4.0) | |
| Yes, but can’t remember what | 5 (20.0) | |
| What was the worst thing about your operation/procedure? | Intravenous cannulation | 4 (16.0) |
| Post procedure numbness, teeth extraction, denture | 3 (12.0) | |
| Paying the bill | 1 (4.0) | |
| Post-operative vomiting | 1 (4.0) | |
| Pre-operative anxiety | 14 (56.0) | |
| Pre-operative fasting | 1 (4.0) | |
| Procedure being delayed | 1 (4.0) | |
| Question | Very Satisfied | Satisfied | Somewhat Satisfied | Neither Satisfied or Dissatisfied | Somewhat Dissatisfied | Dissatisfied | Very Dissatisfied |
|---|---|---|---|---|---|---|---|
| Effectiveness of the sedation received | 22 (91.7) | 2 (8.3) | 0 | 0 | 0 | 0 | 0 |
| Effect the sedation had on the procedure | 22 (91.7) | 2 (8.3) | 0 | 0 | 0 | 0 | 0 |
| Patient’s ability to communicate post operatively | 24 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Patient’s ability to retain post operative information | 19 (79.2) | 4 (16.7) | 0 | 1 (4.2) | 0 | 0 | 0 |
| Recovery time associated with the sedation | 21 (87.5) | 3 (12.5) | 0 | 0 | 0 | 0 | 0 |
| Your overall satisfaction with the procedure | 24 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Your overall satisfaction with the sedation part of the procedure | 24 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Patient’s cooperation level | 23 (95.8) | 0 | 1 (4.2) | 0 | 0 | 0 | 0 |
| Overall ease of the procedure | 23 (95.8) | 1 (4.2) | 0 | 0 | 0 | 0 | 0 |
| Method of sedation compared with other methods of sedation | 21 (87.5) | 3 (12.5) | 0 | 0 | 0 | 0 | 0 |
| Question | Very Satisfied | Satisfied | Somewhat Satisfied | Neither Satisfied or Dissatisfied | Somewhat Dissatisfied | Dissatisfied | Very Dissatisfied |
|---|---|---|---|---|---|---|---|
| Pain associated with the sedation delivery | 23 (92.0) | 2 (8.0) | 0 | 0 | 0 | 0 | 0 |
| Amount you remember during the procedure | 19 (76.0) | 3 (12.0) | 2 (8.0) | 1 (4.0) | 0 | 0 | 0 |
| Amount of drowsiness with the medication | 17 (68.0) | 4 (16.0) | 2 (8.0) | 1 (4.0) | 0 | 1 (4.0) | 0 |
| Amount of sedation you received (enough to make you drowsy or go to sleep) | 23 (92.0) | 1 (4.0) | 0 | 1 (4.0) | 0 | 0 | 0 |
| Amount of nausea after the procedure | 23 (92.0) | 1 (4.0) | 0 | 0 | 0 | 0 | 1 (4.0) |
| Amount you remember about the procedure | 20 (80.0) | 3 (12.0) | 1 (4.0) | 0 | 1 (4.0) | 0 | 0 |
| Length of time you felt the effects of the sedation received | 22 (88.0) | 3 (12.0) | 0 | 0 | 0 | 0 | 0 |
| Drowsiness after the procedure | 14 (56.0) | 7 (28.0) | 3 (12.0) | 1 (4.0) | 0 | 0 | 0 |
| Grogginess after the procedure | 18 (72.0) | 4 (16.0) | 2 (8.0) | 1 (4.0) | 0 | 0 | 0 |
| How tired you felt after the procedure | 12 (48.0) | 6 (24.0) | 4 (16.0) | 3 (12.0) | 0 | 0 | 0 |
| Your ability to think clearly after the procedure | 17 (68.0) | 7 (28.0) | 0 | 0 | 1 (4.0) | 0 | 0 |
| Ease of recovery after the procedure | 23 (92.0) | 0 | 1 (4.0) | 1 (4.0) | 0 | 0 | 0 |
| How fast you returned to your usual daily activities (things you do everyday) | 17 (70.8) | 3 (12.5) | 2 (8.3) | 0 | 2 (8.3) | 0 | 0 |
| Your overall satisfaction with the sedation experience | 24 (96.0) | 1 (4.0) | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swart, R.; Maes, S.S.A.; Cavanaugh, D.; Mason, K.P. Remimazolam Pilot for Office-Based Dental Sedation: Adverse Events, Awareness and Outcomes. J. Clin. Med. 2023, 12, 7308. https://doi.org/10.3390/jcm12237308
Swart R, Maes SSA, Cavanaugh D, Mason KP. Remimazolam Pilot for Office-Based Dental Sedation: Adverse Events, Awareness and Outcomes. Journal of Clinical Medicine. 2023; 12(23):7308. https://doi.org/10.3390/jcm12237308
Chicago/Turabian StyleSwart, Rudi, Sabine S. A. Maes, David Cavanaugh, and Keira P. Mason. 2023. "Remimazolam Pilot for Office-Based Dental Sedation: Adverse Events, Awareness and Outcomes" Journal of Clinical Medicine 12, no. 23: 7308. https://doi.org/10.3390/jcm12237308
APA StyleSwart, R., Maes, S. S. A., Cavanaugh, D., & Mason, K. P. (2023). Remimazolam Pilot for Office-Based Dental Sedation: Adverse Events, Awareness and Outcomes. Journal of Clinical Medicine, 12(23), 7308. https://doi.org/10.3390/jcm12237308






