Abstract
Since pain is common in many diseases, it is important to summarize the precise prevalence data on pain and high-impact pain, which frequently worsens the quality of life and work activities. This umbrella review aims to estimate the prevalence of pain among patients with different chronic diseases/conditions. We followed the PRISMA guidelines. We identified the following areas addressing the prevalence of pain: (1) pain in cancer patients; (2) neurodegenerative diseases; (3) chronic heart failure; (4) chronic obstructive pulmonary disease; (5) chronic kidney diseases; (6) liver diseases and failure; (7) nursing home seniors; and (8) postamputation (phantom) pain. We included systematic reviews and meta-analyses that reported pain in patients from the mentioned populations. The prevalence of pain in chronic diseases is high, in some cases even higher than the cardinal symptoms of these diseases/conditions. Most patients who suffer from any of these diseases/conditions can develop chronic pain at later stages. Pain in chronic diseases does not receive enough attention and is not properly managed. Future studies are warranted to establish a more precise prevalence of chronic pain and develop better methods of pain screening, detection, and management.
1. Introduction
Pain is a significant public health issue with massive socio-economic impacts. Pain is one of the most common reasons why patients seek medical care [1]. It has been associated with limitations in mobility and everyday tasks, reliance on opioids, anxiety, and depression, as well as a lower perception of overall health and reduced quality of life [1,2,3,4].
Chronic pain is defined by the “International Association for the Study of Pain” (IASP) as “pain that persists or recurs for more than 3 months” [5]. The prevalence of pain among the adult population varies significantly, depending on the geographical area, age, and comorbidities [6]. Since pain is widespread in many diseases, it is important to summarize the precise prevalence data on pain and high-impact chronic pain, which frequently worsens the quality of life and work activities. The estimates of high-impact pain can help identify individuals whose major life domains, such as work, social life, recreation, and self-care activities, are affected by pain, providing valuable insights into the specific population in need of pain services [6].
Pain is a significant concern and is frequently reported as one of the most prevalent symptoms among patients presenting with oncological, neurological, musculoskeletal, cardiovascular, liver, pulmonary, and renal diseases and conditions. Inadequate relief can have demoralizing consequences, negatively affecting physical condition, overall quality of life, and emotional well-being. It often leads to anxiety, anger, depression, and even cognitive impairment [7,8,9,10].
There are numerous factors contributing to the lack of improvement in cancer pain management. Among the common barriers faced by healthcare professionals are lack of knowledge and skills in pain medicine, inadequate pain assessment practices, and reluctance to prescribe analgesics [11].
Conversely, patients have their own barriers, such as hesitance to discuss pain with physicians and nurses, reluctance to look for treatment, difficulties adhering to analgesic prescriptions, and depression as well as other cognitive and psychological factors [12,13].
Since numerous systematic reviews have been published and the studies on the prevalence of pain in chronic diseases are heterogeneous and broad, there is a need to synthesize existing evidence from multiple systematic reviews. To comprehensively assess the prevalence of pain in patients with chronic diseases, we undertook an “umbrella” review, which involved surveying existing systematic reviews and meta-analyses, representing one of the most robust forms of evidence synthesis. Our approach began with a scoping review to pinpoint areas of research consistently supporting the prevalence of pain among patients with different chronic diseases/conditions. We excluded all primary painful diseases, such as trigeminal neuralgia, low back pain, and osteoarthrosis. We identified the following areas addressing the prevalence of pain: (1) pain in cancer patients; (2) neurodegenerative diseases; (3) chronic heart failure; (4) chronic obstructive pulmonary disease (COPD); (5) chronic kidney diseases; (6) liver diseases and liver failure; (7) nursing home seniors; and (8) postamputation (phantom) pain. Therefore, the objective of this umbrella review was to estimate the prevalence of pain among patients from the mentioned populations.
2. Materials and Methods
Search Strategy and Selection Criteria
The current umbrella review was conducted following the PRISMA guidelines [14]. The protocol registration DOI is https://doi.org/10.17605/OSF.IO/RXVWA. We searched for systematic reviews, meta-analyses, and large database studies in eight areas using PubMed, Scopus, and the Cochrane database. The search encompassed studies published until March 2023. We searched for the prevalence of pain among the diseases and conditions associated with frequent pain including the prevalence of pain in (1) cancer patients, (2) neurodegenerative diseases, (3) chronic heart failure, (4) chronic obstructive pulmonary disease, (5) chronic kidney diseases, (6) liver diseases and failure, (7) nursing home seniors, and (8) postamputation (phantom) patients. The search terms and their combinations are described in the Supplementary File.
Additionally, specific terminology tailored to each field of research was employed. The inclusion criteria were devised to identify the most reliable evidence for each research domain and comprised the following:
- Systematic reviews and meta-analyses focused on the prevalence of pain;
- Studies published in peer-reviewed journals;
- When more than five systematic reviews were available, we included the five most recent ones.
The exclusion criteria involved the following: (1) animal studies; (2) individual observational studies (not systematic reviews); (3) case reports; (4) editorials. No restrictions based on language or publication date were applied.
Two authors conducted the search and screening independently. In case of disagreements, the rest of the authors were engaged in resolving the dispute. In instances where data were missing or unclear, we contacted the authors of the papers for clarification. We extracted summary effects, confidence intervals, and measures of statistical significance when available. Due to overlapping studies and variations in reporting styles and units, we did not conduct a meta-analysis for each area. All the extracted data are presented in Table 1 and Table 2. The quality of systematic reviews and meta-analyses was assessed using AMSTAR-2 (A MeaSurement Tool to Assess systematic Reviews) [15]. This tool consists of 16 questions related to the design and reporting of the study, to which one of the three possible answers is given: “Yes”, “No”, or “Partial yes”. This assessment does not imply an overall score but rather assesses each study on every one of the 16 domains.

Table 1.
Characteristics of the included studies.

Table 2.
Prevalence and mechanisms of pain.
3. Results
Twenty-five studies were included in this umbrella review (Figure 1).
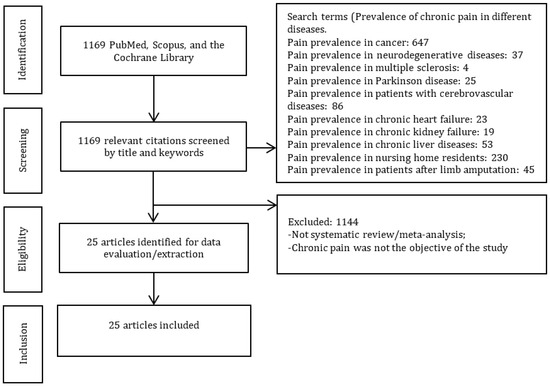
Figure 1.
PRISMA diagram.
3.1. Patient Characteristics
3.1.1. Diseases/Conditions
Diagnosis in which the prevalence of pain was studied: We included systematic reviews and meta-analyses (SRs & MAs), which reported pain in patients with a wide variety of diseases and conditions including cancer (head and neck, bronchus/lung, breast, gastrointestinal tract, prostate, gynecological cancers, rectal), arthritis, neurodegenerative diseases, depression, dementia, osteoporosis, pressure ulcer, falls, anxiety, chronic heart failure, chronic obstructive pulmonary diseases, chronic kidney diseases and end-stage kidney failure, end-stage liver disease, traumatic brain injury, and cerebrovascular diseases (Table 1 and Table 2) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
3.1.2. Countries (Where the Included Studies Were Conducted/Data Collected)
The included systematic reviews and meta-analyses reported the prevalence of pain in patients from many geographical regions and countries (Table 1). Some individual systematic reviews studied the prevalence of pain in patients from several geographical regions including Europe, Asia, North America, South America, and Oceania representing the following countries: Finland, the Netherlands, Italy, Norway, the United States of America, Hong Kong, Czech Republic, England, France, Germany, Israel, Japan, Brazil, Turkey, Austria, Sweden, Australia, China, Korea, Denmark, the UK, Scotland, Greece, India, Egypt, Canada, Taiwan, Cyprus, Spain, Switzerland, Malaysia, Thailand, Nigeria, Saudi Arabia, Republic of Guinea, Morocco, South Korea, Uruguay, Lebanon, Poland, Iran, Sri Lanka, and Pakistan (Table 1).
3.2. Quality Assessment
AMSTAR evaluation was used for quality assessment (Table 3). All the studies included the components of PICO in their research questions and inclusion criteria. Seven studies explicitly stated that a protocol was developed prior to conducting the study and that corresponding changes were made to the protocol. Five studies explained the study design selection. Seventeen studies mentioned conducting the study selection in duplicate, and only four studies mentioned extracting the data in duplicate. Only two studies provided a list of excluded studies with justifications. Most studies had some descriptions of the included studies. If a meta-analysis was performed, all the studies used appropriate statistical methods. However, none of the studies discussed the impact of the risk of bias of individual studies on the results, and few studies discussed heterogeneity. Six studies examined publication bias. A few studies did not mention conflict of interest, and none of the studies considered funding of the included studies.

Table 3.
AMSTAR quality assessment.
3.3. Prevalence of Chronic Pain in Different Diseases/Conditions
In total, out of the 25 included studies, we included 4 that reported pain in cancer patients [16,17,26,31]; 7 that reported pain in neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, Huntington’s disease, and dementia [20,21,22,23,24,25,40]; 2 that reported pain in cerebrovascular diseases [38,39]; 1 that reported pain in chronic heart failure (CHF) [28]; 4 that reported pain in chronic kidney disease (CKD) [32,33,34,35], 1 that reported pain in traumatic brain injury (TBI) [37]; 2 that reported pain in COPD [29,30]; 1 that reported pain in chronic liver diseases [36]; 2 that reported pain in nursing home residents and seniors [18,27]; and 1 that reported pain in chronic pain after limb amputation [19].
3.3.1. Prevalence of Chronic Pain in Cancer Patients
We included four studies that reported the prevalence of chronic pain in cancer patients (Figure 2). The reported prevalence varied relatively widely, mostly depending on the stage of cancer, rather than the type of cancer.
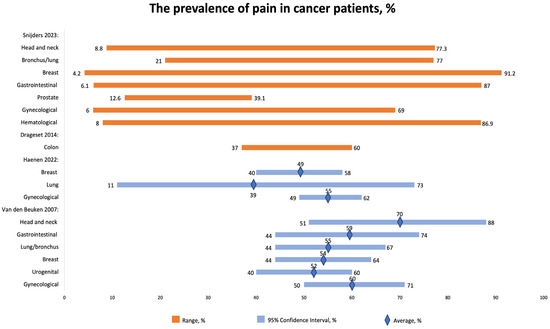
Figure 2.
The prevalence of pain in cancer patients, %. CI, confidence interval. Sniders 2023 [16], Drageset 2014 [26], Haenen 2022 [17]; Van den Beuken 2007 [31].
Snijders 2023 [16] studied the prevalence of chronic pain in cancer patients. The overall prevalence of cancer-related pain was reported to be 44.5%. Importantly, among treatment groups, the lowest prevalence (35.8%) was observed in groups that received curative treatment. Overall, the prevalence of pain in cancer patients remains high, especially in metastatic, advanced, and terminal patients, reaching 54.6%. The prevalence of pain was as follows: the highest prevalence was reported in patients with breast cancer (4.2–91.2%), followed by hematological malignancies (8–86.9%), GI (6.1–87%), head and neck (8.8–77.3%), bronchus/lung (21–77%), gynecological (6–69%), and prostate (12.6–39.1%).
The authors reported that a reduction in the prevalence and intensity of cancer-related pain might be positively influenced by the improvement in awareness and knowledge among healthcare professionals, treatment strategies, and new pain management guidelines. Nevertheless, disparities in knowledge among healthcare professionals from different countries should be addressed. The prevalence of cancer-related pain was significantly higher in Asia, South America, and Africa compared to Europe. Many cancer patients in low-income countries receive inadequate pain control, partially due to inadequate education. Moreover, doctors generally possess more advanced knowledge about pain management compared to nurses; however, both have an insufficient understanding of pharmacology, especially the side effects of opioids. Furthermore, the majority of oncology nurses have insufficient knowledge in this area [16].
Haenen et al. [17] reported the following prevalence of pain according to the type of cancer: the highest prevalence was registered in gynecological cancer (55% (95% CI 49–62%)), followed by breast cancer (49% (95% CI 40–58%)) and lung cancer (39% (95% CI 11–73%)). At least half of cancer patients experience pain at least three months after therapy.
About half of breast cancer survivors experience pain, with estimates ranging from 40% to 58%. Similarly, around 39% of lung cancer survivors and 55% of gynecological cancer survivors also report experiencing pain, with confidence intervals of 11% to 73% and 49% to 62%, respectively.
Around 47% of individuals reported pain more than three months after successful completion of cancer treatment. However, there is insufficient evidence to determine whether the type of cancer, treatment approach, method of pain measurement, or duration of follow-up have any significant impact on the occurrence of pain in these cancer survivors [17].
Van den Beuken-van Everdingen et al. (2007) [31] also reported the prevalence of pain in patients with different types of cancers, including head and neck cancer (70% (51–88)), GI tract (59% (44–74)), lung/bronchus (55% (44–67)), breast (54% (44–64)), urogenital (52% (40–60)), and gynecological (60% (50–71%)).
Cancer pain can be generated by (1) cancer itself or metastases (infiltration of soft tissues, bony metastasis, and compression of nerves); (2) treatment-related side effects (chemotherapy-induced musculoskeletal pain, mucositis); (3) postoperative pain; and (4) radiation-induced dermatitis, mucositis, or enteritis. Pain may become chronic in many cancer survivors. Some chemotherapeutic agents, such as platins, bortezomib, taxanes, or vincristine, can cause painful chemotherapy-induced peripheral neuropathy, whereas aromatase inhibitors are associated with diffuse joint pain. As described earlier, surgery can result in phantom limb pain or pain from scar formation [41,42,43,44].
3.3.2. Prevalence of Pain in Nursing Home Residents
Three systematic reviews reported the prevalence of chronic pain in seniors and nursing home residents [18,26,27]. Cole et al. (2022) reported the prevalence of pain among nursing home residents with different comorbidities including arthritis, depression, dementia, osteoporosis, pressure ulcers, falls, anxiety, and contractures [18]. Since the residents suffered from several diseases, it was not possible to find out precisely what disease and condition caused the pain. The prevalence of “current pain” at the time of the survey varied from 22.2 to 85%, whereas persistent pain varied from 19.5 to 58.5%, and chronic pain varied from 55.9 to 58.1% (Figure 3).
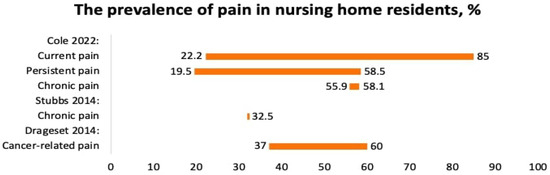
Figure 3.
The prevalence of pain in nursing home residents, %. Cole 22 [18], Stubbs [27], Drageset 2014 [26].
Drageset et al. (2014) reported the prevalence of cancer-related pain in nursing home residents [26]. The prevalence of pain varied from 37 to 60%.
Stubbs et al., 2014, reported the prevalence of chronic pain among seniors from the US, the UK, Australia, Italy, Japan, China, Nigeria, the Netherlands, Taiwan, and Turkey. The prevalence of pain was 32.5% (Figure 3). Seniors with pain were more likely to fall, especially with foot pain [27].
Many residents suffered from depression, which could worsen the pain severity or be triggered by pain. Moreover, depression, limitations in daily activities (immobility), cognitive impairment, dementia, and arthritis were consistently associated with pain. Dementia and cognitive dysfunction impair the ability to communicate and report pain. Subsequently, pain in non-verbal residents is usually detected from facial expressions, crying, and guarding of body parts. Nursing home residents who cannot report the pain verbally may be experiencing pain that is not always recognized [18].
3.3.3. Residual Limb Pain after Amputation
We included one study, that of Evans et al., 2022 [19], which reported the prevalence the residual limb pain after amputation due to cancer, trauma, and vascular pathologies.
Individuals who underwent limb amputation frequently experience postamputation pain. Among them, patients who had amputations due to cancer tend to report the highest pain intensity, followed by patients with traumatic amputations and amputations due to peripheral vascular diseases. Pain intensity in upper extremity amputations was reported to be higher compared to that in lower extremity amputations. Most individual studies included in the SR indicated that nearly all patients who underwent amputation had pre-amputation pain.
The pain occurs despite attempts to control it with different analgesic methods. From three months to two years, the prevalence of residual limb pain ranges between 22% and 27% (Figure 4). Individuals with amputations due to cancer often report the highest pain severity. It should also be noted that the reported pain severity is generally higher in individuals undergoing upper extremity amputations compared to lower extremity amputations, but this may be influenced by an increased presence of cancer or trauma in the studies involving upper extremity amputations [19].
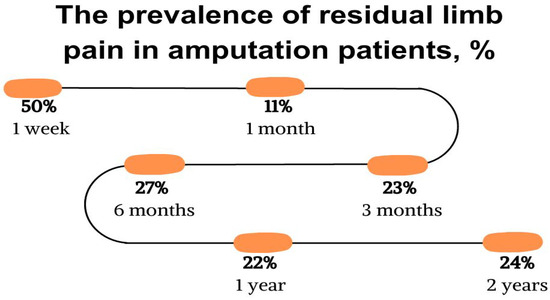
Figure 4.
The prevalence of residual limb pain in amputation patients, %. Evans 2022 [19].
When it comes to perioperative pain control, there is no consensus on the optimal approach. However, several aspects, such as the duration, route of administration, and content of perioperative prescribing practices, have been previously studied. For example, patients receiving epidural bupivacaine with morphine for three days preoperatively reported no residual limb pain at six and twelve months following amputation. Conversely, a three-day duration of early postoperative perineural and subfascial ropivacaine did not decrease residual limb pain severity at one month or longer follow-up. Comparisons have also been made between different pain control methods. It was also reported that epidural blocks led to decreased residual limb pain at one week compared to perineural blocks. It was also reported that among various methods of preoperative pain control, the group treated with epidural pain control throughout the perioperative period had a significant reduction in phantom limb pain severity at six months [19].
Additionally, studies have examined the use of adjunctive medications, such as calcitonin and clonidine, in combination with nerve blocks. Some of these medications showed improvements in reducing residual limb pain, while others did not yield significant benefits. Several medications, including gabapentin and valproic acid, have been tested for their effectiveness in reducing residual limb pain, but the results have been inconclusive. These studies primarily enrolled patients with amputations due to vascular diseases, so further investigation is needed to determine the efficacy of these medications in other subgroups or causes of amputation [19].
3.3.4. Prevalence of Pain in Patients with Neurodegenerative Diseases
We included seven systematic reviews that focused on neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, Huntington’s disease, Alzheimer’s syndrome, and dementia (non-specified) [20,21,22,23,24,25,40].
The study by Hurwitz 2021 [20] that examined pain among ALS patients included several countries (Japan, US, Italy, Germany, Canada, France, Australia, Sweden, Finland, UK). The overall prevalence of pain (Figure 5) was 60% (95% CI 50–69%). The prevalence of pain in the head, neck, trunk, and back was almost 25%, while that for upper limbs was over 40%. The authors conclude that pain in ALS is high.
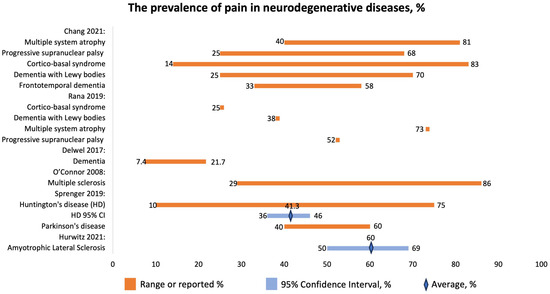
Figure 5.
The prevalence of pain in neurodegenerative diseases, %. Chang 2021 [22], Rana 2019 [24], Delwel 2017 [25], O’Connor 2008 [40], Sprenger 2019 [23], Hurwitz 2021 [20].
Chang 2021 reported the prevalence of pain in patients with atypical parkinsonism, MSA, PSP, CBS, DLB, and FTD (Figure 5) [22]. The disorders have been associated with the following pain locations and types:
- MSA: neck, back, limbs; MSK, neuropathic, dystonic;
- PSP: limbs, neck, back, MSK;
- CBS: limbs, dystonic;
- DLB: multiple places, neuropathic, MSK;
- FTD: head, neck, shoulder, abdomen, MSK.
Neurodegenerative diseases can lead to chronic pain. However, its prevalence might be underestimated due to the cognitive decline of patients. Patients with neurodegenerative diseases, such as atypical parkinsonism, may have a higher likelihood of pain. Since the pathophysiology of pain in these diseases might include different pathways and mechanisms, it is important to use a strategy based on understanding the underlying mechanisms when choosing the most suitable treatment for pain relief. However, there is insufficient evidence regarding the exact physiological mechanisms that contribute to pain in specific neurodegenerative disorders. Furthermore, some patients may have difficulty accurately reporting pain due to cognitive issues or changes in speech and language, which can result in unreliable findings when using clinical assessment tools or quantitative sensory testing in research studies [22].
Multiple system atrophy (MSA) is defined as a neurodegenerative disease characterized by the presence of α-synuclein-positive oligodendroglial cytoplasmic inclusions and degenerative changes in certain brain structures. It can manifest with predominant parkinsonism (MSA-P) or predominant cerebellar features (MSA-C), along with various autonomic and pyramidal symptoms. Pain is a common non-motor symptom in MSA, with chronic musculoskeletal pain being the most frequently reported subtype. Neuropathic pain, both central and peripheral, is also prevalent in MSA. The severity of pain tends to increase as the disease progresses. The exact underlying causes of pain in MSA are not fully understood, but there are pathological changes in brain regions associated with pain processing. Some MSA patients may also experience peripheral neuropathy, which contributes to neuropathic sensations. Pain treatment in MSA patients is often inadequate, with a significant number not receiving proper pain-relieving therapies. Dopaminergic therapy may provide partial relief for some patients, but it does not consistently improve pain sensitivity. Botulinum toxin injections have shown some effectiveness in relieving pain associated with dystonia and sialorrhea. Physiotherapy and medications like pregabalin, gabapentin, and amitriptyline may be beneficial for neuropathic pain, although more high-quality evidence is needed. Progressive supranuclear palsy (PSP), another neurodegenerative syndrome, also presents with pain, but the prevalence appears to be lower compared to MSA and Parkinson’s disease (PD) according to questionnaire-based studies [22].
Sprenger et al. (2019) [23] studied the prevalence of pain in patients with HD. The prevalence of pain was 41.3% (95% CI: 36–46%) with a range of 10–75% (Figure 5). Pain might be one of the HD symptoms.
Huntington’s disease (HD) is a devastating neurodegenerative condition, inherited in an autosomal-dominant manner. It leads to a range of distressing symptoms, including characteristic motor symptoms, such as chorea, as well as cognitive, emotional, and behavioral disturbances. Secondary symptoms may include weight loss, sleep disorders, and autonomic problems. HD causes significant atrophy in the brain, especially affecting the GABAergic system. Interestingly, this striatal atrophy is already evident during the pre-manifest stage, which can be up to 10 to 15 years before the actual clinical diagnosis. As the disease progresses to the manifest stage, the severity of atrophy in the striatum correlates with the overall disease severity; total functional capacity; and cognitive disturbances, including disturbances affecting memory, executive function, and processing speed. The striatum is part of the cerebral “pain matrix”, a network responsible for various aspects of pain processing, such as its sensory-discriminative, affective-emotional, and cognitive-evaluative dimensions. Notably, the striatum seems to be predominantly involved in the affective-motivational and cognitive-evaluative dimensions of pain, which play a crucial role in understanding pain; suffering level; unpleasantness; and the ability to remember, interpret, and respond appropriately to pain. Apart from its role in pain processing, the striatum also serves an analgesic function. Experiments involving morphine injections into the marginal divisions of the striatum resulted in dose-dependent hypoalgesia, which could be reversed by naloxone. Additionally, the striatum contains a high concentration of endogenous opiates and their receptors. Other brain regions in the “pain matrix” and their associated pain dimensions include the primary as well as secondary somatosensory cortices (sensory-discriminative), anterior cingulate cortex (affective and cognitive), thalamus (sensory-discriminative and affective), amygdala (affective), insula (affective and cognitive), and prefrontal cortex (affective and cognitive). Studies using magnetic resonance imaging have found atrophy in these regions in both the premanifest and manifest stages of HD, with disease progression correlating with increased atrophy. Research on pain in HD patients has yielded conflicting results. Some studies showed that a significant number of patients experienced pain, but not all of them received appropriate analgesic treatment [22].
The prevalence of pain in patients with Huntington’s disease (HD) was 41.3%, compared with 40–60% in another neurodegenerative disease, Parkinson’s disease. Additionally, the prevalence of pain could be influenced by various factors such as age; sex; drug treatment; motor functions; cognitive, emotional, and behavioral disturbances; comorbidities; the severity and duration of the disease; and the site and types of pain (e.g., nociceptive vs. neuropathic and acute vs. chronic pain). Moreover, higher scores of depression and anxiety, the use of analgesic medication, and the presence of comorbid conditions were linked to an increased likelihood of experiencing more severe pain in HD patients [23].
The symptoms and worries experienced by patients with HD differ as the disease progresses. In the early stages, known as premanifest HD, many patients express concerns related to their social interactions, such as complex family relationships and lack of support from their environment. On the other hand, during the manifest stage, physical issues become more prominent, including difficulties with swallowing food, driving, and walking. As a result of the significant impact of HD on various aspects of health, pain may play a relatively smaller role in affecting patients’ quality of life, and consequently, it is less commonly reported [23].
Rana et al., 2019, focused on the prevalence of chronic pain in patients with atypical parkinsonism [24]. They report prevalence for CBS to be 25%; for LBD, 38%; for MSA, 73%; and for PSP, 52% (Figure 5).
Characteristics of Pain
Patients with atypical parkinsonism experience pain, especially in limbs [21].
Delwel et al., 2017, studied the prevalence of orofacial pain in patients with dementia [25]. Older individuals suffering from dementia exhibit more significant oral health issues compared to those without dementia. These issues include problems such as coronal caries, root caries, and retained roots. However, it was observed that the number of remaining teeth and the decayed missing filled teeth index were similar between both groups. There is a lack of research on orofacial pain in older people with dementia. As a result, there is an urgent need for further investigation and focus on oral health, particularly concerning orofacial pain, in this vulnerable population.
O’Connor et al. (2008) focused on the prevalence of chronic pain in patients with multiple sclerosis (MS) [40]. The prevalence of pain ranged between 29 and 86%. The following types of pain were reported: extremity, back pain, headache, trigeminal neuralgia, neuropathic, MSK, and mixed.
The evidence available indicates that pain is prevalent in individuals with MS. It affects around one in five patients at the onset of the disease, about half of patients at any given time during their illness, and up to three-quarters of patients within the last month. This pain has a significant impact on the health-related quality of life, leading to impairments in both physical and emotional functioning. MS-related pain can be categorized into various types, including continuous central neuropathic pain, intermittent central neuropathic pain, musculoskeletal pain, and mixed neuropathic and non-neuropathic pain [40]. It is crucial to differentiate between these pain types in future studies. To achieve this, clear descriptions of the pain assessment methods and the timeframe for pain evaluation should be provided. Comparing the pain experiences of MS patients with those of non-MS patients having similar disability and healthcare utilization can help determine the extent to which pain is specifically attributable to MS. Depression and psychosocial risk factors have been linked to MS-related pain, but it remains uncertain whether they are causal factors for pain development or consequences of pain [40].
3.3.5. Prevalence of Pain in Chronic Heart Failure Patients
One systematic review, conducted by Alemzadeh-Ansari (2017), reported the prevalence of pain in patients with chronic heart failure (CHF) [28]. The prevalence of pain in 20,875 patients with CHF varied from 23 to 85% (Figure 6). The most commonly reported types of pain were chest pain and neuropathic pain. The mechanisms of pain included ischemia, inflammation, ascites, and constipation [28].
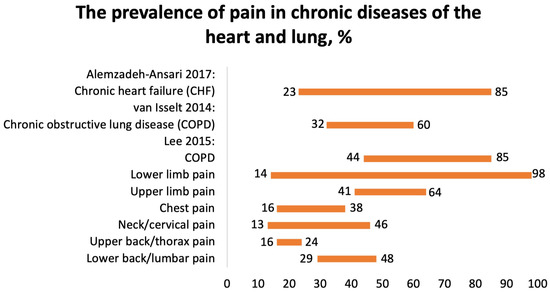
Figure 6.
The prevalence of pain in chronic diseases of the heart and lung, %. Alemzadeh-Ansari 2017 [28], van Isselt 2014 [29], Lee 2015 [30].
The mechanisms of pain in CHF patients are not fully understood. Pain perception may vary and be altered by other symptoms such as fatigue, dyspnea, anxiety, and depression. Since approximately 80% of patients with CHF are elderly, several sources of pain, including psychological, physical, and neurological diseases, may contribute to the prevalence of pain. Some comorbidities, such as malignancies, coronary artery disease, COPD, pneumonia, peripheral vascular disease, depression, diabetes mellitus, osteoarthritis, and low back pain, are also common in CHF patients and can be the source of chronic pain [28].
3.3.6. Prevalence of Pain in COPD
We included two SRs focusing on the prevalence of pain in patients with COPD. Van Isselt (2014) reported the prevalence of chronic pain in patients with COPD [29]. Pain is a significant issue in patients suffering from COPD, affecting an estimated 32–60% of individuals (Figure 6). However, little is known about the factors contributing to pain, and there is a dearth of information regarding interventions aimed at alleviating pain in COPD patients. Studies have indicated that pain is more prevalent in patients with moderate airflow limitation compared to those with severe or very severe airflow limitation.
According to the SR by Lee et al. (2015), the polled prevalence of pain in 8677 COPD patients was 66% (95% CI 44–85%) (Figure 6) [30]. It was localized in the shoulder, neck, upper limb, chest, lower limb, head, buttocks, and back. Higher pain intensity was associated with dyspnea, altered respiration mechanics, fatigue, poorer quality of life, and a greater quantity of comorbidities. Common pain regions were the lower limb (14–98%), upper limb (41–64%), chest (16–38%), neck/cervical (13–46%), upper back/thorax (16–24%), and lower back/lumbar (29–48%). The majority of patients with COPD and pain had at least one comorbidity. Common comorbidities included cancer, musculoskeletal, endocrine disorders, and depression. There were no differences in pulmonary function in patients with COPD with or without pain.
Several factors may influence pain experience. Thus, comorbidities may be one of the influencing factors. The prevalence of comorbidities is higher in patients with COPD experiencing pain than in COPD patients without pain or healthy control individuals. Breathlessness is also associated with higher pain intensity and may be related to the respiratory mechanics observed in patients with COPD. Altered respiration and muscle overload have been associated with pain including low back pain. The relationship between breathlessness and pain is complex, involving common neural pathways, and is responsible for the discomfort of both symptoms [28,29].
3.3.7. Prevalence of Pain in Chronic Kidney Diseases
We analyzed four SRs that studied the prevalence of pain in patients with chronic kidney disease (CKD). Davison et al. (2021) reported that 43.6% patients with CKD suffered from moderate or severe pain based on data from 16,558 patients (US, Saudi Arabia, Norway, Republic of Guinea, Italy, Morocco, Australia, Spain, South Korea, Hong Kong, Canada, Brazil, Turkey, Israel, Uruguay, Switzerland, Lebanon, Taiwan, Poland, UK, India, Netherlands, Iran, Sri Lanka, Malaysia, Germany, China) [32]. Of them, 60.5% were hemodialysis patients (Figure 7). The pain was localized in bones, joints, muscles, lower extremities, neck and shoulders, back, or upper extremities or was widespread. The reported mechanism of pain was neuropathic, “atheropathic”, or musculoskeletal. The research findings demonstrated that chronic pain was highly prevalent. Most patients experiencing pain rate its intensity as either moderate (typically ranging from 4 to 6 out of 10) or severe (ranging from 7 to 10 out of 10). Limited data exist for patients on peritoneal dialysis and those conservatively managed without dialysis, as well as for patients with CKD who did not require renal replacement therapy at the time of study. Patients managed conservatively reported the lowest prevalence of severe pain, likely due to “active” pain management in CKD. Interestingly, pain intensity rates were also high in patients with earlier stages of CKD and did not seem to correlate with the severity of their CKD, possibly indicating that the pain is mostly induced by comorbidities rather than CKD itself. Nonetheless, pain management is crucial for patients with CKD. Routine screening for pain in all CKD patients should be integrated into nephrology care. Pain assessment and management should be viewed as a component of quality care for CKD and chronic kidney failure patients [24,25,26,27,28,29,30,31].
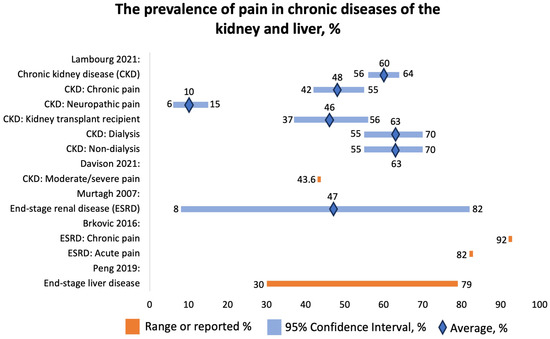
Figure 7.
The prevalence of pain in chronic diseases of the kidney and liver, %. Lambourg 2021 [33], Davison 2021 [32], Murtagh 2007 [35], Brkovic 2016 [34], Peng 2019 [36].
Lambourg et al. (2021) concluded that 60% (95% CI 56–64%) of CKD patients experienced pain based on data from 40,678 individuals (Figure 7) [33]. Of them, 48% (95% CI 42–55%) reported chronic pain; 10% (95% CI 6–15%), neuropathic pain. Forty-six percent (95% CI 37–56%) of kidney transplant recipients and 63% (95% CI 55–70%) of dialysis patients reported pain. The most commonly reported types of pain included abdominal, headache, joint, and bone. The mechanisms of pain were neuropathic, postsurgical, and due to drug toxicity and mineral turnover.
Brkovic et al. (2016) found that among 6917 patients with end-stage kidney disease on hemodialysis, the prevalence of acute pain was 82%, and that of chronic pain was 92% (Figure 7) [34]. The cohort consisted of patients from Scotland, Italy, Kuwait, Brazil, the US, Canada, Morocco, Spain, the UK, Serbia, Turkey, Switzerland, Israel, Pakistan, Japan, Iran, and France. Patients most commonly reported headache, MSK, and diffuse pain. The most commonly mentioned mechanisms were ischemic and neuropathic. The pain was probably due to comorbidities and procedures.
Murtagh et al. (2007) reported that the prevalence of pain in ESRD was 47% (8–82%) (Figure 7). The pain was mainly caused by muscle cramps and headaches [35]. Apart from that, the authors also reported the prevalence of other symptoms, such as fatigue, 71% (12–97%); pruritus, 55% (10–77%); constipation, 53% (8–57%); anorexia, 49% (25–61%); sleep disturbance, 44% (20–83%); anxiety, 38% (12–52%); dyspnea, 35% (11–55%); nausea, 33% (15–48%); restless legs, 30% (8–52%); and depression, 27% (5–58%).
3.3.8. Prevalence of Pain in Patients with Liver Diseases
We included one SR conducted by Peng et al. (2019) that focused on the prevalence of pain in end-stage liver disease [36]. The cohort consisted of 5434 patients from North America, Europe, Asia, and Africa. The reported prevalence of pain ranged from 30% to 79% (Figure 7). Interestingly, the prevalence of pain was greater than the prevalence of other symptoms, such as dyspnea (20–88%), muscular cramps (56–68%), nighttime insomnia (26–77%), daytime sleepiness (29.5–71%), anxiety (14–45%), depressive symptoms (4.5–64%), and erectile dysfunction (53–93%). The symptoms of end-stage liver disease are similar to the symptoms of other advanced diseases.
3.3.9. Prevalence of Pain in Patients with Traumatic Brain Injury (TBI)
Only one SR that reported the prevalence of pain in patients who sustained TBI matched the criteria of our umbrella review. Nampiaparampil et al. (2008) conducted a comprehensive SR focusing on the prevalence of chronic pain including headaches, discussed other potential pain syndromes in patients with TBI, described the relationship between severity of brain injury and pain, and compared the civilian vs. combat veteran status on chronic pain [37].
The prevalence of chronic pain was 51.5 (95% CI 49.8–53.2%) among civilians and 43.1 (95% CI 39.9–46.3%) among veterans (Figure 8). The prevalence of headache was 57.8 (95% CI 55.5–60.2%). Most headaches (51%) were localized in the occipital region. However, no significant association was found between the location of head trauma and the site of pain in these TBI patients. Apart from post-TBI headaches, these patients were found to develop complex regional pain syndrome and muscular-spasticity-related pain.
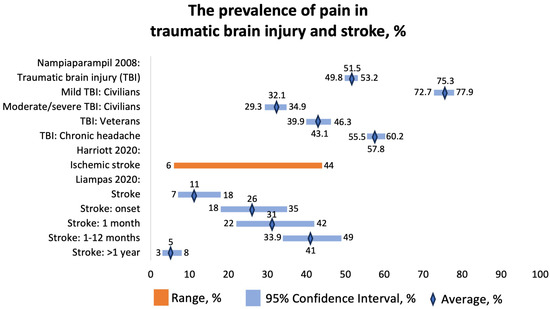
Figure 8.
The prevalence of pain in traumatic brain injury and stroke, %. Nampiaparampil 2008 [37], Harriott 2020 [38], Liampas 2020 [39].
Interestingly, patients with mild TBI had a higher prevalence of chronic pain compared with those with moderate or severe TBI, and the reason for that remains unclear. It might be due to difficulty in reporting or processing symptoms, executive dysfunction, memory disturbances, and language deficits in patients with severe TBI.
Chronic pain is a frequent consequence of TBI contributing to poor recovery. Patients who sustained TBIs in combat situations had higher rates of pain compared with the general population. Post-TBI patients might benefit from early screening, detection, and management of pain to reduce morbidity.
3.3.10. Prevalence of Pain in Cerebrovascular Diseases
We included two SRs that focused on pain in patients with stroke. Harriott et al. (2020) found that the prevalence of stroke onset and poststroke headaches in 33,231 patients with ischemic stroke ranged from 6 to 44% (Figure 8) [38]. The mechanism of headaches included changes in innervation, increases in compartmental pressures, and meningeal inflammation. The importance of poststroke headaches is not given enough attention. There is a limited understanding of the predictive potential and treatment of new-onset poststroke headache or headache as a symptom of stroke. Some cerebrovascular conditions, such as venous sinus thrombosis or cervical artery dissection, have been well described as presenting with headaches, but few studies focus on new-onset and persistent headaches specifically after ischemic stroke. A new-onset headache during acute ischemic stroke was found to be a predictor of persistent headache six months after the stroke, and a poststroke headache is a common form of chronic poststroke pain, which can significantly impact disability. The overall prevalence suggests that around 14% of adult patients with ischemic stroke experience headaches either at the time of the stroke or shortly afterward. These headaches often persist for months to years, ranging from moderate to severe intensity, leading to significant disability associated with the condition. Several factors seem to be associated with poststroke headaches, including younger age, female sex, nonlacunar cortical stroke syndromes, and involvement of the posterior fossa. It appears that there may be differences in trigeminal and autonomic innervation of the posterior cerebral vessels, possibly explaining why posterior circulation strokes are more associated with headaches. The exact mechanisms underlying poststroke headaches are not clear, but they appear to be qualitatively different from typical migraines, even in individuals with a history of migraines. Possible factors contributing to poststroke headaches could be related to trigeminovascular afferents or meningeal inflammation, mechanical forces, spreading depolarization, platelet-derived factors, serotonergic mechanisms, or peptide-containing nociceptors, although more research is needed to fully understand these mechanisms [37].
Liampas et al. (2020) reported the prevalence of central poststroke pain (CPSP) at different time points following stroke [39]. The overall prevalence was 11 (95% CI 7–18%), at stroke onset, 26% (95% CI 18–35%); at 1 month following stroke, 31% (95% CI 22–42%); from 1 month to 1 year, 41% (95% CI 33.9–49.0%); and more than 1 year after stroke, 5% (95% CI 3–8%) (Figure 8). They included patients with strokes of various anatomical locations, including medullary stroke, thalamic stroke, somatosensory tract stroke, and cortical stroke. The pooled prevalence of CPSP in patients with medullary stroke was 57% (95% CI 24–85%, n = 216). The pooled prevalence of CPSP in patients with thalamic stroke was 52% (95% CI 41–62%, n = 93). The prevalence of CPSP was 53% (n = 30) in patients with stroke at any site of the somatosensory tract and 17% (n = 24) among patients with stroke with a cortical localization [38].
4. Discussion
4.1. Brief Summary
The objective of this umbrella review was to synthesize and analyze the highest level of available evidence on the prevalence of pain among patients with different diseases and conditions. It appears that pain does not receive enough attention, although it is highly prevalent in patients with heart, liver, and kidney failure; COPD; multiple sclerosis; Huntington’s disease; Parkinson’s disease; Alzheimer’s disease; traumatic brain injury; and cancer. Consequently, it is not properly managed, leading to patient suffering, which could be avoided by proper pain detection and treatment. In patients with certain diseases, pain is reported more frequently than the symptoms of the patient’s primary disease. Thus, the prevalence of cough, one of the leading symptoms in patients with COPD, was reported in about 55.5–65.6% of patients, and the polled prevalence of pain in COPD patients was 66% (95% CI 44–85%) [30]. In the North American patient population, the prevalence of dyspnea at rest, one of the most common symptoms of CHF, was 38% [45], whereas the prevalence of pain in CHF patients varied from 23 to 85%. Again, the prevalence of pain was probably even higher than the prevalence of the leading symptom of CHF. The same tendency is observed in other diseases.
4.2. Limitations
Many SRs included in this umbrella review did not provide detailed information about the intensity of pain or only presented an overall percentage of pain without specifying the different types of pain experienced. Some studies did not mention the specific type of pain, whether it was nociceptive, neuropathic, or a combination of both. The lack of consistent reporting of pain intensity limits the ability to assess the dimensions of pain. Many studies used different terms for specification of the duration of pain, such as simply “pain”, “chronic pain”, “residual pain”, and “recurrent pain”, and did not mention the duration of pain (e.g., 3 months). Greater evidence of the duration and frequency of pain episodes is needed to fully appreciate the pain experience. Several pain management options have been suggested; however, their effectiveness was not assessed. Therefore, further research is required to evaluate the effectiveness of possible interventions for pain management (COPD) [28]. The following subsections present limitations related to specific patient populations and recommendations for future studies.
4.2.1. Nursing Home Residents
The prevalence and types of pain differ from study to study, possibly due to differences in research methodologies and objectives, the source of data, and the intensity of pain measured. Some studies did not report the duration of pain evaluation (days, months); therefore, it was challenging to classify the pain according to the chronology and analyze the impact of pain. Many studies did not provide the precise prevalence of pain in certain patient populations, such as patients with dementia or elderly nursing home residents, because they could not properly report the pain due to their cognitive abilities.
To improve the ability to predict, detect, and manage pain among some vulnerable patient populations, such as nursing home residents and patients with dementia, it is crucial to study factors that increase the likelihood of pain reporting. Although dementia may affect pain thresholds and experience, resulting in a lower prevalence of pain, the most likely reason for the underestimated prevalence of pain is reduced reporting or detection of pain. Assessing pain in movement, rather than at rest, can probably better detect pain experiences, especially if relying on proxy reports. Using protocols that involve pain assessment during body movements may result in a more adequate determination of the prevalence of pain [18].
4.2.2. Postamputation Pain
The rate of postamputation pain is one of the highest among all types of chronic postoperative pain. Overall, the optimal protocol for pain management during the perioperative period remains uncertain and requires more research. To reduce the prevalence of postamputation pain, future studies should focus on exploring preoperative pain control, surgical techniques, and postoperative pain management. Additional clinical trials with a low risk of bias are necessary to study the impact of multiday preoperative and postoperative nerve blocks on neuroma [19].
4.2.3. Neurodegenerative Diseases
The prevalence of chronic pain in MS patients, especially at later stages, is high, affecting the majority of patients. More precise methods for assessing pain in MS are needed, and the roles of patient questionnaires, physical examination, bedside sensory testing, and quantitative sensory testing should be better defined. Moreover, more research is necessary to study the relationships between pain and depression, fatigue, and cognitive deficits. Certain types of pain associated with MS, such as pain related to optic neuritis and muscle spasms, have not been well characterized. Furthermore, there is limited understanding of how clinical exacerbations of MS relate to the occurrence, type, and intensity of pain. Identifying and characterizing the mechanisms behind different types of pain in MS patients, especially extremity pain, which is the most common type, is crucial for improved pain classification and the development of mechanism-based treatments. As the treatment of MS-related pain remains poorly understood, it is essential to conduct randomized controlled trials to evaluate the effectiveness and safety of existing treatments for neuropathic and non-neuropathic pain in MS patients. These treatments include antidepressants, anticonvulsants, opioid analgesics, non-steroidal anti-inflammatory drugs, and non-pharmacologic therapies. Additionally, few studies have explored the effects of disease-modifying therapy on pain in MS patients, not only in relieving pain but also as a potential source of pain. Given the widespread use of such therapy for relapsing MS, research on its effects on pain and the possibility of preventing pain should be prioritized in future clinical trials. Understanding the implications of pain treatment for patients, clinicians, policy-makers, and third-party payers is essential in determining the risk–benefit ratio and cost-effectiveness of various treatments [20,21,22,23].
Given the aging global population and the increasing prevalence of neurodegenerative disorders, untreated pain is becoming a significant burden for a growing number of individuals. It is crucial to establish a global consensus on strategies to address these challenges, enabling large-scale, high-quality clinical trials in the future. This will pave the way for the development of pain interventions based on a thorough understanding of the underlying mechanisms, ultimately leading to an improved quality of life for many people living with neurodegenerative disorders [20,21,22,23].
4.2.4. COPD
It is crucial to establish standardized assessment tools for measuring pain in COPD patients. Future studies should focus on determining the precise prevalence of pain in relation to disease severity and comorbidity. Additionally, research should delve into the causes, progression, and characteristics of pain, and clinical intervention trials should be conducted. Moreover, it is vital to improve pain recognition and treatment in clinical practice. Integrating pain assessment into regular comprehensive symptom evaluations for COPD patients is important. It is also essential to discuss the prevalence of pain and its potential impact on the quality of life in COPD guidelines to raise awareness and enhance recognition of this aspect of the disease [29].
4.2.5. CKD
It is necessary to extend a routine symptom assessment to patients with earlier stages of CKD as well. Renal and POS-renal are simple assessment tools that screen for common symptoms experienced by CKD patients and offer the opportunity to shift care towards a more patient-centered approach. Furthermore, more comprehensive pain assessment tools validated for CKD patients are available, such as the Visual Analog Scale (VAS), Verbal Rating Scale (VRS), and Numeric Rating Scale (NRS).
5. Conclusions
The prevalence of pain is high in patients with cancer, COPD, multiple sclerosis, Huntington’s disease, Parkinson’s disease, Alzheimer’s disease, and traumatic brain injury, as well as heart, liver, and kidney failure. The majority of patients who suffer from any of these chronic diseases are especially at high risk of developing pain at later stages. Pain does not receive enough attention and is not properly managed. In some cases, pain was reported even more frequently than the principal presenting symptoms of these diseases. Future studies are warranted to establish the prevalence of chronic pain more precisely and develop better methods of pain screening, detection, and management. More studies are also required to better understand the pathophysiology and mechanisms of chronic pain in some diseases, especially in neurodegenerative diseases. It would be also important to provide clear definitions of pain, such as acute, subacute, or chronic and its duration. Existing pain management methods should be widely applied in clinical practice, especially if solid evidence of their efficacy is established (e.g., regional nerve blocks to prevent and manage postamputation pain; opioids, regional anesthesia, pumps for cancer-related pain). For some patient populations, such as nursing home residents, in patients with dementia and neurodegenerative diseases, the most limiting factor for effective pain management is pain detection. Therefore, apart from better use of existing standard pain screening, detection, and rating, developing newer pain biomarkers could probably improve the quality of care.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12237302/s1, File S1: Search terms.
Author Contributions
Conceptualization, D.V.; methodology, D.V.; data extraction, K.T. and M.A.; quality assessment, K.T.; data visualization, M.A.; writing—original draft preparation, D.V.; writing—review and editing, D.V., M.A., K.T. and Y.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by Nazarbayev University Faculty Development Competitive Research Grants No. SOM2021005 (021220FD2851). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schappert, S.M.; Burt, C.W. Ambulatory Care Visits to Physician Offices, Hospital Outpatient Departments, and Emergency Departments: United States, 2001–2002. Vital Health Stat. Ser. 13 Data Natl. Health Surv. 2006, 159, 1–66. [Google Scholar]
- Gureje, O.; Von Korff, M.; Simon, G.E.; Gater, R. Persistent Pain and Well-Being: A World Health Organization Study in Primary Care. JAMA 1998, 280, 147. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.H.; Elliott, A.M.; Chambers, W.A.; Smith, W.C.; Hannaford, P.C.; Penny, K. The Impact of Chronic Pain in the Community. Fam. Pract. 2001, 18, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-21484-1. [Google Scholar]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Interagency Pain Research Coordinating Committee. National Pain Strategy: A Comprehensive Population Health-Level Strategy for Pain; US Department of Health and Human Services: Washington, DC, USA; National Institutes of Health: Washington, DC, USA, 2016.
- Cramer, J.D.; Johnson, J.T.; Nilsen, M.L. Pain in Head and Neck Cancer Survivors: Prevalence, Predictors, and Quality-of-Life Impact. Otolaryngol. Head Neck Surg. 2018, 159, 853–858. [Google Scholar] [CrossRef]
- Kroenke, K.; Theobald, D.; Wu, J.; Loza, J.K.; Carpenter, J.S.; Tu, W. The Association of Depression and Pain with Health-Related Quality of Life, Disability, and Health Care Use in Cancer Patients. J. Pain Symptom Manag. 2010, 40, 327–341. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lai, Y.-L.; Ward, S.E. Effect of Cancer Pain on Performance Status, Mood States, and Level of Hope among Taiwanese Cancer Patients. J. Pain Symptom Manag. 2003, 25, 29–37. [Google Scholar] [CrossRef]
- Strang, P. Cancer Pain—A Provoker of Emotional, Social and Existential Distress. Acta Oncol. 1998, 37, 641–644. [Google Scholar] [CrossRef]
- Kwon, J.H. Overcoming Barriers in Cancer Pain Management. J. Clin. Oncol. 2014, 32, 1727–1733. [Google Scholar] [CrossRef]
- Mercadante, S.; Adile, C.; Tirelli, W.; Ferrera, P.; Penco, I.; Casuccio, A. Barriers and Adherence to Pain Management in Advanced Cancer Patients. Pain Pract. 2021, 21, 388–393. [Google Scholar] [CrossRef]
- Stoorvogel, H.; van Haastregt, J.; Theunissen, M.; Schoenmaekers, J.; Hoeben, A.; van den Beuken-van Everdingen, M. Unacceptable Pain in Oncology: The Patients’ Perspective on Reasons for Absence of Pain Interventions. Eur. J. Cancer Care 2022, 31, e13628. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on Prevalence of Pain in Patients with Cancer 2022: A Systematic Literature Review and Meta-Analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef] [PubMed]
- Haenen, V.; Evenepoel, M.; De Baerdemaecker, T.; Meeus, M.; Devoogdt, N.; Morlion, B.; Dams, L.; Van Dijck, S.; Van der Gucht, E.; De Vrieze, T.; et al. Pain Prevalence and Characteristics in Survivors of Solid Cancers: A Systematic Review and Meta-Analysis. Support. Care Cancer 2022, 31, 85. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.S.; Carpenter, J.S.; Chen, C.X.; Blackburn, J.; Hickman, S.E. Prevalence and Factors Associated with Pain in Nursing Home Residents: A Systematic Review of the Literature. J. Am. Med. Dir. Assoc. 2022, 23, 1916–1925.e1. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.G.; Chaker, S.C.; Curran, G.E.; Downer, M.A.; Assi, P.E.; Joseph, J.T.; Kassis, S.A.; Thayer, W.P. Postamputation Residual Limb Pain Severity and Prevalence: A Systematic Review and Meta-Analysis. Plast. Surg. 2022, 30, 254–268. [Google Scholar] [CrossRef]
- Hurwitz, N.; Radakovic, R.; Boyce, E.; Peryer, G. Prevalence of Pain in Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Amyotroph. Lateral Scler. Front. Degener. 2021, 22, 449–458. [Google Scholar] [CrossRef]
- Innes, K.E.; Sambamoorthi, U. The Potential Contribution of Chronic Pain and Common Chronic Pain Conditions to Subsequent Cognitive Decline, New Onset Cognitive Impairment, and Incident Dementia: A Systematic Review and Conceptual Model for Future Research. J. Alzheimers Dis. 2020, 78, 1177–1195. [Google Scholar] [CrossRef]
- Chang, J.Y.; Rukavina, K.; Lawn, T.; Chaudhuri, K.R. Pain in Neurodegenerative Diseases with Atypical Parkinsonism: A Systematic Review on Prevalence, Clinical Presentation, and Findings from Experimental Studies. J. Integr. Neurosci. 2021, 20, 1067–1078. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, G.P.; van der Zwaan, K.F.; Roos, R.A.C.; Achterberg, W.P. The Prevalence and the Burden of Pain in Patients with Huntington Disease: A Systematic Review and Meta-Analysis. Pain 2019, 160, 773–783. [Google Scholar] [CrossRef]
- Rana, A.Q.; Qureshi, A.R.; Siddiqui, O.; Sarfraz, Z.; Rana, R.; Shtilbans, A. Prevalence of Pain in Atypical Parkinsonism: A Systematic Review and Meta-Analysis. J. Neurol. 2019, 266, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Delwel, S.; Binnekade, T.T.; Perez, R.S.G.M.; Hertogh, C.M.P.M.; Scherder, E.J.A.; Lobbezoo, F. Oral Health and Orofacial Pain in Older People with Dementia: A Systematic Review with Focus on Dental Hard Tissues. Clin. Oral Investig. 2017, 21, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Drageset, J.; Corbett, A.; Selbaek, G.; Husebo, B.S. Cancer-Related Pain and Symptoms among Nursing Home Residents: A Systematic Review. J. Pain Symptom Manag. 2014, 48, 699–710.e1. [Google Scholar] [CrossRef]
- Stubbs, B.; Binnekade, T.; Eggermont, L.; Sepehry, A.A.; Patchay, S.; Schofield, P. Pain and the Risk for Falls in Community-Dwelling Older Adults: Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2014, 95, 175–187.e9. [Google Scholar] [CrossRef] [PubMed]
- Alemzadeh-Ansari, M.J.; Ansari-Ramandi, M.M.; Naderi, N. Chronic Pain in Chronic Heart Failure: A Review Article. J. Tehran Univ. Heart Cent. 2017, 12, 49–56. [Google Scholar]
- Van Dam van Isselt, E.F.; Groenewegen-Sipkema, K.H.; Spruit-van Eijk, M.; Chavannes, N.H.; de Waal, M.W.M.; Janssen, D.J.A.; Achterberg, W.P. Pain in Patients with COPD: A Systematic Review and Meta-Analysis. BMJ Open 2014, 4, e005898. [Google Scholar] [CrossRef]
- Lee, A.L.; Harrison, S.L.; Goldstein, R.S.; Brooks, D. Pain and Its Clinical Associations in Individuals with COPD: A Systematic Review. Chest 2015, 147, 1246–1258. [Google Scholar] [CrossRef]
- Van den Beuken-van Everdingen, M.H.J.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. Prevalence of Pain in Patients with Cancer: A Systematic Review of the Past 40 Years. Ann. Oncol. 2007, 18, 1437–1449. [Google Scholar] [CrossRef]
- Davison, S.N.; Rathwell, S.; Ghosh, S.; George, C.; Pfister, T.; Dennett, L. The Prevalence and Severity of Chronic Pain in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Can. J. Kidney Health Dis. 2021, 8, 205435812199399. [Google Scholar] [CrossRef]
- Lambourg, E.; Colvin, L.; Guthrie, G.; Murugan, K.; Lim, M.; Walker, H.; Boon, G.; Bell, S. The Prevalence of Pain among Patients with Chronic Kidney Disease Using Systematic Review and Meta-Analysis. Kidney Int. 2021, 100, 636–649. [Google Scholar] [CrossRef]
- Brkovic, T.; Burilovic, E.; Puljak, L. Prevalence and Severity of Pain in Adult End-Stage Renal Disease Patients on Chronic Intermittent Hemodialysis: A Systematic Review. Patient Prefer. Adherence 2016, 10, 1131–1150. [Google Scholar] [CrossRef]
- Murtagh, F.E.M.; Addington-Hall, J.; Higginson, I.J. The Prevalence of Symptoms in End-Stage Renal Disease: A Systematic Review. Adv. Chronic Kidney Dis. 2007, 14, 82–99. [Google Scholar] [CrossRef]
- Peng, J.-K.; Hepgul, N.; Higginson, I.J.; Gao, W. Symptom Prevalence and Quality of Life of Patients with End-Stage Liver Disease: A Systematic Review and Meta-Analysis. Palliat. Med. 2019, 33, 24–36. [Google Scholar] [CrossRef]
- Nampiaparampil, D.E. Prevalence of Chronic Pain after Traumatic Brain Injury: A Systematic Review. JAMA 2008, 300, 711–719. [Google Scholar] [CrossRef]
- Harriott, A.M.; Karakaya, F.; Ayata, C. Headache after Ischemic Stroke: A Systematic Review and Meta-Analysis. Neurology 2020, 94, e75–e86. [Google Scholar] [CrossRef]
- Liampas, A.; Velidakis, N.; Georgiou, T.; Vadalouca, A.; Varrassi, G.; Hadjigeorgiou, G.M.; Tsivgoulis, G.; Zis, P. Prevalence and Management Challenges in Central Post-Stroke Neuropathic Pain: A Systematic Review and Meta-Analysis. Adv. Ther. 2020, 37, 3278–3291. [Google Scholar] [CrossRef]
- O’Connor, A.B.; Schwid, S.R.; Herrmann, D.N.; Markman, J.D.; Dworkin, R.H. Pain Associated with Multiple Sclerosis: Systematic Review and Proposed Classification. Pain 2008, 137, 96–111. [Google Scholar] [CrossRef]
- Deng, G. Integrative Medicine Therapies for Pain Management in Cancer Patients. Cancer J. 2019, 25, 343–348. [Google Scholar] [CrossRef]
- Beckwée, D.; Bautmans, I.; Lefeber, N.; Lievens, P.; Scheerlinck, T.; Vaes, P. Effect of Transcutaneous Electric Nerve Stimulation on Pain after Total Knee Arthroplasty: A Blind Randomized Controlled Trial. J. Knee Surg. 2018, 31, 189–196. [Google Scholar] [CrossRef]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Wylde, V.; Dennis, J.; Beswick, A.D.; Bruce, J.; Eccleston, C.; Howells, N.; Peters, T.J.; Gooberman-Hill, R. Systematic Review of Management of Chronic Pain after Surgery. Br. J. Surg. 2017, 104, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, G.; Angermann, C.E.; Cleland, J.G.F.; Lam, C.S.P.; Dahlström, U.; Dickstein, K.; Ertl, G.; Hassanein, M.; Hart, K.W.; Lindsell, C.J.; et al. Global Differences in Characteristics, Precipitants, and Initial Management of Patients Presenting with Acute Heart Failure. JAMA Cardiol. 2020, 5, 401–410. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).