Therapeutic Use of Low-Dose Local Anesthetics in Pain, Inflammation, and Other Clinical Conditions: A Systematic Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Identifying the Research Question
2.3. Identifying Relevant Studies
- MEDLINE via PubMed
- Cochrane Library—including the Cochrane Database of Systematic Reviews (CDSR) and CENTRAL (clinical trials)
- LILACS
2.4. Study Selection
2.5. Charting the Data
2.6. Collating, Summarizing, and Reporting the Results
3. Results
3.1. Search Results and Study Selection
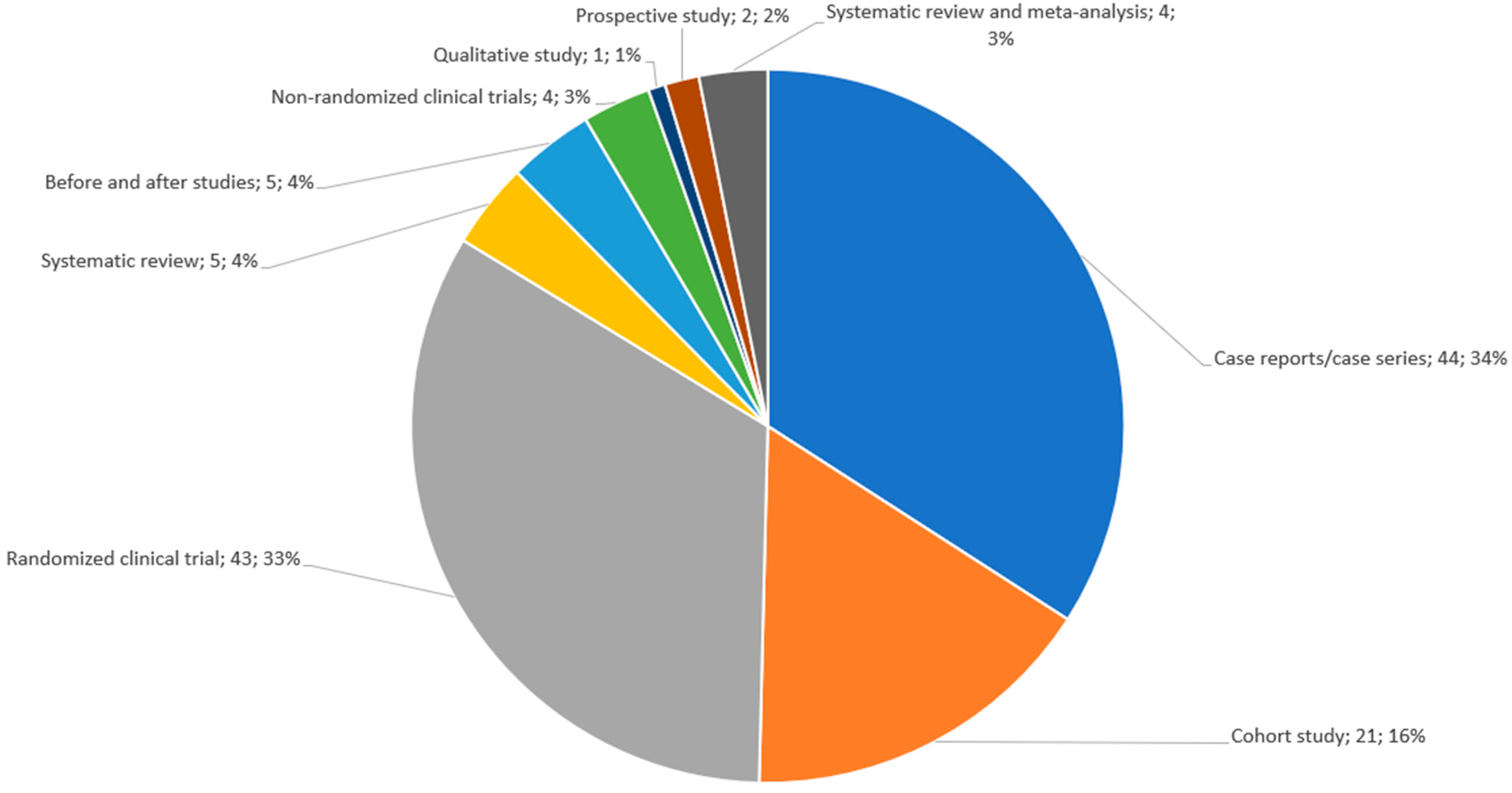
3.2. Description of the Included Studies
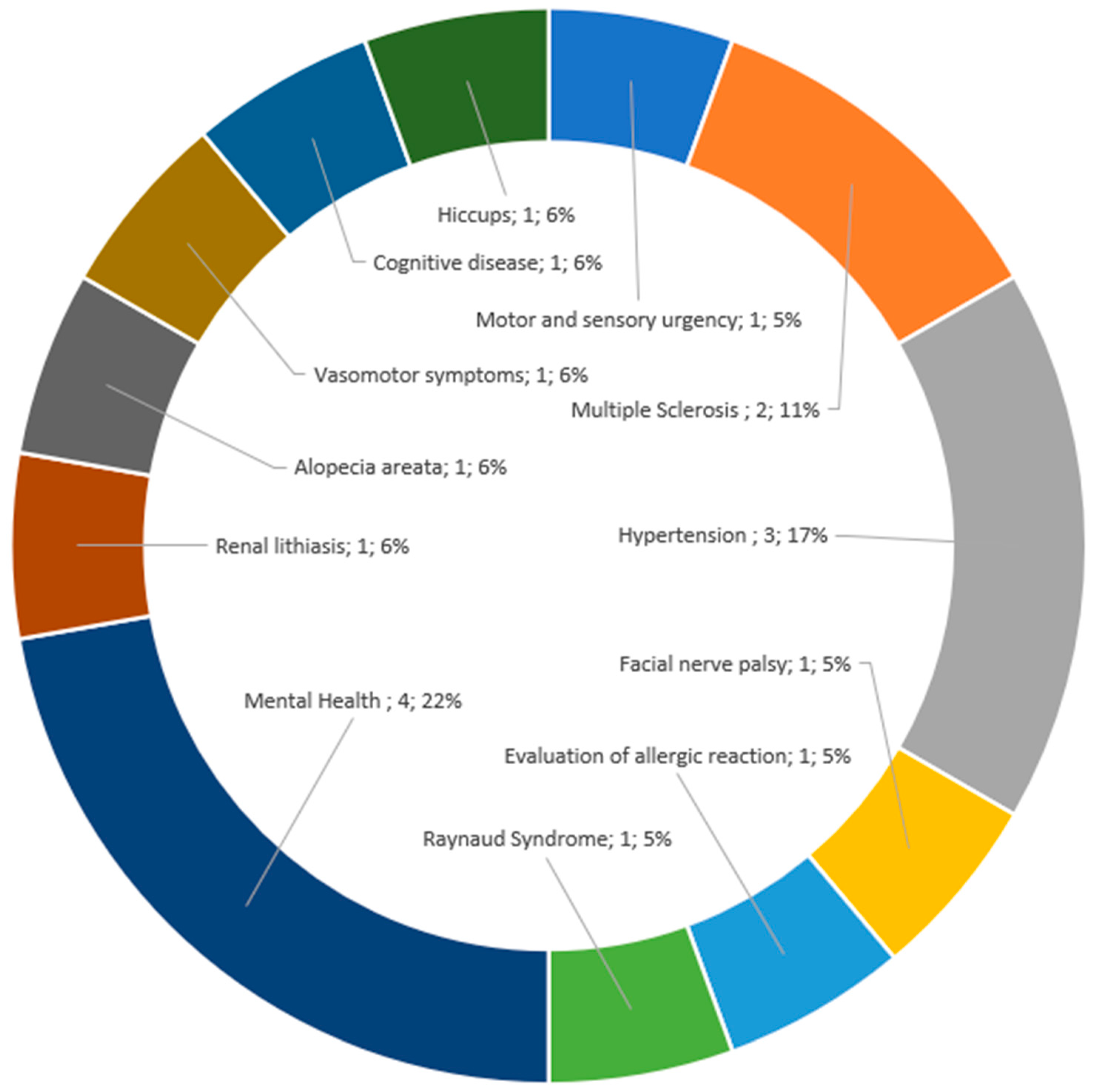
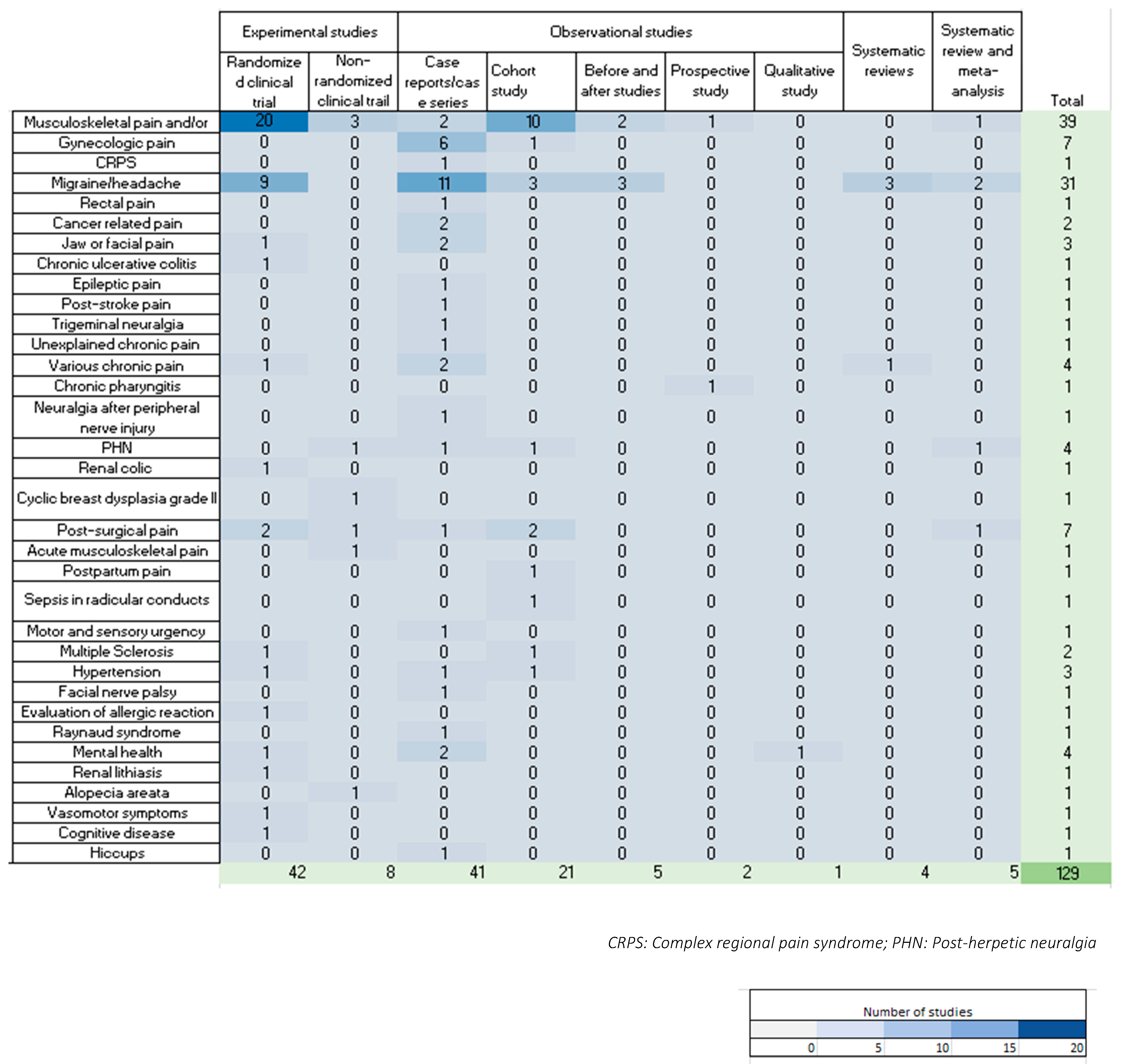
3.3. Description of Indications
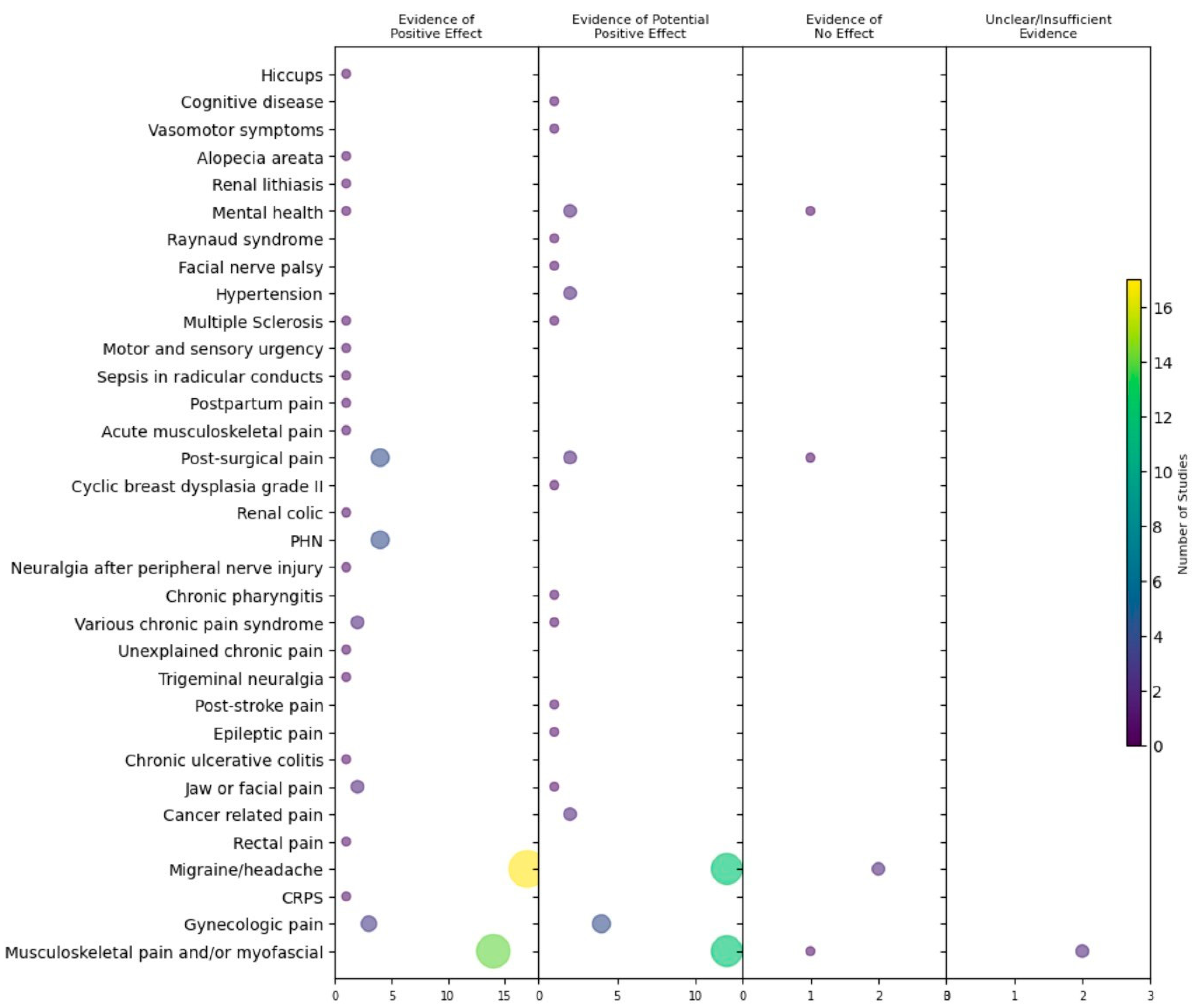
3.4. Outcomes
4. Discussion
4.1. Properties of LAs and Mechanisms of Action
4.1.1. Neuronal Reflex Circuitries
4.1.2. Pathophysiological Coupling Mechanisms between Sympathetic and Nociceptive Systems
4.1.3. Sensitization and Neuroplastic Mechanisms
4.1.4. Neuroimmunological Interactions and Therapeutic Implications
4.2. Safety
4.3. Limitations of Our Scoping Review
4.4. Future
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koller, C. Personal reminiscences of the first use of cocaine as local anesthetic in eye surgery. Curr. Res. Anesth. Analg. 1928, 7, 9. [Google Scholar] [CrossRef]
- Spiess, G. Die Bedeutung der Anästhesie in der Entzündungstheorie. Münch Med. Wschr. 1906, 53, 345–351. [Google Scholar]
- Weinschenk, S. Neural therapy-Areview of the therapeutic use of local anesthetics. Acupunct. Relat. Ther. 2012, 1, 5–9. [Google Scholar] [CrossRef]
- Barop, H. Textbook and Atlas of Neural Therapy: Diagnosis and Therapy with Local Anesthetics, 1st ed.; Thieme: Stuttgart, Germany, 2018; 309p. [Google Scholar]
- Fischer, L. Neuraltherapie: Neurophysiologie, Injektionstechnik und Therapievorschläge, 5th ed.; Thieme: Stuttgart, Germany, 2019; 208p. [Google Scholar]
- Huneke, F. Unbekannte Fernwirkung der Neuraltherapie. Die Med. Welt 1928, 27, 1013–1014. [Google Scholar]
- Leriche, R.; Fontaine, R. L’anesthésie du ganglion étoile: Sa technique, ses indications, ses résultats. Presse Méd. 1934, 42, 849–850. [Google Scholar]
- Dosch, P. Lehrbuch der Neuraltherapie nach Huneke; 14. Aufl.; Haug: Heidelberg, Germany, 1995. [Google Scholar]
- Fischer, L.; Barop, H.; Maxion-Bergemann, S. Health Technology Assessment HTA Neural Therapy. A nationwide evaluation funded by the Swiss Federal Office of Public Health, submited to the Swiss Federal Office of Public Health, Bern, Switzerland. January 2005. [Google Scholar]
- Mermod, J.; Fischer, L.; Staub, L.; Busato, A. Patient satisfaction of primary care for musculoskeletal diseases: A comparison between Neural Therapy and conventional medicine. BMC Complement. Altern. Med. 2008, 8, 33. [Google Scholar] [CrossRef]
- Dönges, A.; Fischer, L.; Marian, F.; Widmer, M.; Herren, S.; Busato, A. Evaluation of Neural Therapy and Comparison with Conventional Medicine: Structure, Process an Outcomes. A Nationwide Evaluation Funded by the Swiss Federal Office of Public Health. Ph.D. Thesis, University of Bern, Bern, Switzerland, 2005. [Google Scholar]
- Bissig, P.; Schoeni-Affolter, F.; Fischer, L.; Busato, A. Is Neural Therapy Cheaper than Conventional Medicine? A Comparison of Cost Structure in Swiss Primary Care Providers. An Observational Study as a Part of a Nationwide Evaluation Funded by the Swiss Federal Office of Public Health. Ph.D. Thesis, University of Bern, Bern, Switzerland, 2008. [Google Scholar]
- Elenkov, I.J.; Wilder, R.L.; Chrousos, G.P.; Vizi, E.S. The sympathetic nerve–an integrative interface between two supersystems: The brain and the immune system. Pharmacol. Rev. 2000, 52, 595–638. [Google Scholar]
- Tracey, K.J. Reflex control of immunity. Nat. Rev. Immunol. 2009, 9, 418–428. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and Functional Neuroscience in Immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef]
- Jänig, W. The Integrative Action of the Autonomic Nervous System, 2nd ed.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Fischer, L.; Barop, H.; Ludin, S.M.; Schaible, H.G. Regulation of acute reflectory hyperinflammation in viral and other diseases by means of stellate ganglion block. A conceptual view with a focus on COVID-19. Auton. Neurosci. 2022, 237, 102903. [Google Scholar] [CrossRef]
- Engel, R.; Barop, H.; Giebel, J.; Ludin, S.M.; Fischer, L. The Influence of Modern Neurophysiology on the Previous Definitions of “Segment” and “Interference Field” in Neural Therapy. Complement. Med. Res. 2022, 29, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, J.; Neuberger, E.W.I.; Bormuth, P.; Comes, F.; Schneider, A.; Banzer, W.; Fischer, L.; Simon, P. Investigation of the Sympathetic Regulation in Delayed Onset Muscle Soreness: Results of an RCT. Front. Physiol. 2021, 12, 697335. [Google Scholar] [CrossRef] [PubMed]
- Puente de la Vega, K.; Gómez, M.; Roqueta, C.; Fischer, L. Effects on hemodynamic variables and echocardiographic parameters after stellate ganglion block in 15 healthy volunteers. Auton. Neurosci. 2016, 197, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Egli, S.; Pfister, M.; Ludin, S.M.; Puente de la Vega, K.; Busato, A.; Fischer, L. Long-term results of therapeutic local anesthesia (neural therapy) in 280 referred refractory chronic pain patients. BMC Complement. Altern. Med. 2015, 15, 200. [Google Scholar] [CrossRef]
- Kronenberg, R.M.; Ludin, S.M.; Fischer, L. Severe case of chronic pelvic pain syndrome: Recovery after injection of procaine into the vesicoprostatic plexus-case report and discussion of pathophysiology and mechanisms of action. Case Rep. Urol. 2018, 2018, 9137215. [Google Scholar] [CrossRef]
- Shiratori Tusita, L.N.; Fischer, L. Chronic Therapy-Resistant Neck Pain in a Fifty-Year-Old Man: The Role of Partially Impacted Third Molars—Case Report and New Pathophysiological Insights. Complement. Med. Res. 2023, 30, 270–274. [Google Scholar] [CrossRef]
- Lopes, C.A.; Fischer, L. A case of severe trigeminal neuralgia: Recovery by means of stellate ganglion block with procaine. Discussion of possible mechanisms of action. J. Int. Med. Res. 2023, 51, 3000605231164479. [Google Scholar] [CrossRef]
- Fischer, L. Pathophysiology of pain and neural therapy. Praxis 2003, 92, 2051–2059. [Google Scholar] [CrossRef]
- Schaible, H.G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res. Ther. 2014, 16, 470. [Google Scholar] [CrossRef]
- Schaible, H.G.; Straub, R.H. Function of the sympathetic supply in acute and chronic experimental joint inflammation. Auton. Neurosci. 2014, 182, 55–64. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Strong, J.A.; Zhang, J.M.; Schaible, H.G. The sympathetic nervous system and pain. In The Oxford Handbook of the Neurobiology of Pain; Wood, J.N., Ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Vinyes, D.; Muñoz-Sellart, M.; Caballero, T.G. Local anesthetics as a therapeutic tool for post COVID-19 patients: A case report. Medicine 2022, 101, e29358. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.; Muñoz, M.; Catalán, M.; Vinyes, D. Treatment of Localized Vulvar Pain with Neural Therapy: A Case Series and Literature Review. Complement. Med. Res. 2021, 28, 571–577. [Google Scholar]
- Bashan, I.; Ozturk, G.Y. Effect of Neural Therapy on shoulder dysfunction and pain in supraspinatus tendinopathy. Pak. J. Med. Sci. 2022, 38 Pt I, 565–569. [Google Scholar] [CrossRef]
- Nazlıkul, H.; Ural, F.G.; Öztürk, G.T.; Öztürk, A.D.T. Evaluation of neural therapy effect in patients with piriformis syndrome. J. Back Musculoskelet. Rehabil. 2018, 31, 1105–1110. [Google Scholar] [CrossRef]
- Feigin, G.; Velasco Figueroa, S.; Englesakis, M.F.; D’Souza, R.; Hoydonckx, Y.; Bhatia, A. Stellate Ganglion Block for Non-Pain Indications: A Scoping Review. Pain Med. 2023, 2, pnad011. [Google Scholar] [CrossRef]
- Fischer, L.; Ludin, S.M.; Thommen, D.; Hausammann, R. Application for the Adoption of Interference Field Therapy (Huneke’s Neural Therapy) into the Health Benefit Basket of the Compulsory Health Insurance, submitted to the Federal Commission for General Health Insurance Benefits and Fundamental Health Issues and the Federal Department of Home Affaires (EDI) in the Federal Office of Public Health (BAG). 2010; submited to BAG. [Google Scholar]
- Barbagli, P.; Bollettin, R. Therapy of articular and periarticular pain with local anesthetics (neural therapy a. t. Huneke). Long and short term results. Minerva Anesth. 1998, 64, 35–43. [Google Scholar]
- Gibson, R.G.; Gibson, S.L. Neural therapy in the treatment of multiple sclerosis. J. Altern. Complement. Med. 1999, 5, 543–552. [Google Scholar] [CrossRef]
- Liu, M.H.; Tian, J.; Su, Y.P.; Wang, T.; Xiang, Q.; Wen, L. Cervical sympathetic block regulates early systemic inflammatory response in severe trauma patients. Med. Sci. Monit. 2013, 19, 194–201. [Google Scholar]
- Yang, X.; Shi, Z.; Li, X.; Li, J. Impacts of stellate ganglion block on plasma NF-κB and inflammatory factors of TBI patients. Int. J. Clin. Exp. Med. 2015, 8, 15630–15638. [Google Scholar]
- Zhao, H.Y.; Yang, G.T.; Sun, N.N.; Kong, Y.; Liu, Y.F. Efficacy and safety of stellate ganglion block in chronic ulcerative colitis. World J. Gastroenterol. 2017, 23, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, P.; Mahmoudi, K.; Khodaverdi, M. Faiz SHR: Effects of ultrasound guided ganglion stellate blockade on intraoperative and postoperative hemodynamic responses in laparoscopic gynecologic surgery. Videosurgery Miniinv 2020, 15, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, H.; Arvaniti, C.; Schoinas, A.; Benas, D.; Vlachos, S.; Palaiodimos, L.; Pavlidis, G.; Ikonomidis, I.; Batistaki, C.; Voumvourakis, C.; et al. Bilateral sphenopalatine ganglion block reduces blood pressure in never treated patients with essential hypertension. A randomized controlled single-blinded study. Int. J. Cardiol. 2018, 250, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Kahokehr, A.; Sammour, T.; Soop, M.; Hill, A.G. Intraperitoneal local anaesthetic in abdominal surgery—A systematic review. ANZ J. Surg. 2011, 81, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Atalay, N.S.; Sahin, F.; Atalay, A.; Akkaya, N. Comparison of efficacy of neural therapy and physical therapy in chronic low back pain. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 431–435. [Google Scholar] [CrossRef]
- Lynch, J.H.; Mulvaney, S.W.; Kim, E.H.; de Leeuw, J.B.; Schroeder, M.J.; Kane, S.F. Effect of Stellate Ganglion Block on Specific Symptom Clusters for Treatment of Post-Traumatic Stress Disorder. Mil. Med. 2016, 181, 1135–1141. [Google Scholar] [CrossRef]
- Naja, Z.; Al-Tannir, M.; El-Rajab, M.; Ziade, F.; Baraka, A. Nerve stimulator-guided occipital nerve blockade for postdural puncture headache. Pain Pract. 2009, 9, 51–58. [Google Scholar] [CrossRef]
- Tepe, N.; Tertemiz, O.F. Comparison of greater occipital nerve and greater occipital nerve + supraorbital nerve block effect in chronic medication overuse headache. Turk. J. Med. Sci. 2021, 51, 1065–1070. [Google Scholar] [CrossRef]
- Kim, H.J.; Ahn, H.S.; Lee, J.Y.; Choi, S.S.; Cheong, Y.S.; Kwon, K.; Yoon, S.H.; Leem, J.G. Effects of applying nerve blocks to prevent postherpetic neuralgia in patients with acute herpes zoster: A systematic review and meta-analysis. Korean J. Pain 2017, 30, 3–17. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Lin, Y.; Chen, L.; Ye, H. The efficacy of greater occipital nerve block for the treatment of migraine: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2018, 165, 129–133. [Google Scholar] [CrossRef]
- Mellick, L.B.; Mellick, G.A. Treatment of acute orofacial pain with lower cervical intramuscular bupivacaine injections: A 1-year retrospective review of 114 patients. J. Orofac. Pain 2008, 22, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Vinyes, D.; Hermosilla Traverso, P.; Murillo, J.H.; Sánchez-Padilla, M.; Muñoz-Sellart, M. Improvement in post-orthodontic chronic musculoskeletal pain after local anesthetic injections in the trigeminal area: A case series. J. Int. Med. Res. 2023, 51, 03000605231214064. [Google Scholar]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Jänig, W.; Baron, R. Pathophysiologie des Schmeruzes. In Lehrbuch der Integrativen Schmerztherapie; Fischer, L., Peuker, E.T., Eds.; Haug: Stuttgart, Germany, 2011. [Google Scholar]
- Borgeat, A.; Aguirre, J. Update on local anesthetics. Curr. Opin. Anaesthesiol. 2010, 23, 466–471. [Google Scholar] [CrossRef]
- Modig, J. Influence of regional anesthesia, local anesthetics, and sympathicomimetics on the pathophysiology of deep vein thrombosis. Acta Chir. Scand. Suppl. 1989, 550, 119–124. [Google Scholar]
- Villar-Garea, A.; Fraga, M.F.; Espada, J.; Esteller, M. Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res. 2003, 63, 4984–4989. [Google Scholar]
- Gradinaru, D.; Ungurianu Am Margina, D.; Villanueva, M.M.; Bürkle, A. Procaine-The Controversial Geroprotector Candidate: New Insights Regarding its Molecular and Cellular Effects. Oxid. Med. Cell. Longev. 2021, 2021, 3617042. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, L.; Dan, J.; Zhu, Q. Differential effects and mechanisms of local anesthetics on esophaeal carcinoma cell migration, growth, survival and chemosensitivity. BMC Anesthesiol. 2020, 20, 126. [Google Scholar] [CrossRef]
- Badwe, R.A.; Parmar, V.; Nair, N.; Joshi, S.; Hawaldar, R.; Pawar, S.; Kadayaprath, G.; Borthakur, B.B.; Thammineedi, S.R.; Pandya, S.; et al. Effect of Peritumoral Infiltration of Local Anesthetic Before Surgery on Survival in Early Breast Cancer. J. Clin. Oncol. 2023, 41, 3318–3328. [Google Scholar] [CrossRef]
- Hollmann, M.W.; Durieux, M.E. Local anaesthetics and the inflammatory response: A new therapeutic indication? Anaesthesiology 2000, 93, 858–875. [Google Scholar] [CrossRef] [PubMed]
- Cassuto, J.; Sinclair, R.; Bonderovic, M. Anti-inflammatory properties of local anesthetics and their present and potential clinical implications. Acta Anaesthesiol. Scand. 2006, 50, 265–282. [Google Scholar] [CrossRef]
- Beilin, B.; Shavit, Y.; Trabekin, E.; Mordashev, B.; Mayburd, E.; Zeidel, A.; Bessler, H. The effects of postoperative pain management on immune response to surgery. Anesth. Analg. 2003, 97, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Lirk, P.; Picardi, S.; Hollmann, M.W. Local anaesthetics: 10 essentials. Eur. J. Anaesthesiol. 2014, 31, 575–585. [Google Scholar] [CrossRef]
- Pietruck, C.; Grond, S.; Xie, G.X.; Palmer, P.P. Local anesthetics differentially inhibit sympathetic neuron-mediated and C fiber-mediated synovial neurogenic plasma extravasation. Anesth. Analg. 2003, 96, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Ricker, G. Pathologie als Naturwissenschaft, Relationspathologie; Springer: Berlin/Heidelberg, Germany, 1924; 391p. [Google Scholar]
- Melzack, R.; Wall, P.D. Pain mechanisms; a new theory. Science 1965, 150, 971–979. [Google Scholar] [CrossRef]
- Baron, R.; Jänig, W. Schmerzsyndrome mit kausaler Beteiligung des Sympathikus. Anaesthesist 1998, 47, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Perl, E.R. Adrenergic excitation of cutaneous pain receptors incuced by peripheral nerve injury. Science 1991, 251, 1608–1610. [Google Scholar] [CrossRef]
- Birder, L.A.; Perl, E.R. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheral nerve injury or inflammation. J. Physiol. 1999, 515 Pt 2, 533–542. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Lorton, D. Autonomic regulation of cellular immune function. Auton. Neurosci. 2014, 182, 15–41. [Google Scholar] [CrossRef]
- McLachlan, E.M.; Jänig, W.; Devor, M.; Michaelis, M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature 1993, 363, 543–546. [Google Scholar] [CrossRef]
- Chung, K.; Chung, J.M. Sympathetic sprouting in the dorsal root ganglion after spinal nerve ligation: Evidence of regenerative collateral sprouting. Brain Res. 2001, 895, 204–212. [Google Scholar] [CrossRef]
- Ramer, M.S.; Bisby, M.A. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain 1997, 70, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Takatori, M.; Kuroda, Y.; Hirose, M. Local anesthetics suppress nerve growth factor-mediated neurite outgrowth by inhibition of tyrosine kinase activity of TrkA. Anesth. Analg. 2006, 102, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Li, H.; Munir, M.A. Decreasing sympathetic sprouting in pathologic sensory ganglia: A new mechanism for treating neuropathic pain using lidocaine. Pain 2004, 109, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Sandkühler, J. Learning and memory in pain pathways. Pain 2000, 88, 113–118. [Google Scholar] [CrossRef]
- Alkadhi, K.A.; Alzoubi, K.H.; Aleisa, A.M. Plasticity of synaptic transmission in autonomic ganglia. Prog. Neurobiol. 2005, 75, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Kansha, M.; Nagata, T.; Irita, K.; Takahashi, S. Dibucaine and tetracaine inhibit the activation of mitogen-activated protein kinase mediated by L-type calcium channels in PC12 cells. Anesthesiology 1999, 91, 1798–1806. [Google Scholar] [CrossRef]
- Tan, Z.; Dohi, S.; Ohguchi, K.; Nakashima, S.; Nozawa, Y. Local anaesthetics inhibit muscarinic receptor-mediated activation of extracellular sign regulated kinases in rat feochromocytoma PC12 cells. Anesthesiology 1999, 91, 1014–1024. [Google Scholar] [CrossRef]
- Eggli, P.; Fischer, L. Vegetatives Nervensystem (Neuroanatomische und neurophysiologische Grundlagen). In Lehrbuch Integrative Schmerztherapie; Fischer Land Peuker, E.T., Ed.; Haug: Stuttgart, Germany, 2011; pp. 17–26. [Google Scholar]
- McLachlan, E. Transmission of signals through sympathetic ganglia—Modulation, integration or simply distribution? Acta Physiol. Scand. 2003, 177, 227–235. [Google Scholar] [CrossRef]
- Schaible, H.G.; Ebersberger, A.; Natura, G. Update on peripheral mechanisms of pain: Beyond prostaglandins and cytokines. Arthritis Res. Ther. 2011, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Thapa, D.; Gombar, S.; Ahuja, V.; Gupta, R. Analgesic efficacy of pre-operative stellate ganglion block on postoperative pain relief: A randomised controlled trial. Anaesthesia 2014, 69, 954–960. [Google Scholar] [CrossRef] [PubMed]
- García-Morán, E.; Sandín-Fuentes, M.G.; Álvarez López, J.C.; Duro-Aguado, I.; Urueña-Martínez, N.; Hernández-Luis, C. Electrical storm secondary to acute myocardial infarction and heart failure treated with left stellate ganglion block. Rev. Esp. Cardiol. 2013, 66, 595–597. [Google Scholar] [CrossRef]
- Guo, W.; Jin, X.J.; Yu, J.; Liu, Y.; Zhang, J.P.; Yang, D.W.; Zhang, L.; Guo, J.-R. Effects of stellate ganglion block on the peri-operative vasomotor cytokine content and intrapulmonary shunt in patients with esophagus cancer. Asian Pac. J. Cancer Prev. 2014, 15, 9505–9509. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Yao, J.; Zhang, T.; Jin, J.; Zeng, X.; Yue, Z. Stellate ganglion block may prevent the development of neurogenic pulmonary edema and improve the outcome. Med. Hypotheses 2013, 80, 158–161. [Google Scholar] [CrossRef]
- Fischer, L. Neuraltherapie in der Notfallmedizin. Ärztez f NHV 1995, 9, 676–685. [Google Scholar]
- Patel, R.A.; Priore, D.L.; Szeto, W.Y.; Slevin, K.A. Left Stellate Ganglion Blockade for the Management of Drug-Resistant Electrical Storm. Pain Med. 2011, 4610–4637. [Google Scholar] [CrossRef]
- Alino, J.; Kosatka, D.; McLean, B.; Hirsch, K. Efficacy of stellate ganglion block in the treatment of anxiety symptoms from combat-related post-traumatic stress disorder: A case series. Mil. Med. 2013, 178, e473–e476. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, K.; Khan, M.H.; Deng, Y.; Shah, K.B. A review of Stellate Ganglion Block as an Adjunctive Treatment Modality. Cureus 2023, 15, e35174. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinyes, D.; Muñoz-Sellart, M.; Fischer, L. Therapeutic Use of Low-Dose Local Anesthetics in Pain, Inflammation, and Other Clinical Conditions: A Systematic Scoping Review. J. Clin. Med. 2023, 12, 7221. https://doi.org/10.3390/jcm12237221
Vinyes D, Muñoz-Sellart M, Fischer L. Therapeutic Use of Low-Dose Local Anesthetics in Pain, Inflammation, and Other Clinical Conditions: A Systematic Scoping Review. Journal of Clinical Medicine. 2023; 12(23):7221. https://doi.org/10.3390/jcm12237221
Chicago/Turabian StyleVinyes, David, Montserrat Muñoz-Sellart, and Lorenz Fischer. 2023. "Therapeutic Use of Low-Dose Local Anesthetics in Pain, Inflammation, and Other Clinical Conditions: A Systematic Scoping Review" Journal of Clinical Medicine 12, no. 23: 7221. https://doi.org/10.3390/jcm12237221
APA StyleVinyes, D., Muñoz-Sellart, M., & Fischer, L. (2023). Therapeutic Use of Low-Dose Local Anesthetics in Pain, Inflammation, and Other Clinical Conditions: A Systematic Scoping Review. Journal of Clinical Medicine, 12(23), 7221. https://doi.org/10.3390/jcm12237221





