Survival Outcomes after Placement of Inferior Vena Cava Filters in Cancer Patients: Insights from a Comprehensive Cancer Center’s Experience
Abstract
:1. Introduction
2. Methods
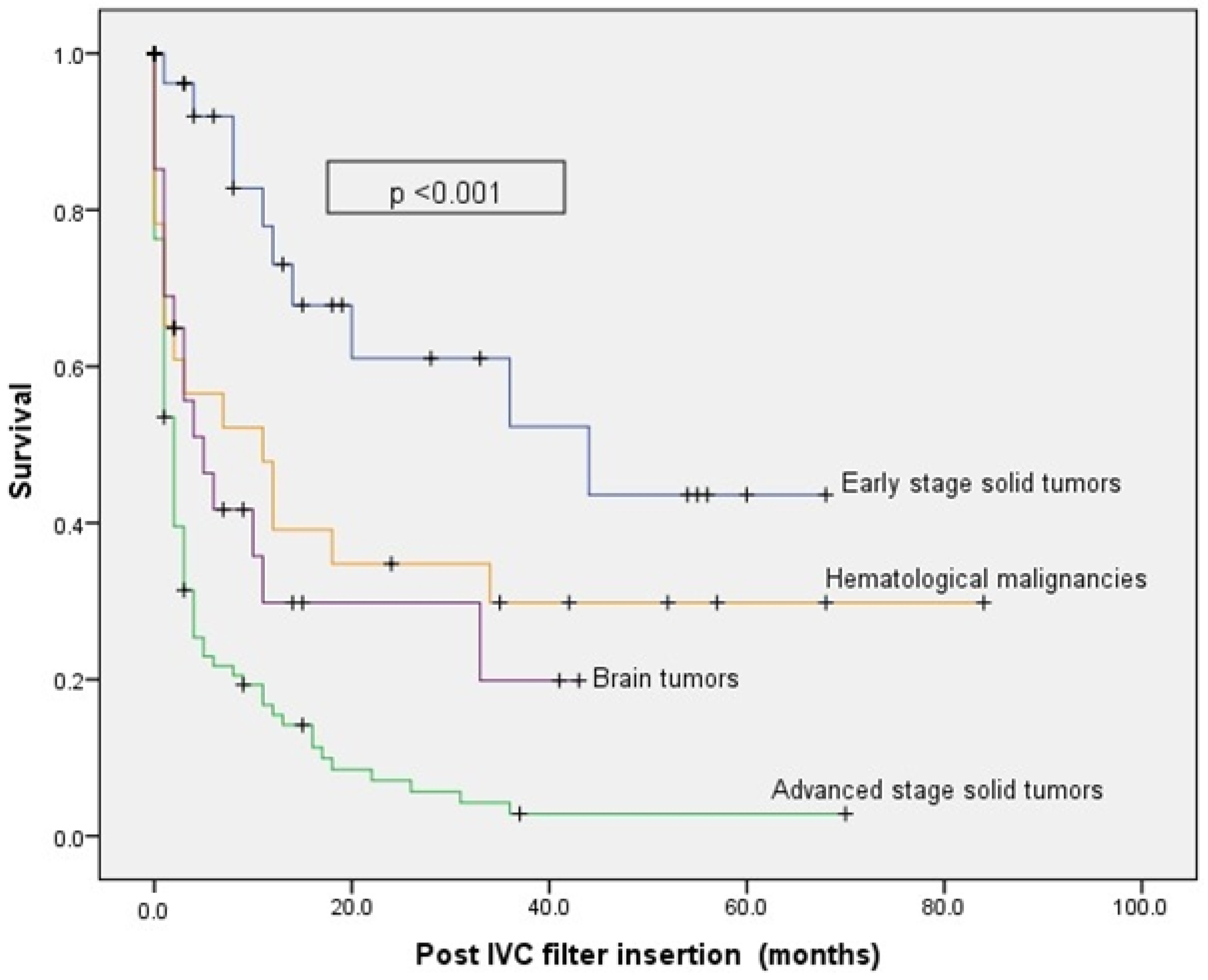
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Risk Factors for Deep Vein Thrombosis and Pulmonary Embolism. Arch. Intern. Med. 2000, 160, 809. [Google Scholar] [CrossRef]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: A population-based study. Arch. Intern. Med. 2002, 162, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Thodiyil, P.A.; Kakkar, A.K. Variation in relative risk of venous thromboembolism in different cancers. Thromb. Haemost. 2002, 87, 1076–1077. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razeq, H.; Mansour, A.; Abdulelah, H.; Al-Shwayat, A.; Makoseh, M.; Ibrahim, M.; Abunasser, M.; Rimawi, D.; Al-Rabaiah, A.; Alfar, R.; et al. Thromboembolic events in cancer patients on active treatment with cisplatin-based chemotherapy: Another look! Thromb. J. 2018, 16, 2. [Google Scholar] [CrossRef]
- Yabeyu, A.B.; Hussen, S.U.; Tigneh, W.; Fentie, A.M. Incidence and determinants of chemotherapy associated thromboembolic events among ethiopian patients treated for solid malignancy: A retrospective cross-sectional study. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221091216. [Google Scholar] [CrossRef]
- Hutten, B.A.; Prins, M.H.; Gent, M.; Ginsberg, J.; Tijssen, J.G.; Büller, H.R. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: A retrospective analysis. J. Clin. Oncol. 2000, 18, 3078–3083. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Sgouros, J.; Maraveyas, A. Excess premature (3-month) mortality in advanced pancreatic cancer could be related to fatal vascular thromboembolic events. A hypothesis based on a systematic review of phase III chemotherapy studies in advanced pancreatic cancer. Acta Oncol. 2008, 47, 337–346. [Google Scholar] [CrossRef]
- Bonnesen, K.; Klok, F.A.; Andersen, M.J.; Andersen, A.; Nielsen-Kudsk, J.E.; Mellemkjær, S.; Sørensen, H.T.; Schmidt, M. Long-Term Prognostic Impact of Pulmonary Hypertension After Venous Thromboembolism. Am. J. Cardiol. 2023, 199, 92–99. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.A.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Muñoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Büller, H.R.; Davidson, B.L.; Decousus, H.; Gallus, A.; Gent, M.; Piovella, F.; Prins, M.H.; Raskob, G.; Segers, A.E.; Cariou, R.; et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: A randomized trial. Ann. Intern. Med. 2004, 140, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Van Hylckama Vlieg, M.A.M.; Nasserinejad, K.; Visser, C.; Bramer, W.M.; Ashrani, A.A.; Bosson, J.L.; Crusan, D.J.; D’Alessio, A.; Fluharty, M.E.; Ģībietis, V.; et al. The risk of recurrent venous thromboembolism after discontinuation of anticoagulant therapy in patients with cancer-associated thrombosis: A systematic review and meta-analysis. EClinical Med. 2023, 64, 102194. [Google Scholar] [CrossRef] [PubMed]
- Gardella, L.; Faulk, J. Phlegmasia Alba and Cerulea Dolens. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Young, T.; Sriram, K.B. Vena caval filters for the prevention of pulmonary embolism. Cochrane Database Syst. Rev. 2020, 10, CD006212. [Google Scholar]
- Jaff, M.R.; McMurtry, M.S.; Archer, S.L.; Cushman, M.; Goldenberg, N.; Goldhaber, S.Z.; Jenkins, J.S.; Kline, J.A.; Michaels, A.D.; Thistlethwaite, P.; et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension. Circulation 2011, 123, 1788–1830. [Google Scholar] [CrossRef]
- Bikdeli, B.; Chatterjee, S.; Desai, N.; Kirtane, A.; Desai, M.; Bracken, M. Inferior vena cava filters to prevent pulmonary embolism: Systematic review and meta-analysis. J. Am. Coll. Cardiol. 2017, 70, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Vestra, M.D.; Grolla, E.; Bonanni, L.; Pesavento, R. Are too many inferior vena cava filters used? Controversial evidences in different clinical settings: A narrative review. Intern. Emerg. Med. 2018, 13, 145–154. [Google Scholar] [CrossRef]
- Patel, G.; Panikkath, R.; Fenire, M.; Gadwala, S.; Nugent, K. Indications and appropriateness of inferior vena cava filter placement. Am. J. Med. Sci. 2015, 349, 212–216. [Google Scholar] [CrossRef]
- Li, X.; Haddadin, I.; McLennan, G.; Farivar, B.; Staub, D.; Beck, A.; Thompson, D.; Partovi, S. Inferior vena cava filter—Comprehensive overview of current indications, techniques, complications and retrieval rates. Vasa 2020, 49, 449–462. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Bai, H.; Liu, Q.; Chen, R. Effect of inferior vena cava filters on pulmonary embolism-related mortality and major complications: A systematic review and meta-analysis of randomized controlled trials. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 792–800.e2. [Google Scholar] [CrossRef]
- Sarosiek, S.; Crowther, M.; Sloan, J.M. Indications, complications, and management of inferior vena cava filters: The experience in 952 patients at an academic hospital with a level I trauma center. JAMA Intern. Med. 2013, 173, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Joels, C.S.; Sing, R.F.; Heniford, B.T. Complications of inferior vena cava filters. Am. Surg. 2003, 69, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, M.; Chan, E.; Nazzal, M.; Abbas, J.; Erikson, G.; Sediqe, S.; Gohara, S. Complications related to inferior vena cava filters: A single-center experience. Ann. Vasc. Surg. 2010, 24, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Mismetti, P.; Rivron-Guillot, K.; Quenet, S.; Décousus, H.; Laporte, S.; Epinat, M.; Barral, F.G. A prospective long-term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism. Chest 2007, 131, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, O.; Nagam, N.; Chitima-Matsiga, R.; Bearelly, S.; Bearelly, D. Retrievable inferior vena cava filters are not getting retrieved: Where is the gap? Thromb. Res. 2010, 126, 493–497. [Google Scholar] [CrossRef]
- Imberti, D.; Prisco, D. Retrievable vena cava filters: Key considerations. Thromb. Res. 2008, 122, 442–449. [Google Scholar] [CrossRef]
- Abdel-Razeq, H.; Mansour, A.; Ismael, Y.; Abdulelah, H. Inferior vena cava filters in cancer patients: To filter or not to filter. Ther. Clin. Risk Manag. 2011, 7, 99–102. [Google Scholar] [CrossRef]
- Bajda, J.; Park, A.N.; Raj, A.; Raj, R.; Gorantla, V.R. Inferior Vena Cava Filters and Complications: A Systematic Review. Cureus 2023, 15, e40038. [Google Scholar] [CrossRef]
- Mansour, A.; Ismael, Y.; Abdel-Razeq, H. Inferior vena cava filters in patients with advanced-stage cancer. Hematol./Oncol. Stem Cell Ther. 2014, 7, 136–141. [Google Scholar] [CrossRef]
- Jarrett, B.P.; Dougherty, M.J.; Calligaro, K.D. Inferior vena cava filters in malignant disease. J. Vasc. Surg. 2002, 36, 704–707. [Google Scholar] [CrossRef]
- Wallace, M.J.; Jean, J.L.; Gupta, S.; Eapen, G.A.; Johnson, M.M.; Ahrar, K.; Madoff, D.C.; Morello, F.A.; Murthy, R.; Hicks, M.E. Use of inferior vena caval filters and survival in patients with malignancy. Cancer 2004, 101, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Decousus, H.; Leizorovicz, A.; Parent, F.; Page, Y.; Tardy, B.; Girard, P.; Laporte, S.; Faivre, R.; Charbonnier, B.; Barral, F.-G.; et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. N. Engl. J. Med. 1998, 338, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Mismetti, P.; Laporte, S.; Pellerin, O.; Ennezat, P.V.; Couturaud, F.; Elias, A.; Falvo, N.; Meneveau, N.; Quere, I.; Roy, P.M.; et al. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: A randomized clinical trial. JAMA 2015, 313, 1627–1635. [Google Scholar] [CrossRef]
- Brunson, A.; Ho, G.; White, R.; Wun, T. Inferior vena cava filters in patients with cancer and venous thromboembolism (VTE): Patterns of use and outcomes. Thromb. Res. 2016, 140 (Suppl. S1), S132–S141. [Google Scholar] [CrossRef]
- PREPIC Study Group. Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: The PREPIC (Prevention du Risque d’Embolie Pulmonaire par Interruption Cave) randomized study. Circulation 2005, 112, 416–422. [Google Scholar] [CrossRef]
- Barginear, M.F.; Gralla, R.J.; Bradley, T.P.; Ali, S.S.; Shapira, I.; Greben, C.; Nier-Shoulson, N.; Akerman, M.; Lesser, M.; Budman, D.R. Investigating the benefit of adding a vena cava filter to anticoagulation with fondaparinux sodium in patients with cancer and venous thromboembolism in a prospective randomized clinical trial. Support. Care Cancer 2012, 20, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Kayali, F.; Olson, R.E. Twenty-one-year trends in the use of inferior vena cava filters. Arch. Intern. Med. 2004, 164, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Duszak, J.; Richard Parker, L.; Levin, D.C.; Rao, V.M. Placement and removal of inferior vena cava filters: National trends in the medicare population. J. Am. Coll. Radiol. 2011, 8, 483–489. [Google Scholar] [CrossRef]
- Kuy, S.; Dua, A.; Lee, C.J.; Patel, B.; Desai, S.S.; Dua, A.; Szabo, A.; Patel, P.J. National trends in utilization of inferior vena cava filters in the United States, 2000–2009. J. Vasc. Surg. Venous Lymphat. Disord. 2014, 2, 15–20. [Google Scholar] [CrossRef]
- Tao, M.J.; Montbriand, J.M.; Eisenberg, N.; Sniderman, K.W.; Roche-Nagle, G. Temporary inferior vena cava filter indications, retrieval rates, and follow-up management at a multicenter tertiary care institution. J. Vasc. Surg. 2016, 64, 430–437. [Google Scholar] [CrossRef]
- Wadhwa, V.; Trivedi, P.S.; Chatterjee, K.; Tamrazi, A.; Hong, K.; Lessne, M.L.; Ryu, R.K. Decreasing utilization of inferior vena cava filters in post-FDA warning era: Insights from 2005 to 2014 nationwide inpatient sample. J. Am. Coll. Radiol. 2017, 14, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Frederick ASpencer, M.D. A Population-based study of inferior vena cava filters in patients with acute venous thromboembolism. Arch. Intern. Med. 2010, 170, 1456–1462. [Google Scholar]
- Shaikh, S.S.; Kamath, S.D.; Ghosh, D.; Lewandowski, R.J.; McMahon, B.J. Safety and outcomes of permanent and retrievable inferior vena cava filters in the oncology population. Int. J. Vasc. Med. 2020, 2020, 6582742. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.E.; Saeed, M.J.; Novak, E.; Brown, D.L. Association of inferior vena cava filter placement for venous thromboembolic disease and a contraindication to. JAMA Netw. Open 2018, 1, e180452. [Google Scholar] [CrossRef] [PubMed]
- Billett, H.H.; Jacobs, L.G.; Madsen, E.M.; Giannattasio, E.R.; Mahesh, S.; Cohen, H.W. Efficacy of inferior vena cava filters in anticoagulated patients. J. Thromb. Haemost. 2007, 5, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Yabroff, K.R.; Shao, Y.; Feuer, E.J.; Brown, M.L. Projections of the cost of cancer care in the United States: 2010–2020. J. Natl. Cancer Inst. 2011, 103, 117–128. [Google Scholar] [CrossRef]
| Clinical Characteristics | Number of Patients | Percentage |
|---|---|---|
| Median age (IQR), years | 56 (47–65) | |
| Gender | ||
| Male | 86 | 48.9 |
| Female | 90 | 51.1 |
| Primary Cancer | ||
| Brain | 28 | 15.9 |
| Breast | 16 | 9.1 |
| Colorectal | 14 | 7.9 |
| Lung | 13 | 7.4 |
| Bladder | 11 | 6.3 |
| Leukemia | 11 | 6.3 |
| Lymphoma | 10 | 5.7 |
| Pancreatic | 10 | 5.7 |
| Sarcoma | 8 | 4.5 |
| Prostate | 5 | 2.8 |
| Ovarian | 5 | 2.8 |
| Stage of cancer at the time of IVC insertion | ||
| Early stage | 25 | 14.2 |
| Metastatic | 99 | 56.3 |
| Unstageable (hematological) | 24 | 13.6 |
| Unstageable (brain) | 28 | 15.9 |
| Type of anticancer therapy during the 6 weeks before IVC filter | ||
| Chemotherapy/Immunotherapy | 70 | 39.8 |
| Major surgery | 14 | 7.9 |
| Radiotherapy | 10 | 5.7 |
| Palliative care | 41 | 23.3 |
| Other | 36 | 20.5 |
| Site of VTE | ||
| PE | 44 | 25 |
| Lower extremity DVT | 81 | 46 |
| DVT and PE | 41 | 23.3 |
| Indication for filter insertion | ||
| Contraindication for anti-coagulation | 99 | 56.3 |
| Recurrent VTE while on anticoagulation | 56 | 31.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Razeq, H.; Tamimi, F.; Al-Jaghbeer, M.J.; Sharaf, B.; Abdel-Razeq, R.; Bani Issa, J.; Abu-Jaish, H.; Salama, O. Survival Outcomes after Placement of Inferior Vena Cava Filters in Cancer Patients: Insights from a Comprehensive Cancer Center’s Experience. J. Clin. Med. 2023, 12, 7209. https://doi.org/10.3390/jcm12237209
Abdel-Razeq H, Tamimi F, Al-Jaghbeer MJ, Sharaf B, Abdel-Razeq R, Bani Issa J, Abu-Jaish H, Salama O. Survival Outcomes after Placement of Inferior Vena Cava Filters in Cancer Patients: Insights from a Comprehensive Cancer Center’s Experience. Journal of Clinical Medicine. 2023; 12(23):7209. https://doi.org/10.3390/jcm12237209
Chicago/Turabian StyleAbdel-Razeq, Hikmat, Faris Tamimi, Mohammed J. Al-Jaghbeer, Baha’ Sharaf, Rashid Abdel-Razeq, Jafar Bani Issa, Hala Abu-Jaish, and Osama Salama. 2023. "Survival Outcomes after Placement of Inferior Vena Cava Filters in Cancer Patients: Insights from a Comprehensive Cancer Center’s Experience" Journal of Clinical Medicine 12, no. 23: 7209. https://doi.org/10.3390/jcm12237209
APA StyleAbdel-Razeq, H., Tamimi, F., Al-Jaghbeer, M. J., Sharaf, B., Abdel-Razeq, R., Bani Issa, J., Abu-Jaish, H., & Salama, O. (2023). Survival Outcomes after Placement of Inferior Vena Cava Filters in Cancer Patients: Insights from a Comprehensive Cancer Center’s Experience. Journal of Clinical Medicine, 12(23), 7209. https://doi.org/10.3390/jcm12237209








