Endovascular Treatment of Type A Aortic Dissection: A Systematic Review and Meta-Analysis Using Reconstructed Time-to-Event Data
Abstract
1. Introduction
2. Methods
2.1. Systematic Search and Eligibility Criteria
2.2. Data Extraction
2.3. Statistical Analysis
2.3.1. Data Pooling and Patient Feature Meta-Analysis
2.3.2. Reconstruction of Patient Time-to-Event Data and Survival Meta-Analysis
2.3.3. Risk-of-Bias Assessment
3. Results
3.1. Literature Search
3.2. Basic Demographics and Medical History
3.3. Disease and Peri-Operative Details
3.4. Complications
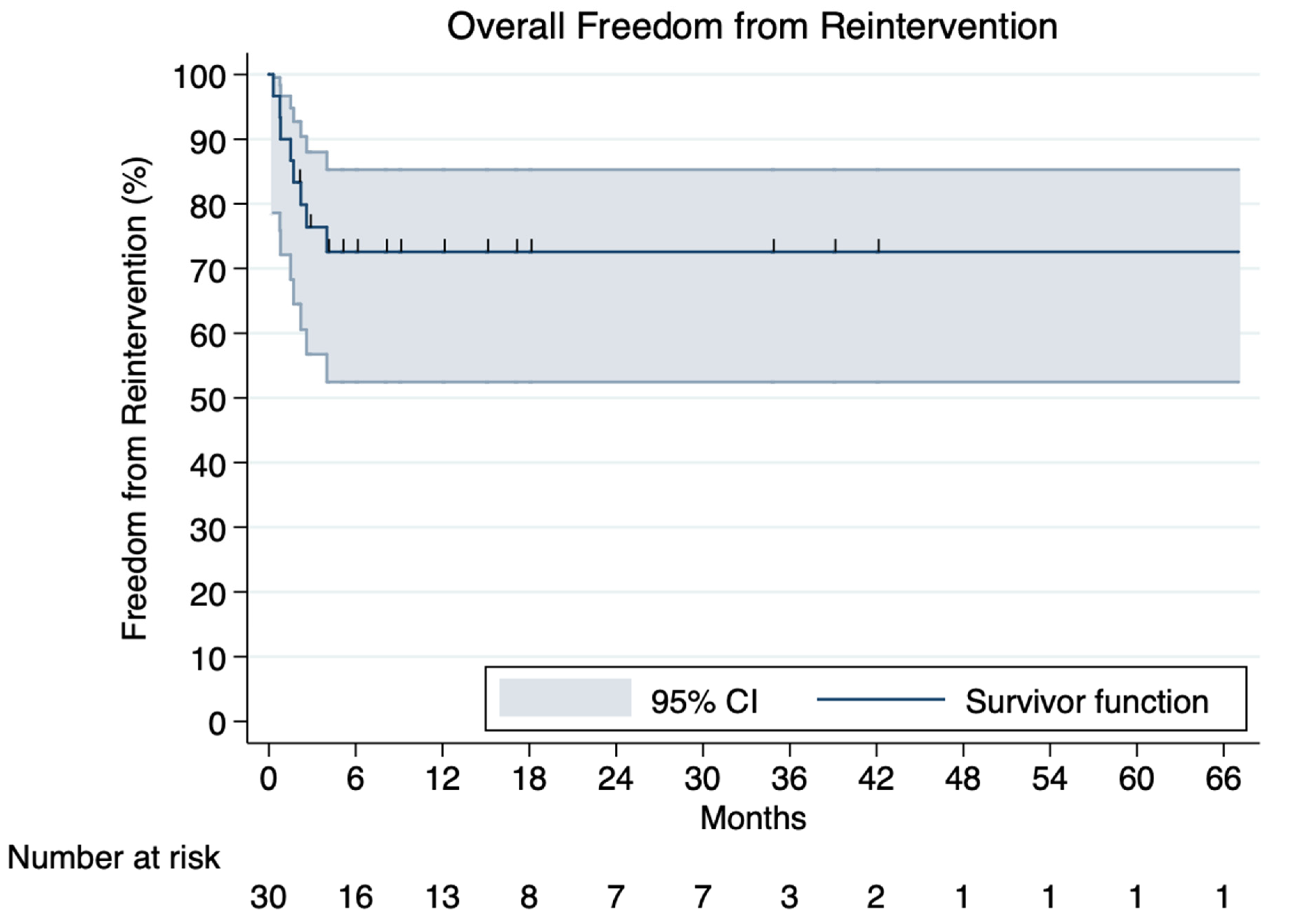
3.5. Reinterventions
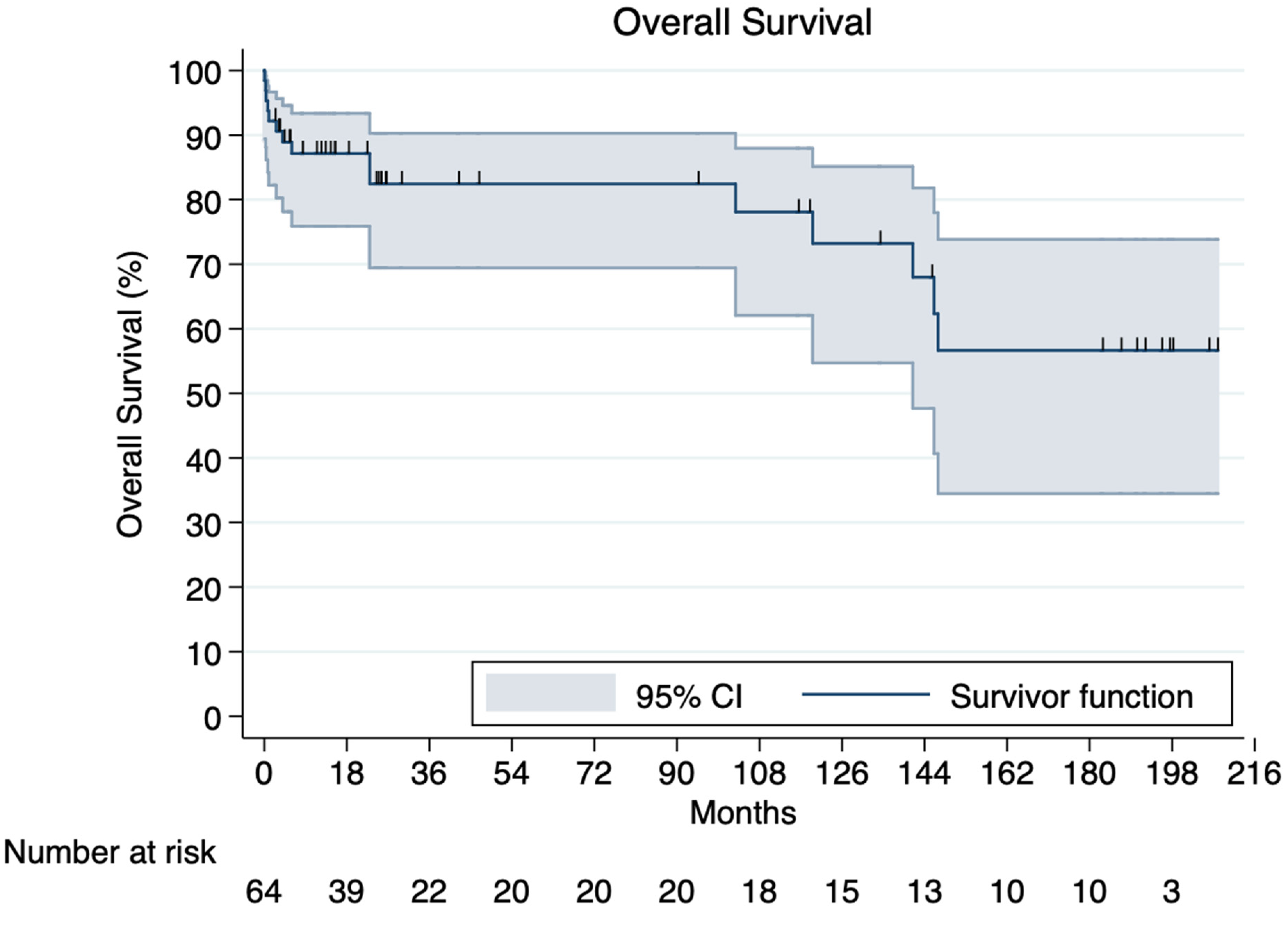
3.6. Overall Survival and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bossone, E.; Eagle, K.A. Epidemiology and management of aortic disease: Aortic aneurysms and acute aortic syndromes. Nat. Rev. Cardiol. 2021, 18, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, R.S.; Cohen, R.G.; Fleischman, F.; Bowdish, M.E. Acute Type A Aortic Dissection. Cardiol. Clin. 2017, 35, 331–345. [Google Scholar] [CrossRef]
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Perkins, J.; Silver, L.E.; Rothwell, P.M.; Oxford Vascular Study. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation 2013, 127, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Sherk, W.M.; Khaja, M.S.; Williams, D.M. Anatomy, Pathology, and Classification of Aortic Dissection. Technol. Vasc. Interv. Radiol. 2021, 24, 100746. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, I.; Mórocz, J.; Szlávi, J.; Schmidt, J.; Tornóci, L.; Nagy, L.; Szép, L. Epidemiology and clinicopathology of aortic dissection. Chest 2000, 117, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Carbone, A.; Eagle, K.A. Gender Differences in Acute Aortic Dissection. J. Pers. Med. 2022, 12, 1148. [Google Scholar] [CrossRef]
- Kuang, J.; Yang, J.; Wang, Q.; Yu, C.; Li, Y.; Fan, R. A preoperative mortality risk assessment model for Stanford type A acute aortic dissection. BMC Cardiovasc. Disord. 2020, 20, 508. [Google Scholar] [CrossRef]
- Writing Committee Members; Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, A.W.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 28, e334–e482. [Google Scholar] [CrossRef] [PubMed]
- Jassar, A.S.; Sundt, T.M., 3rd. How should we manage type A aortic dissection? Gen. Thorac. Cardiovasc. Surg. 2019, 67, 137–145. [Google Scholar] [CrossRef]
- De Freitas, S.; Rossi, M.J.; Abramowitz, S.D.; Fatima, J.; Kiguchi, M.M.; Vallabhaneni, R.; Walsh, S.R.; Woo, E.Y. Systematic review and meta-analysis of endovascular interventions for Stanford type A aortic dissection. J. Vasc. Surg. 2021, 74, 1721–1731.e4. [Google Scholar] [CrossRef] [PubMed]
- Hagan, P.G.; Nienaber, C.A.; Isselbacher, E.M.; Bruckman, D.; Karavite, D.J.; Russman, P.L.; Evangelista, A.; Fattori, R.; Suzuki, T.; Oh, J.K.; et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. JAMA 2000, 283, 897–903. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl h, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed) 2021, 372, n71. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Royston, P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017, 17, 786–802. [Google Scholar] [CrossRef]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- National Heart, Lung and Blood Institute. Quality Assessment Tool for Case Series Studies. Available online: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series (accessed on 13 August 2022).
- Kato, N.; Shimono, T.; Hirano, T.; Ishida, M.; Yada, I.; Takeda, K. Transluminal placement of endovascular stent-grafts for the treatment of type A aortic dissection with an entry tear in the descending thoracic aorta. J. Vasc. Surg. 2001, 34, 1023–1028. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Q.; Liu, L.; Chang, G.; Chen, X.; Feng, H.; Zhang, X.; Fu, W.; Dong, Z.; Jing, Z. Mid-term outcomes from a multicenter study: Is TEVAR safe for ascending aortic dissection? Int. J. Cardiol. 2018, 265, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chang, G.; Li, X.; Hu, Z.; Li, S.; Yang, J.; Chen, W.; Li, J. Endovascular treatment of arch and proximal thoracic aortic lesions. J. Vasc. Surg. 2008, 48, 64–68. [Google Scholar] [CrossRef]
- Qin, J.; Wu, X.; Li, W.; Ye, K.; Yin, M.; Liu, G.; Cui, C.; Zhao, Z.; Liu, X.; Lu, X. Laser fenestration of aortic arch stent grafts for endovascular treatment of retrograde type A dissection. Int. J. Cardiol. 2021, 328, 69–74. [Google Scholar] [CrossRef]
- Omura, A.; Matsuda, H.; Matsuo, J.; Hori, Y.; Fukuda, T.; Inoue, Y.; Seike, Y.; Uehara, K.; Sasaki, H.; Kobayashi, J. Thoracic endovascular repair for retrograde acute type A aortic dissection as an alternative choice. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Verscheure, D.; Haulon, S.; Tsilimparis, N.; Resch, T.; Wanhainen, A.; Mani, K.; Dias, N.; Sobocinski, J.; Eagleton, M.; Ferreira, M.; et al. Endovascular Treatment of Post Type A Chronic Aortic Arch Dissection with a Branched Endograft: Early Results from a Retrospective International Multicenter Study. Ann. Surg. 2021, 273, 997–1003. [Google Scholar] [CrossRef]
- Nienaber, C.A.; Sakalihasan, N.; Clough, R.E.; Aboukoura, M.; Mancuso, E.; Yeh, J.S.; Defraigne, J.; Cheshire, N.; Rosendahl, U.P.; Quarto, C.; et al. Thoracic endovascular aortic repair (TEVAR) in proximal (type A) aortic dissection: Ready for a broader application? J. Thorac. Cardiovasc. Surg. 2017, 153, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Roselli, E.E.; Atkins, M.D.; Brinkman, W.; Coselli, J.; Desai, N.; Estrera, A.; Johnston, D.R.; Patel, H.; Preventza, O.; Vargo, P.R.; et al. ARISE: First-In-Human Evaluation of a Novel Stent Graft to Treat Ascending Aortic Dissection. J. Endovasc. Ther. 2022, 19, 15266028221095018. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.K.; Lee, C.H. Experience of stent-graft repair in acute ascending aortic syndromes. J. Card. Surg. 2019, 34, 1012–1017. [Google Scholar] [CrossRef]
- Ronchey, S.; Serrao, E.; Alberti, V.; Fazzini, S.; Trimarchi, S.; Tolenaar, J.; Mangialardi, N. Endovascular stenting of the ascending aorta for type A aortic dissections in patients at high risk for open surgery. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 475–480. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Yen, H.T.; Wu, C.C.; Huang, D.K. Thoracic Endovascular Aortic Repair for Type A Intramural Hematoma and Retrograde Thrombosed Type A Aortic Dissection: A Single-Center Experience. Ann. Vasc. Surg. 2020, 65, 224–231. [Google Scholar] [CrossRef]
- Gao, F.; Zeng, Q.; Lin, F.; Ge, X. Sandwich Technique for Endovascular Repair of Acute Type A Aortic Dissection. J. Endovasc. Ther. 2017, 24, 647–653. [Google Scholar] [CrossRef]
- Wang, L.; Bai, L.; Zhang, Y.; Liu, J.; Li, X. Application of an extracorporeal prefenestrated stent graft in endovascular repair of ascending aorta and aortic arch lesions. Vascular 2021, 29, 323–329. [Google Scholar] [CrossRef]
- Mitreski, G.; Flanders, D.; Maingard, J.; Robinson, D.; Chuen, J.; Matalanis, G.; Seevanayagam, S.; Kok, H.K.; Ranatunga, D.; Asadi, H.; et al. STABILISE; treatment of aortic dissection, a single Centre experience. CVIR Endovasc. 2022, 5, 7. [Google Scholar] [CrossRef]
- Roselli, E.E.; Idrees, J.J.; Johnston, D.R.; Eagleton, M.J.; Desai, M.Y.; Svensson, L.G. Zone zero thoracic endovascular aortic repair: A proposed modification to the classification of landing zones. J. Thorac. Cardiovasc. Surg. 2018, 155, 1381–1389. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Q.; Feng, R.; Zhou, J.; Zhao, Z.; Bao, J.; Feng, X.; Feng, J.; Pei, Y.; Song, C.; et al. Outcomes of Endovascular Repair of Ascending Aortic Dissection in Patients Unsuitable for Direct Surgical Repair. J. Am. Coll. Cardiol. 2016, 68, 1944–1954. [Google Scholar] [CrossRef]
- Shu, C.; Wang, T.; Li, Q.M.; Li, M.; Jiang, X.H.; Luo, M.Y.; Jiang, X.; Luo, M.; Li, X. Thoracic endovascular aortic repair for retrograde type A aortic dissection with an entry tear in the descending aorta. J. Vasc. Interv. Radiol. 2012, 23, 453–460.e1. [Google Scholar] [CrossRef]
- Yuan, X.; Mitsis, A.; Mozalbat, D.; Nienaber, C.A. Alternative management of proximal aortic dissection: Concept and application. Indian J. Thorac. Cardiovasc. Surg. 2022, 38 (Suppl. S1), 183–192. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Shi, H.; Qin, J.; Zhao, Z.; Yin, M.; Liu, X.; Ye, K.; Liu, G.; Li, W.; Lu, X. Outcomes of emergency in situ laser fenestration-assisted thoracic endovascular aortic repair in patients with acute Stanford type A aortic dissection unfit for open surgery. J. Vasc. Surg. 2020, 71, 1472–1479.e1. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Lu, H.; Jiang, D.; Qiu, Z.; Rashid, J.; Xie, L.; Shen, Y.; Chen, L. Early Efficacy of In Situ Fenestration with a Triple Chimney Technique for High-Risk Stanford Type A Aortic Dissection: A Single-Center Retrospective Study. J. Interv. Cardiol. 2021, 2021, 5662697. [Google Scholar] [CrossRef] [PubMed]
- Higashigawa, T.; Kato, N.; Nakajima, K.; Chino, S.; Hashimoto, T.; Ouchi, T.; Tokui, T.; Maze, Y.; Mizumoto, T.; Teranishi, S.; et al. Thoracic endovascular aortic repair for retrograde type A aortic dissection. J. Vasc. Surg. 2019, 69, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Chang, G.; Li, S.; Hu, Z.; Yao, C.; Chen, W.; Li, X.; Wang, S. Endovascular stent-graft treatment for Stanford type A aortic dissection. Eur. J. Vasc. Endovasc. Surg. 2011, 42, 787–794. [Google Scholar] [CrossRef]
- Qin, J.; Zhao, Z.; Liu, G.; Ye, K.; Yin, M.; Cui, C.; Shi, H.; Peng, Z.; Jiang, M.; Liu, X.; et al. In situ diode laser fenestration of aortic arch stent grafts during thoracic endovascular aortic repair of Stanford type A aortic dissection. EuroIntervention 2019, 14, e1854–e1860. [Google Scholar] [CrossRef]
- Vallabhajosyula, P.; Gottret, J.P.; Bavaria, J.E.; Desai, N.D.; Szeto, W.Y. Endovascular repair of the ascending aorta in patients at high risk for open repair. J. Thorac. Cardiovasc. Surg. 2015, 149, S144–S150. [Google Scholar] [CrossRef]
- Khoynezhad, A.; Donayre, C.E.; Walot, I.; Koopmann, M.C.; Kopchok, G.E.; White, R.A. Feasibility of endovascular repair of ascending aortic pathologies as part of an FDA-approved physician-sponsored investigational device exemption. J. Vasc. Surg. 2016, 63, 1483–1495. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, R.C.; Navarro, T.P.; Reis, F.R.; Lima, L.C.; Monteiro, E.L.; Procopio, R.J.; Botelho, F.E.; Dardik, A. Early experience with off-the-shelf endografts using a zone 0 proximal landing site to treat the ascending aorta and arch. J. Thorac. Cardiovasc. Surg. 2014, 148, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tsilimparis, N.; Drewitz, S.; Detter, C.; Spanos, K.; von Kodolitsch, Y.; Rohlffs, F.; Reichenspurner, H.; Debus, E.S.; Kölbel, T. Endovascular repair of ascending aortic pathologies with tubular endografts: A single-center experience. J. Endovasc. Ther. 2019, 26, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, M.; Shah, A.; Jeudy, J.; Pasrija, C.; Lebowitz, J.; Kaczorowski, D.; Gupta, A.; Toursavadkohi, S.; Taylor, B.S. Endovascular repair of ascending aortic disease in high-risk patients yields favorable outcome. Ann. Thorac. Surg. 2020, 109, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Berretta, P.; Patel, H.J.; Gleason, T.G.; Sundt, T.M.; Myrmel, T.; Desai, N.; Korach, A.; Panza, A.; Bavaria, J.; Khoynezhad, A.; et al. IRAD experience on surgical type A acute dissection patients: Results and predictors of mortality. Ann. Cardiothorac. Surg. 2016, 5, 346–351. [Google Scholar] [CrossRef]
- Baikoussis, N.G.; Antonopoulos, C.N.; Papakonstantinou, N.A.; Argiriou, M.; Geroulakos, G. Endovascular stent grafting for ascending aorta diseases. J. Vasc. Surg. 2017, 66, 1587–1601. [Google Scholar] [CrossRef]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends from the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef]
- Dorros, G.; Dorros, A.M.; Planton, S.; O’Hair, D.; Zayed, M. Transseptal guidewire stabilization facilitates stent-graft deployment for persistent proximal ascending aortic dissection. J. Endovasc. Ther. 2000, 7, 506–512. [Google Scholar] [CrossRef]
- Shah, A.; Khoynezhad, A. Thoracic endovascular repair for acute type A aortic dissection: Operative technique. Ann. Cardiothorac. Surg. 2016, 5, 389–396. [Google Scholar] [CrossRef]
| Author | Year | Journal | Center | Country/Region | Design | Study Period | TEVAR |
|---|---|---|---|---|---|---|---|
| Hsieh et al. 2019 [26] | 2019 | Journal of Cardiac Surgery | Changhua Christian Hospital | Taiwan | Retrospective cohort | 2015–2018 | 4 |
| Ronchey et al. 2013 [27] | 2013 | European Journal of Vascular and Endovascular Surgery | San Filippo Neri Hospital, Rome | Italy | Retrospective cohort | 2009–2012 | 4 |
| Chen et al. 2019 [28] | 2019 | Annals of Vascular Surgery | Kaohsiung Chang Gung Memorial Hospital | Taiwan | Retrospective cohort | 2017–2019 | 4 |
| Gao et al. 2017 [29] | 2017 | Journal of Endovascular Therapy | Jiangya Haikou Hospital, Municipal Hospital of XinJiang, XinJiang | China | Retrospective cohort | 2013–2016 | 7 |
| Wang et al. 2021 [30] | 2021 | Vascular | General Hospital of Ningxia Medical University, Ningxia | China | Retrospective cohort | April 2016–June 2017 | 9 |
| Mitreski et al. 2022 [31] | 2022 | CVIR Endovascular | Austin Health, Heidelberg | Australia | Retrospective cohort | 2011–2020 | 10 |
| Roselli et al. 2018 [32] | 2018 | The Journal of Thoracic and Cardiovascular Surgery | Cleveland Clinic, Cleveland | USA | Retrospective cohort | 2006–2016 | 14 |
| Li et al. 2016 [33] | 2016 | Journal of the American College of Cardiology | 2nd Military Medical University, Shanghai | China | Retrospective cohort | 2009–2011 | 15 |
| Shu et al. 2012 [34] | 2012 | Journal of Vascular and Interventional Radiology | The 2nd Xiang-ya Hospital of Central-south University, Changsha | China | Retrospective cohort | 2006–2011 | 17 |
| Yuan et al. 2022 [35] | 2022 | Indian Journal of Thoracic and Cardiovascular Surgery | Cardiology and Aortic Centre, Royal Brompton and Harefield Hospitals, London | UK | Retrospective cohort | 2015–2020 | 19 |
| Yan et al. 2019 [36] | 2019 | Journal of Vascular Surgery | Shanghai Ninth People’s Hospital, Shanghai | China | Retrospective cohort | March 2016–December 2018 | 20 |
| Wu et al. 2021 [37] | 2021 | Journal of Interventional Cardiology | Union Hospital, Fujian Medical University, Fuzhou | China | Retrospective cohort | January 2018–December 2019 | 24 |
| Higashigawa et al. 2019 [38] | 2019 | Journal of Vascular and Interventional Radiology | Multicenter (Japan) | Japan | Retrospective cohort | May 1997–January 2016 | 31 |
| Ye et al. 2011 [39] | 2011 | European Journal of Vascular and Endovascular Surgery | The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou | China | Retrospective cohort | 2001–2009 | 45 |
| Qin et al. 2019 [40] | 2019 | EuroIntervention | Shanghai Jiao Tong University, Shanghai | China | Retrospective cohort | April 2014–May 2018 | 58 |
| Vallabhajosyula et al. 2015 [41] | 2015 | The Journal of Thoracic and Cardiovascular Surgery | University of Pennsylvania Medical Center, Philadelphia | USA | Retrospective cohort | 2007–2012 | 2 |
| Khoynezhad et al. 2016 [42] | 2016 | Journal of Vascular Surgery | Cedars-Sinai Medical Center, David Geffen UCLA School of Medicine, Los Angeles | USA | RCT | 2014–2016 | 2 |
| Bernardes at al. 2014 [43] | 2014 | The Journal of Thoracic and Cardiovascular Surgery | Madre Teresa Hospital, Belo Horizonte, Minas Gerais | Brazil | Retrospective cohort | 2007–2012 | 2 |
| Tsilimparis et al. 2019 [44] | 2019 | Journal of Endovascular Therapy | German Aortic Center, University Heart Center, Hamburg | Germany | Retrospective cohort | 2010–2017 | 16 |
| Ghoreishi et al. 2019 [45] | 2019 | The Annals of Thoracic Surgery | University of Maryland School of Medicine, Baltimore | USA | Retrospective cohort | 2018–2019 | 8 |
| Author | TEVAR | Age (SD) (Years) | Males (n, %) | Females (n, %) | DM (n, %) | COPD (n, %) | HTN (n, %) |
|---|---|---|---|---|---|---|---|
| Hsieh et al. 2019 [26] | 4 | 56.25 (14.4) | 2 (50) | 2 (50) | NR | NR | NR |
| Ronchey et al. 2013 [27] | 4 | 70 (7.65) | 2 (50) | 2 (50) | 1 (25) | NR | 4 (100) |
| Chen et al. 2019 [28] | 4 | 67.25 (8.5) | 3 (75) | 1 (25) | NR | NR | NR |
| Gao et al. 2017 [29] | 7 | 54.57 (10.25) | 7 (100) | 0 (0) | NR | NR | NR |
| Wang et al. 2021 [30] | 9 | NR | 7 (77.8) | 2 (22.2) | NR | NR | 8 (88.9) |
| Mitreski et al. 2022 [31] | 10 | 60.7 (11.47) | 6 (60) | 4 (40) | NR | NR | NR |
| Roselli et al. 2018 [32] | 14 | NE | 4 (28.6) | 10 (71.4) | 5 (35.7) | 4 (28.6) | 13 (92.9) |
| Li et al. 2016 [33] | 15 | 65 (12.1) | 12 (80) | 3 (20( | NR | 3 (20) | NR |
| Shu et al. 2012 [34] | 17 | 54.5 (10.3) | 16 (94.1) | 1 (5.9) | 4 (23.5) | 0 (0) | 17 (100) |
| Yuan et al. 2022 [35] | 19 | NR | NR | NR | NR | NR | NR |
| Yan et al. 2019 [36] | 20 | 67 (NR) | 18 (90) | 2 (10) | 9 (45) | 6 (30) | 13 (65) |
| Wu et al. 2021 [37] | 24 | 65.4 (9.3) | 22 (91.7) | 2 (8.3) | 3 (12.5) | 2 (8.3) | 23 (95.8) |
| Higashigawa et al. 2019 [38] | 31 | 64 (11.0) | 30 (96.8) | 1 (3.2) | 1 (3.2) | 1 (3.2) | 22 (70.9) |
| Ye et al. 2011 [39] | 45 | 51 (NR) | 41 (91.1) | 4 (8.9) | NR | NR | 41 (91.1) |
| Qin et al. 2019 [40] | 58 | 58 (NR) | 32 (55.1) | 26 (44.9) | 33 (56.9) | 4 (6.9) | 42 (72.4) |
| Vallabhajosyula et al. 2015 [41] | 2 | 84 (NR) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 2 100) |
| Khoynezhad et al. 2016 [42] | 2 | 86 (NR) | 1 (50) | 1 (50) | 0 (0) | NR | 1 (50) |
| Bernardes at al. 2014 [43] | 2 | 52.5 (NR) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 2 (100) |
| Tsilimparis et al. 2019 [44] | 16 | 70 (15) | NE | NE | NR | NR | NR |
| Ghoreishi et al. 2019 [45] | 8 | 69 (9) | NE | NE | NR | 1 (12.5) | 8 (100) |
| Author | TEVAR | Access Site (n, %) | Device Used (n, %) | Mechanism of Cardiac Output Suppression (n, %) |
|---|---|---|---|---|
| Hsieh et al. 2019 [26] | 4 | Femoral (n = 4, 100) | GORE (n = 2, 50), MEDTRONIC (n = 1, 25) | VTACH (n = 4, 100) |
| Ronchey et al. 2013 [27] | 4 | Femoral (n = 4, 100) | Cook TX2 (n = 1, 25), Off-shelf Cook (n = 3, 75) | RVP (n = 4, 100) |
| Chen et al. 2019 [28] | 4 | Femoral (n = 4, 100) | NR | DHTN (n = 4, 100) |
| Gao et al. 2017 [29] | 7 | Femoral (n = 7, 100) | GORE TAG (n = 7, 100) | DHTN (n = 7, 100) |
| Wang et al. 2021 [30] | 9 | Femoral with adjunctive brachial (n = 9, 100) | MEDTRONIC Valiant Captiva (n = 9, 100) | DHTN (n = 9, 100% |
| Mitreski et al. 2022 [31] | 10 | Femoral (n = 10, 100) | COOK Zenith TX2 (n = 10, 100) | NR |
| Roselli et al. 2018 [32] | 14 | NR | NR | RVP (n = 14, 100) |
| Li et al. 2016 [33] | 15 | Femoral (n = 15, 100) | COOK Zenith TX2 Pro Form (n = 15, 100) | NR |
| Shu et al. 2012 [34] | 17 | Femoral (n = 17, 100) | MicroPort Hercules (n = 10, 58.9), COOK Zenith (n = 5, 29.4), MEDTRONIC Valiant (n = 2, 11.8) | DHTN (n = 17, 100 |
| Yuan et al. 2022 [35] | 19 | Femoral (n = 19, 100) | NR | RVP (n = 19, 100) |
| Yan et al. 2019 [36] | 20 | Femoral (n = 20, 100) | GORE TAG (n = 20, 100) | NR |
| Wu et al. 2021 [37] | 24 | Femoral with adjunctive LCA (n = 24, 100) | NR | NR |
| Higashigawa et al. 2019 [38] | 31 | NR | NR | NR |
| Ye et al. 2011 [39] | 45 | LCA (n = 2, 4.4), femoral (n = 22, 48.8) | NR | DHTN (n = 45, 100) |
| Qin et al. 2019 [40] | 58 | Femoral with adjunctive LCA or RCA (n = 58, 100) | GORE TAG (n = 58, 100) | SCA (n = 58, 100) |
| Vallabhajosyula et al. 2015 [41] | 2 | Transapical (n = 2, 100) | COOK Zenith TX2 (n = 2, 100) | RVP (n = 2, 100) |
| Khoynezhad et al. 2016 [42] | 2 | Femoral (n = 2, 100) | MEDTRONIC Valiant PS-IDE (n = 2, 100) | RVP (n = 2, 100) |
| Bernardes at al. 2014 [43] | 2 | Femoral (n = 2, 100) | COOK Zenith (n = 1, 50), GORE TAG (n = 1, 50) | DHTN (n = 2, 100) |
| Tsilimparis et al. 2019 [44] | 16 | NR | Cook Ascend TAA Endovascular Graft (n = 16, 100) | IVCO (n = 16, 100) |
| Ghoreishi et al. 2019 [45] | 8 | NR | GORE TAG (n = 8, 100) | RVP (n = 8, 100) |
| Author | TEVAR | Early Complications (n, %) | Early Complications (Types) (n, %) | Late Complications (n, %) | Late Complications (Types) (n, %) | Conversion to Open Surgery (n, %) |
|---|---|---|---|---|---|---|
| Hsieh et al. 2019 [26] | 4 | 1 (25) | New TAAD (n = 1, 100) | 2 (50) | Endoleak (n = 1, 50) | 1 (25) |
| Ronchey et al. 2013 [27] | New TAAD (n = 1, 50) | |||||
| Chen et al. 2019 [28] | 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Gao et al. 2017 [29] | 4 | 0 (0) | 0 (0) | 3 (75) | New PAU (n = 1, 33.3) | 0 (0) |
| Wang et al. 2021 [30] | Hematoma progression (n = 1, 33.3) | |||||
| Mitreski et al. 2022 [31] | New TAAD (n = 1, 33.3) | |||||
| Roselli et al. 2018 [32] | 7 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Li et al. 2016 [33] | 9 | 0 (0) | 0 (0) | 2 (22.2) | New TAAD (n = 1, 50) | 0 (0) |
| Shu et al. 2012 [34] | Endoleak (n = 1, 50) | |||||
| Yuan et al. 2022 [35] | 10 | 2 (20) | Pulmonary hemorrhage (n = 1, 50) | 0 (0) | 0 (0) | NR |
| Yan et al. 2019 [36] | End-organ ischemia (n = 1, 50) | |||||
| Wu et al. 2021 [37] | 14 | 6 (42.8) | Cardiac tamponade (n = 2, 33.3) | 4 (28.6) | NR | NR |
| Higashigawa et al. 2019 [38] | Multi-organ-failure (n = 2, 33.3) | |||||
| Ye et al. 2011 [39] | 15 | 2 (13.3) | Arrhythmia (n = 1, 50) | 8 (53.3) | New TAAD (n = 2, 25) | 1 (6.7) |
| Qin et al. 2019 [40] | Myocardial Infarction (1, 12.5) | |||||
| Vallabhajosyula et al. 2015 [41] | Arrhythmia (1, 12.5) | |||||
| Khoynezhad et al. 2016 [42] | Tamponade (1, 12.5) | |||||
| Bernardes at al. 2014 [43] | Endoleak (n = 1, 12.5) | |||||
| Tsilimparis et al. 2019 [44] | 17 | 0 (0) | 0 (0) | 1 (5.9) | New TAAD (n = 1, 100) | 0 (0) |
| Ghoreishi et al. 2019 [45] | 19 | 0 (0) | 0 (0) | 1 (5.3) | Endoleak (n = 1, 100) | 1 (5.3) |
| Hsieh et al. 2019 [26] | 20 | 2 (10) | Pneumonia (n = 1, 50) | 3 (15) | Endoleak (n = 3, 100) | 0 (0) |
| Ronchey et al. 2013 [27] | Stroke (n = 1, 50) | |||||
| Chen et al. 2019 [28] | 24 | 0 (0) | 0 (0) | 1 (4.2) | Endoleak (n = 1, 100) | 0 (0) |
| Gao et al. 2017 [29] | 31 | 8 (25.8) | Abdominal aortic rupture (n = 1, 12.5) | 9 (29.0) | Intimal injury (n = 3, 33.3) | 5 (16.1) |
| Wang et al. 2021 [30] | Intimal injury (n = 3, 37.5) | Abdominal aortic aneurysm (n = 1, 11.1) | ||||
| Mitreski et al. 2022 [31] | Left arm ischemia (n = 1, 12.5) | New TAAD (n = 3, 33.3) | ||||
| Roselli et al. 2018 [32] | Type I endoleak (n = 2, 25) | Type I endoleak (n = 1, 11.1) | ||||
| Li et al. 2016 [33] | Type II endoleak (n = 1, 12.5) | Type II endoleak (n = 1, 11.1) | ||||
| Shu et al. 2012 [34] | 45 | 4 (8.9) | Myocardial infarction (n = 1, 25) | 14 (31.1) | Type I endoleak (n = 9, 75.4) | 0 (0) |
| Yuan et al. 2022 [35] | Neck hematoma (n = 1, 25) | Stroke (n = 3, 21.4) | ||||
| Yan et al. 2019 [36] | Gastrointestinal hemorrhage (n = 1, 25) | Pseudoaneurysm (n = 1, 7.1) | ||||
| Wu et al. 2021 [37] | Type I endoleak (n = 1, 25) | Type II endoleak (n = 1, 7.1) | ||||
| Higashigawa et al. 2019 [38] | 58 | 2 (3.4) | Cardiac tamponade (n = 1, 50) | 3 (5.2) | Type I endoleak (n = 2, 66.7) | 0 (0) |
| Ye et al. 2011 [39] | Pneumonia (n = 1, 50) | Type II endoleak (n = 1, 33.3) | ||||
| Qin et al. 2019 [40] | 2 | 1 (50) | Aortic valve leaflet entrapment (n = 1, 100) | 1 (50) | Endoleak (n = 1, 100) | 0 (0) |
| Vallabhajosyula et al. 2015 [41] | 2 | 1 (50) | NR | 1 (50) | New PAU (n = 1, 100) | 1 (50) |
| Khoynezhad et al. 2016 [42] | 2 | 1 (50) | Endoleak (n = 1, 100) | 2 (100) | Pneumonia (n = 1, 50) | 2 (100) |
| Bernardes at al. 2014 [43] | Pulmonary Embolism (n = 1, 50) | |||||
| Tsilimparis et al. 2019 [44] | 16 | NR | NR | NE | NE | NR |
| Ghoreishi et al. 2019 [45] | 8 | 3 (37.5) | Cardiac tamponade (n = 1, 33.3) | 1 (12.5) | Aortic regurgitation (n = 1, 100) | 0 (0) |
| Multi-organ failure (n = 1, 33.3) | ||||||
| Right common femoral artery dissection (n = 1, 33.3) |
| Author | TEVAR | Acute TAAD (n, %) | Subacute TAAD (n, %) | Chronic TAAD (n, %) | Operation Time (SD) (Minutes) | Follow-Up Time (SD) (Months) | Concomitant Procedures (n) | Mean Hospital Stay (SD) (Days) | Mean ICU Stay (SD) (Days) |
|---|---|---|---|---|---|---|---|---|---|
| Hsieh et al. 2019 [26] | 4 | 4 (100) | 0 (0) | 0 (0) | NR | 11 (NR) | 1 | NR | NR |
| Ronchey et al. 2013 [27] | 4 | 4 (100) | 0 (0) | 0 (0) | 128 (NR) | 15 (NR) | 1 | NR | NR |
| Chen et al. 2019 [28] | 4 | 4 (100) | 0 (0) | 0 (0) | NR | 12.25 (5.1) | 0 | NR | NR |
| Gao et al. 2017 [29] | 7 | NR | NR | NR | 231.6 (38.4) | 14.3 (13.4) | 4 | NR | NR |
| Wang et al. 2021 [30] | 9 | NR | NR | NR | 152.22 (17.81) | 46.5 (4.47) | NR | NR | NR |
| Mitreski et al. 2022 [31] | 10 | 9 (90) | 0 (0) | 1 | NR | 36.6 (NR) | 0 | NR | NR |
| Roselli et al. 2018 [32] | 14 | NR | NR | NR | NR | NR | 0 | NR | NR |
| Li et al. 2016 [33] | 15 | 1 (6.7) | 7 (46.7) | 7 (46.7) | 128.6 (26.2) | 62 (NR) | NR | 9.4 (2.5) | 3.3 (1) |
| Shu et al. 2012 [34] | 17 | 2 (11.8) | 0 (0) | 15 (88.2) | 82.1 (16.9) | 25.7 (17.2) | NR | NR | NR |
| Yuan et al. 2022 [35] | 19 | NR | NR | NR | NR | NR | NR | NR | NR |
| Yan et al. 2019 [36] | 20 | 20 (100) | 0 (0) | 0 (0) | 208 (NR) | 16 (NR) | NR | 13 (5) | 2 (NR) |
| Wu et al. 2021 [37] | 24 | 15 (62.5) | 0 (0) | 9 (37.5) | 237.4 (40.6) | 21.4 (6.9) | NR | 15.5 (7.1) | 1.8 (1.1) |
| Higashigawa et al. 2019 [38] | 31 | 24 (77.4) | 7 (29.1) | 0 (0) | NR | 99 (69.0) | NR | NR | NR |
| Ye et al. 2011 [39] | 45 | 30 (66.7) | 0 (0) | 15 (33.3) | NR | 35.5 (5.4) | 20 | NR | NR |
| Qin et al. 2019 [40] | 58 | 21 (36.2) | 37 (63.8) | 0 (0) | 162 (36) | 10.6 (5.4) | NR | 10 (2.6) | NR |
| Vallabhajosyula et al. 2015 [41] | 2 | 2 (100) | 0 (0) | 0 (0) | NR | NR | 0 | NR | 10 (NR) |
| Khoynezhad et al. 2016 [42] | 2 | 1 (50) | 1 (50) | 0 (0) | NR | NR | 1 | NR | NR |
| Bernardes at al. 2014 [43] | 2 | 2 (100) | 0 (0) | 0 (0) | NR | NR | 0 | NR | NR |
| Tsilimparis et al. 2019 [44] | 16 | 8 (50) | 0 (0) | 8 (50) | NE | 11 (NR) | 10 | NR | NR |
| Ghoreishi et al. 2019 [45] | 8 | 7 (87.5) | 0 (0) | 1 (12.5) | NE | 13 (13) | 0 | 8.6 (NR) | NR |
| Author | Early Complications (n, %) | Late Complications (n, %) | Endoleak (n, %) | Technical Failure (n, %) | Stroke | Reintervention (n, %) | Conversion to Open Surgery (n, %) |
|---|---|---|---|---|---|---|---|
| Hsieh et al. 2019 [26] | 1 (25%) | 2 (50) | 1 (25) | 1 (25) | 0 (0) | 1 (25) | 1 (25) |
| Ronchey et al. 2013 [27] | 0 (0) | 0 (0) | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Chen et al. 2019 [28] | 0 (0) | 3 (75) | 0 (0) | 0 (0) | 0 (0) | 2 (50) | 1 (25) |
| Gao et al. 2017 [29] | 0 (0) | 0 (0) | NR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Wang et al. 2021 [30] | 0 (0) | 2 (22.2) | 1 (11.1) | 0 (0) | 0 (0) | 1 (11.1) | 0 (0) |
| Mitreski et al. 2022 [31] | 2 (20) | 0 (0) | NR | 0 (0) | 0 (0) | 1 (10) | NR |
| Roselli et al. 2018 [32] | 6 (42.8) | 4 (28.6) | NR | 0 (0) | 2 (14.7) | NR | NR |
| Li et al. 2016 [33] | 2 (13.3) | 8 (53.3) | 1 (6.7) | 0 (0) | 0 (0) | 2 (13.1) | 1 (6.7) |
| Shu et al. 2012 [34] | 0 (0) | 1 (5.9) | NR | 0 (0) | 0 (0) | 1 (5.9) | 0 (0) |
| Yuan et al. 2022 [35] | 0 (0) | 1 (5.3) | NR | 2 (10.5) | 1 (5.3) | 1 (5.3) | 1 (5.3) |
| Yan et al. 2019 [36] | 2 (10) | 3 (15) | 3 (15) | 0 (0) | 1 (5) | 0 (0) | 0 (0) |
| Wu et al. 2021 [37] | 0 (0) | 1 (4.2) | 1 (4.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Higashigawa et al. 2019 [38] | 8 (25.8) | 9 (29.0) | 5 (16.1) | 2 (6.4) | 0 (0) | 10 (32.3) | 4 (12.8) |
| Ye et al. 2011 [39] | 4 (8.9) | 14 (31.1) | 11 (24.4) | 1 (2.2) | 3 (6.7) | 10 (22.2) | 0 (0) |
| Qin et al. 2019 [40] | 2 (3.4) | 3 (5.2) | 3 (5.2) | 5 (8.6) | 2 (3.4) | 0 (0) | 0 (0) |
| Vallabhajosyula et al. 2015 [41] | 1 (50) | 1 (50) | 2 (100) | 0 (0) | 1 (50) | 0 (0) | 0 (0) |
| Khoynezhad et al. 2016 [42] | 1 (50) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 1 (50) | 1 (50) |
| Bernardes at al. 2014 [43] | 1 (50) | 2 (100) | 1 (50) | 0 (0) | 0 (0) | 2 (100) | 2 (100) |
| Tsilimparis et al. 2019 [44] | NE | NE | NR | NR | NR | 3 (18.8) | NR |
| Ghoreishi et al. 2019 [45] | 3 (37.5) | 1 (12.5) | 0 (0) | NR | 0 (0) | 1 (12.5) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mylonas, K.S.; Zoupas, I.; Tasoudis, P.T.; Vitkos, E.; Stavridis, G.T.; Avgerinos, D.V. Endovascular Treatment of Type A Aortic Dissection: A Systematic Review and Meta-Analysis Using Reconstructed Time-to-Event Data. J. Clin. Med. 2023, 12, 7051. https://doi.org/10.3390/jcm12227051
Mylonas KS, Zoupas I, Tasoudis PT, Vitkos E, Stavridis GT, Avgerinos DV. Endovascular Treatment of Type A Aortic Dissection: A Systematic Review and Meta-Analysis Using Reconstructed Time-to-Event Data. Journal of Clinical Medicine. 2023; 12(22):7051. https://doi.org/10.3390/jcm12227051
Chicago/Turabian StyleMylonas, Konstantinos S., Ioannis Zoupas, Panagiotis T. Tasoudis, Evangelos Vitkos, George T. Stavridis, and Dimitrios V. Avgerinos. 2023. "Endovascular Treatment of Type A Aortic Dissection: A Systematic Review and Meta-Analysis Using Reconstructed Time-to-Event Data" Journal of Clinical Medicine 12, no. 22: 7051. https://doi.org/10.3390/jcm12227051
APA StyleMylonas, K. S., Zoupas, I., Tasoudis, P. T., Vitkos, E., Stavridis, G. T., & Avgerinos, D. V. (2023). Endovascular Treatment of Type A Aortic Dissection: A Systematic Review and Meta-Analysis Using Reconstructed Time-to-Event Data. Journal of Clinical Medicine, 12(22), 7051. https://doi.org/10.3390/jcm12227051






