New Challenges in Heart Failure with Reduced Ejection Fraction: Managing Worsening Events
Abstract
:1. Introduction
2. Worsening Heart Failure: A Clue to Unravel Clinical Deterioration?
2.1. An Evolving Definition
2.2. Clinical Course of WHF
2.3. Setting of Care
2.4. Subclinical Features
3. Worsening Heart Failure: Is It Time to Adopt New Strategies Alongside the “Four Pillars” to Reduce the Residual Risk of Adverse Events?
4. The Damaging Course of Heart Failure: Can We Slow the “Rolling Stone”?
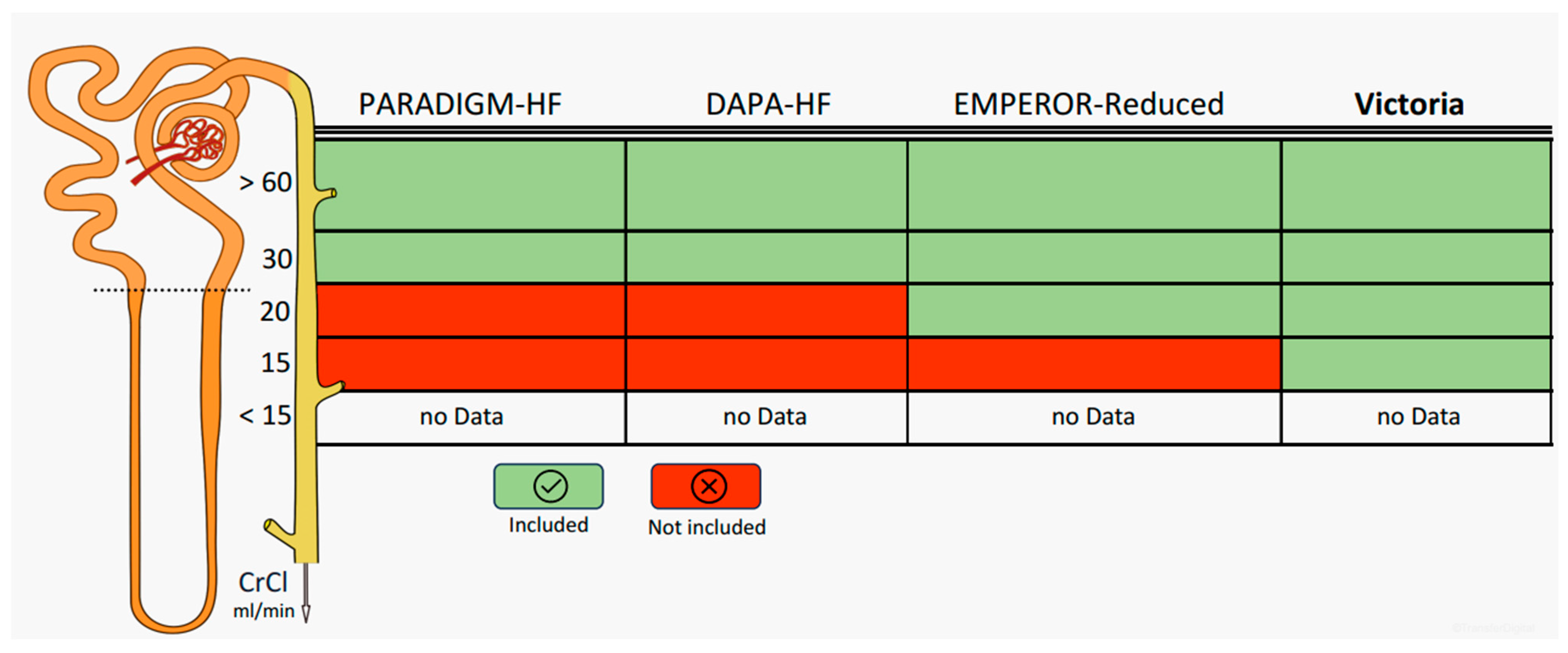
5. Worsening Renal Function: How Far Can We Go with Optimal Medical Therapy?
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greene, S.J.; Bauersachs, J.; Brugts, J.J.; Ezekowitz, J.A.; Lam, C.S.P.; Lund, L.H.; Ponikowski, P.; Voors, A.A.; Zannad, F.; Zieroth, S.; et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023, 81, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Greene, S.J.; Mentz, R.J.; Felker, G.M. Outpatient Worsening Heart Failure as a Target for Therapy: A Review. JAMA Cardiol. 2018, 3, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Braunwald, E.; Gheorghiade, M. Recognizing worsening chronic heart failure as an entity and an end point in clinical trials. JAMA 2014, 312, 789–790. [Google Scholar] [CrossRef]
- Metra, M.; Tomasoni, D.; Adamo, M.; Bayes-Genis, A.; Filippatos, G.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Antohi, L.; Böhm, M. Worsening of chronic heart failure: Definition, epidemiology, management and prevention. A clinical consensus statement by the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2023, 25, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Kristjánsdóttir, I.; Thorvaldsen, T.; Lund, L.H. Congestion and diuretic resistance in acute or worsening heart failure. Card. Fail. Rev. 2020, 6, e25. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Mulla, W.; Goldkorn, R.; Klempfner, R.; Peled, Y.; Arad, M.; Freimark, D.; Goldenberg, I. Differences in mortality of new-onset (de novo) acute heart failure versus acute decompensated chronic heart failure. Am. J. Cardiol. 2019, 124, 554–559. [Google Scholar] [CrossRef]
- Greene, S.J.; Hernandez, A.F.; Dunning, A.; Ambrosy, A.P.; Armstrong, P.W.; Butler, J.; Cerbin, L.P.; Coles, A.; Ezekowitz, J.A.; Metra, M.; et al. Hospitalization for Recently Diagnosed Versus Worsening Chronic Heart Failure: From the ASCEND-HF Trial. J. Am. Coll. Cardiol. 2017, 69, 3029–3039. [Google Scholar] [CrossRef]
- Zile, M.R.; Bennett, T.D.; St. John Sutton, M.; Cho, Y.K.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M., Jr.; Abraham, W.T.; Smart, F.W.; Stevenson, L.W.; et al. Transition from chronic compensated to acute decompensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008, 118, 1433–1441. [Google Scholar] [CrossRef]
- Bozkurt, B. Proposed new conceptualization for definition of decompensated HF. JACC Heart Fail. 2023, 11, 368–371. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2022, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Albert, N.M.; Coats, A.J.S.; Anker, S.D.; Bayes-Genis, A.; Butler, J.; Chioncel, O.; Defilippi, C.R.; Drazner, M.H.; Felker, G.M.; et al. Natriuretic peptides: Role in the diagnosis and management of heart failure: A scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur. J. Heart Fail. 2023, 25, 616–631. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef] [PubMed]
- Boorsma, E.M.; Ter Maaten, J.M.; Damman, K.; Dinh, W.; Gustafsson, F.; Goldsmith, S.; Burkhoff, D.; Zannad, F.; Udelson, J.E.; Voors, A.A. Congestion in heart failure: A contemporary look at physiology, diagnosis and treatment. Nat. Rev. Cardiol. 2020, 17, 641–655. [Google Scholar] [CrossRef]
- Metra, M.; Adamo, M.; Tomasoni, D.; Mebazaa, A.; Bayes-Genis, A.; Abdelhamid, M.; Adamopoulos, S.; Anker, S.D.; Bauersachs, J.; Belenkov, Y.; et al. Pre-discharge and early post-discharge management of patients hospitalized for acute heart failure: A scientific statement by the Heart Failure Association of the ESC. Eur. J. Heart Fail. 2023, 25, 1115–1131. [Google Scholar] [CrossRef]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef]
- Zito, A.; Princi, G.; Romiti, G.F.; Galli, M.; Basili, S.; Liuzzo, G.; Sanna, T.; Restivo, A.; Ciliberti, G.; Trani, C.; et al. Device-based remote monitoring strategies for congestion-guided management of patients with heart failure: A systematic review and meta-analysis. Eur. J. Heart Fail. 2022, 24, 2333–2341. [Google Scholar] [CrossRef]
- Rush, C.J.; Campbell, R.T.; Jhund, P.S.; Connolly, E.C.; Preiss, D.; Gardner, R.S.; Petrie, M.C.; McMurray, J.J. Falling Cardiovascular Mortality in Heart Failure with Reduced Ejection Fraction and Implications for Clinical Trials. JACC Heart Fail. 2015, 3, 603–614. [Google Scholar] [CrossRef]
- Mamas, M.A.; Sperrin, M.; Watson, M.C.; Coutts, A.; Wilde, K.; Burton, C.; Kadam, U.T.; Kwok, C.S.; Clark, A.B.; Murchie, P. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur. J. Heart Fail. 2017, 19, 1095–1104. [Google Scholar] [CrossRef]
- Butler, J.; Usman, M.S.; Anstrom, K.J.; Blaustein, R.O.; Bonaca, M.P.; Ezekowitz, J.A.; Freitas, C.; Lam, C.S.P.; Lewis, E.F.; Lindenfeld, J.; et al. Soluble guanylate cyclase stimulators in patients with heart failure with reduced ejection fraction across the risk spectrum. Eur. J. Heart Fail. 2022, 24, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Fonarow, G.C. Contextualizing Risk Among Patients with Heart Failure. JAMA 2021, 326, 2261–2262. [Google Scholar] [CrossRef] [PubMed]
- Docherty, K.F.; Jhund, P.S.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; DeMets, D.L.; Sabatine, M.S.; Bengtsson, O.; et al. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur. Heart J. 2020, 41, 2379–2392. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Claggett, B.L.; Vaduganathan, M.; Desai, A.S.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Efficacy and safety of dapagliflozin in patients with heart failure with mildly reduced or preserved ejection fraction by baseline glycaemic status (DELIVER): A subgroup analysis from an international, multicentre, double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2022, 10, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Ameri, P.; Bertero, E.; Maack, C.; Teerlink, J.R.; Rosano, G.; Metra, M. Medical treatment of heart failure with reduced ejection fraction: The dawn of a new era of personalized treatment? Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 539–546. [Google Scholar] [CrossRef]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Chaudhary, A.G.; Alreefi, F.M.; Aziz, M.A. Emerging Pharmacologic Therapies for Heart Failure with Reduced Ejection Fraction. CJC Open 2021, 3, 646–657. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Diaz, R.; Felker, G.M.; McMurray, J.J.V.; Metra, M.; Solomon, S.D.; Adams, K.F.; Anand, I.; Arias-Mendoza, A.; Biering-Sørensen, T.; et al. Cardiac Myosin Activation with Omecamtiv Mecarbil in Systolic Heart Failure. N. Engl. J. Med. 2021, 384, 105–116. [Google Scholar] [CrossRef]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, G.; Sposato, B.; Volterrani, M. Chronic heart failure: The role of di vericiguat. Eur. Heart J. Suppl. 2023, 25 (Suppl. C), C316–C318. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.M.; Kahwash, R.; Abraham, W.T. Optimizer Smart in the treatment of moderate-to-severe chronic heart failure. Future Cardiol. 2020, 1, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Kuck, K.H.; Goldsmith, R.L.; Lindenfeld, J.; Reddy, V.Y.; Carson, P.E.; Mann, D.L.; Saville, B.; Parise, H.; Chan, R. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart Fail. 2018, 6, 874–883. [Google Scholar] [CrossRef]
- Kuschyk, J.; Falk, P.; Demming, T.; Marx, O.; Morley, D.; Rao, I.; Burkhoff, D. Long-term clinical experience with cardiac contractility modulation therapy delivered by the Optimizer Smart system. Eur. J. Heart Fail. 2021, 7, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Breitenstein, S.; Roessig, L.; Sandner, P.; Lewis, K.S. Novel sGC stimulators and sGC activators for the treatment of heart failure. Novel sGC Stimulators and sGC Activators for the Treatment of Heart Failure. Handb. Exp. Pharmacol. 2017, 243, 225–247. [Google Scholar] [CrossRef]
- Dewan, P.; Jhund, P.S.; McMurray, J.J.V. VICTORIA in context. Eur. J. Heart Fail. 2020, 22, 1747–1751. [Google Scholar] [CrossRef]
- Abdin, A.; Anker, S.D.; Butler, J.; Coats, A.J.S.; Kindermann, I.; Lainscak, M.; Lund, L.H.; Metra, M.; Mullens, W.; Rosano, G.; et al. ‘Time is prognosis’ in heart failure: Time-to-treatment initiation as a modifiable risk factor. ESC Heart Fail. 2021, 8, 4444–4453. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Vitale, C.; Adamo, M.; Metra, M. Roadmap for the management of heart failure patients during the vulnerable phase after heart failure hospitalizations: How to implement excellence in clinical practice. J. Cardiovasc. Med. 2022, 23, 149–156. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N. Engl. J. Med. 2022, 387, 1185–1195. [Google Scholar] [CrossRef]
- Trulls, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Snchez-Marteles, M.; Conde-Martel, A.; Dvila-Ramos, M.F.; Llcer, P.; Salamanca-Bautista, P.; Prez-Silvestre, J.; et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur. Heart J. 2023, 44, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. B-Type Natriuretic Peptide During Treatment with Sacubitril/Valsartan: The PARADIGM-HF Trial. J. Am. Coll. Cardiol. 2019, 73, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; DeMets, D.L.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; Bengtsson, O.; Ponikowski, P.; Sabatine, M.S.; et al. A trial to evaluate the effect of the sodium-glucose co-transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF). Eur. J. Heart Fail. 2019, 21, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on the Clinical Stability of Patients with Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021, 143, 326–336. [Google Scholar] [CrossRef]
- Cohn, J.N. Vasodilators in heart failure. Conclusions from V-HeFT II and rationale for V-HeFT III. Drugs 1994, 47 (Suppl. S4), 47–57; discussion 57–58. [Google Scholar] [CrossRef]
- Lam, P.H.; Packer, M.; Fonarow, G.C.; Faselis, C.; Allman, R.M.; Morgan, C.J.; Singh, S.N.; Pitt, B.; Ahmed, A. Early Effects of Starting Doses of Enalapril in Patients with Chronic Heart Failure in the SOLVD Treatment Trial. Am. J. Med. 2020, 133, e25–e31. [Google Scholar] [CrossRef]
- Granger, C.B.; McMurray, J.J.; Yusuf, S.; Held, P.; Michelson, E.L.; Olofsson, B.; Ostergren, J.; Pfeffer, M.A.; Swedberg, K.; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: The CHARM-Alternative trial. Lancet 2003, 362, 772–776. [Google Scholar] [CrossRef]
- Cohn, J.N. Lessons learned from the valsartan- heart failure trial (Val-HeFT): Angiotensin receptor blockers in heart failure. Am. J. Cardiol. 2002, 90, 992–993. [Google Scholar] [CrossRef]
- Packer, M.; Fowler, M.B.; Roecker, E.B.; Coats, A.J.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Staiger, C.; et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: Results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002, 106, 2194–2199. [Google Scholar] [CrossRef]
- Flather, M.D.; Shibata, M.C.; Coats, A.J.; Van Veldhuisen, D.J.; Parkhomenko, A.; Borbola, J.; Cohen-Solal, A.; Dumitrascu, D.; Ferrari, R.; Lechat, P.; et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 2005, 26, 215–225. [Google Scholar] [CrossRef]
- Simko, F.; Bada, V.; Simková, M.; Simko, J.; Kovács, L.; Hulín, I. Význam aldosterónu pri chronickom zlyhaní srdca: Stúdia RALES [The significance of aldosterone in chronic heart failure: The RALES study]. Vnitr. Lek. 2002, 48, 767–772. [Google Scholar] [PubMed]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B.; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Cheema, B.; Cleveland, E.; Sankar, K.; Subacius, H.; Fonarow, G.C.; Solomon, S.D.; Lewis, E.F.; Greene, S.J.; Maggioni, A.P.; et al. Plasma renin activity, response to aliskiren, and clinical outcomes in patients hospitalized for heart failure: The ASTRONAUT trial. Eur. J. Heart Fail. 2018, 20, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Anstrom, K.J.; Felker, G.M.; Givertz, M.M.; Kalogeropoulos, A.P.; Konstam, M.A.; Mann, D.L.; Margulies, K.B.; McNulty, S.E.; Mentz, R.J.; et al. National Heart Lung and Blood Institute Heart Failure Clinical Research Network. Efficacy and Safety of Spironolactone in Acute Heart Failure: The ATHENA-HF Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Samsky, M.D.; Velazquez, E.J.; Duffy, C.I.; Gurmu, Y.; Braunwald, E.; Morrow, D.A.; DeVore, A.D. Efficacy and Safety of Sacubitril/Valsartan in High-Risk Patients in the PIONEER-HF Trial. Circ. Heart Fail. 2021, 14, e007034. [Google Scholar] [CrossRef] [PubMed]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Ponikowski, P.; Kirwan, B.A.; Anker, S.D.; McDonagh, T.; Dorobantu, M.; Drozdz, J.; Fabien, V.; Filippatos, G.; Göhring, U.M.; Keren, A.; et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomized, controlled trial. Lancet 2020, 396, 1895–1904. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Giczewska, A.; Sliwa, K.; Edelmann, F.; Refsgaard, J.; Bocchi, E.; Ezekowitz, J.A.; Hernandez, A.F.; O’Connor, C.M.; Roessig, L.; et al. Clinical Outcomes and Response to Vericiguat According to Index Heart Failure Event: Insights from the VICTORIA Trial. JAMA Cardiol. 2021, 6, 706–712. [Google Scholar] [CrossRef]
- Rao, V.N.; Diez, J.; Gustafsson, F.; Mentz, R.J.; Senni, M.; Jankowska, E.A.; Bauersachs, J. Practical Patient Care Considerations with Use of Vericiguat After Worsening Heart Failure Events. J. Card. Fail. 2023, 29, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Bauersachs, J.; Brugts, J.J.; Ezekowitz, J.A.; Filippatos, G.; Gustafsson, F.; Lam, C.S.P.; Lund, L.H.; Mentz, R.J.; Pieske, B.; et al. Management of Worsening Heart Failure with Reduced Ejection Fraction: JACC Focus Seminar 3/3. J. Am. Coll. Cardiol. 2023, 82, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomized, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, G.; Sciatti, E.; D’isa, S.; D’elia, E.; Senni, M. Heart failure therapy: The fifth card. Eur. Heart J. Suppl. 2023, 25 (Suppl. B), B140–B143. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 2219–2229. [Google Scholar] [CrossRef]
- Pitt, B.; Filippatos, G.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Rossing, P.; Joseph, A.; Kolkhof, P.; Nowack, C.; Schloemer, P.; et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 2252–2263. [Google Scholar] [CrossRef]
- Di Lullo, L.; Lavalle, C.; Scatena, A.; Mariani, M.V.; Ronco, C.; Bellasi, A. Finerenone: Questions and Answers-The Four Fundamental Arguments on the New-Born Promising Non-Steroidal Mineralocorticoid Receptor Antagonist. J. Clin. Med. 2023, 12, 3992. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of kidney function throughout the heart failure trajectory—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef]
- Ouwerkerk, W.; Voors, A.A.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; van der Harst, P.; Hillege, H.L.; Lang, C.C.; Ter Maaten, J.M.; et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: A prospective European study. Eur. Heart J. 2017, 38, 1883–1890. [Google Scholar] [CrossRef]
- Voors, A.A.; Mulder, H.; Reyes, E.; Cowie, M.R.; Lassus, J.; Hernandez, A.F.; Ezekowitz, J.A.; Butler, J.; O’Connor, C.M.; Koglin, J.; et al. Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: Insights from VICTORIA (Vericiguat Global Study in Subjects with HFrEF) trial. Eur. J. Heart Fail. 2021, 23, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R.; Guglin, M.E.; Saltzberg, M.T.; Jessup, M.L.; Bart, B.A.; Teerlink, J.R.; Jaski, B.E.; Fang, J.C.; Feller, E.D.; Haas, G.J.; et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J. Am. Coll. Cardiol. 2007, 49, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.R.; Negoianu, D.; Jaski, B.E.; Bart, B.A.; Heywood, J.T.; Anand, I.S.; Smelser, J.M.; Kaneshige, A.M.; Chomsky, D.B.; Adler, E.D.; et al. Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Heart Fail. 2016, 4, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Vishram-Nielsen, J.K.K.; Pagnesi, M.; Adamo, M.; Lombardi, C.M.; Gustafsson, F.; Metra, M. Advanced heart failure: Guideline-directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Fail. 2022, 9, 1507–1523. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Greene, S.J.; Givertz, M.M.; Vader, J.M.; Starling, R.C.; Ambrosy, A.P.; Shah, P.; McNulty, S.E.; Mahr, C.; Gupta, D.; et al. Sacubitril/Valsartan in Advanced Heart Failure with Reduced Ejection Fraction: Rationale and Design of the LIFE Trial. JACC Heart Fail. 2020, 8, 789–799. [Google Scholar] [CrossRef]
- Moliner-Abós, C.; Rivas-Lasarte, M.; Pamies Besora, J.; Fluvià-Brugues, P.; Solé-González, E.; Mirabet, S.; López López, L.; Brossa, V.; Pirla, M.J.; Mesado, N.; et al. Sacubitril/Valsartan in Real-Life Practice: Experience in Patients with Advanced Heart Failure and Systematic Review. Cardiovasc. Drugs Ther. 2019, 33, 307–314. [Google Scholar] [CrossRef]

| Clinical Trial | Drug | Inclusion Criteria |
|---|---|---|
| PIONEER-HF (881 pts) | sacubitril/valsartan vs. enalapril | Currently hospitalized for a primary diagnosis of HF, including symptoms and signs of fluid overload; randomized no earlier than 24 h and up to 10 d after initial presentation while still hospitalized; stable as defined by an SBP > 100 mm Hg for the preceding 6 h in the absence of symptomatic hypotension, no increase (i.e., intensification) in IV diuretics or use of IV vasodilators within the last 6 h, and no IV inotropes for 24 h prior to randomization |
| AFFIRM-HF (1110 pts) | ferric carboxymaltose vs. placebo | Hospitalized with clinical signs, symptoms, and biomarkers consistent with AHF. During the index hospitalization, patients had to have received at least 40 mg of IV furosemide |
| VICTORIA (5050 pts) | vericiguat vs. placebo | Evidence of WHF (hospitalized within 6 months before randomization) or receiving intravenous diuretic therapy, without hospitalization, within the previous 3 months |
| GALACTIC-HF (8256 pts) | omecamtiv mecarbil vs. placebo | Currently hospitalized for HF (inpatients) or had either made an urgent visit to the emergency department or been hospitalized for heart failure within 1 year before screening (outpatients). 18 < age < 85 |
| SOLOIST-WHF (1222 TDM2 pts) | sotaglifozin vs. placebo | Hospitalized because of the presence of signs and symptoms of HF and received treatment with intravenous diuretic therapy. 18 < age < 85 |
| EMPULSE (530 pts) | empaglifozin vs. placebo | Admitted to the hospital for AHF after initial stabilization (SBP ≥ 100 mmHg and no symptoms of hypotension in the preceding 6 h, no increase in i.v. diuretic dose for 6 h prior to randomization, no i.v. vasodilators including nitrates within the last 6 h prior to randomization, no i.v. inotropic drugs for 24 h prior to randomization) |
| ADVOR (519 pts) | iv acetazolamide vs. placebo | Hospitalized for acute decompensed HF with clinical signs of fluid overload treated with iv loop diuretics (iv dose twice the oral manteinance dose) |
| CLOROTIC (230 pts) | hydrochlorothiazide vs. placebo | Hospitalized ≤ 24 h for acute decompensed HF, treatment with an oral loop diuretic ≥ 1 months before hospitalization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavalle, C.; Di Lullo, L.; Jabbour, J.P.; Palombi, M.; Trivigno, S.; Mariani, M.V.; Summaria, F.; Severino, P.; Badagliacca, R.; Miraldi, F.; et al. New Challenges in Heart Failure with Reduced Ejection Fraction: Managing Worsening Events. J. Clin. Med. 2023, 12, 6956. https://doi.org/10.3390/jcm12226956
Lavalle C, Di Lullo L, Jabbour JP, Palombi M, Trivigno S, Mariani MV, Summaria F, Severino P, Badagliacca R, Miraldi F, et al. New Challenges in Heart Failure with Reduced Ejection Fraction: Managing Worsening Events. Journal of Clinical Medicine. 2023; 12(22):6956. https://doi.org/10.3390/jcm12226956
Chicago/Turabian StyleLavalle, Carlo, Luca Di Lullo, Jean Pierre Jabbour, Marta Palombi, Sara Trivigno, Marco Valerio Mariani, Francesco Summaria, Paolo Severino, Roberto Badagliacca, Fabio Miraldi, and et al. 2023. "New Challenges in Heart Failure with Reduced Ejection Fraction: Managing Worsening Events" Journal of Clinical Medicine 12, no. 22: 6956. https://doi.org/10.3390/jcm12226956
APA StyleLavalle, C., Di Lullo, L., Jabbour, J. P., Palombi, M., Trivigno, S., Mariani, M. V., Summaria, F., Severino, P., Badagliacca, R., Miraldi, F., Bellasi, A., & Vizza, C. D. (2023). New Challenges in Heart Failure with Reduced Ejection Fraction: Managing Worsening Events. Journal of Clinical Medicine, 12(22), 6956. https://doi.org/10.3390/jcm12226956







