The Significance of Preoperative Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Systemic Inflammatory Index (SII) in Predicting Severity and Adverse Outcomes in Acute Calculous Cholecystitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. General Data of the Patients Included in the Study Group
3.2. Comparative Analysis of NLR, PLR, and SII Values with TG 13/18 Grading in the Study Group
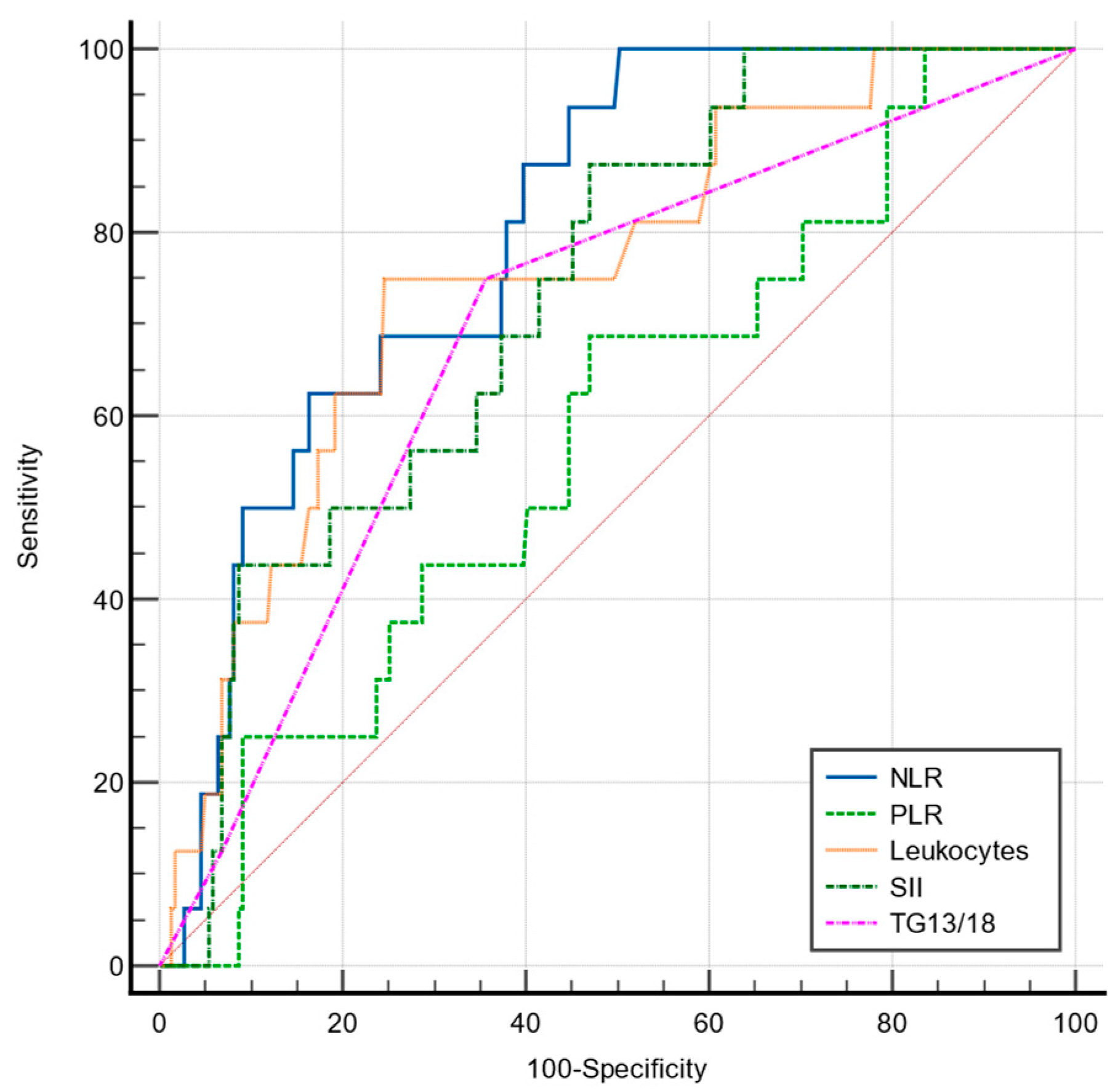
3.3. Prediction Value of NLR, PLR, SII, and TG 13/18 Grading Scale for Advanced Acute Cholecystitis
3.4. Surgical Approach and Postoperative Outcomes
3.5. Correlations between Inflammatory Parameters and Types of Surgery in the Study Group
3.6. Correlations between Inflammatory Parameters and Postoperative Outcomes in the Study Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sartelli, M.; Abu-Zidan, F.M.; Catena, F.; Griffiths, E.A.; Di Saverio, S.; Coimbra, R.; Ordoñez, C.A.; Leppaniemi, A.; Fraga, G.P.; Coccolini, F. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: A prospective multicentre study (WISS Study). World J. Emerg. Surg. 2015, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Junior, C.S.; Di Saverio, S.; Sartelli, M.; Kelly, M.D.; Gomes, C.C.; Gomes, F.C.; Corrêa, L.D.; Alves, C.B.; Guimarãesm, S.F. Acute calculous cholecystitis: Review of current best practices. World J. Gastrointest. Surg. 2017, 9, 118–126, Erratum in World J. Gastrointest. Surg. 2017, 9, 214. [Google Scholar] [CrossRef]
- Tufo, A.; Pisano, M.; Ansaloni, L.; de Reuver, P.; van Laarhoven, K.; Davidson, B.; Gurusamy, K.S. Risk Prediction in Acute Calculous Cholecystitis: A Systematic Review and Meta-Analysis of Prognostic Factors and Predictive Models. J. Laparoendosc. Adv. Surg. Technol. A 2021, 31, 41–53. [Google Scholar] [CrossRef]
- Lucocq, J.; Patil, P.; Scollay, J. Acute cholecystitis: Delayed cholecystectomy has lesser perioperative morbidity compared to emergency cholecystectomy. Surgery 2022, 172, 16–22. [Google Scholar] [CrossRef]
- Bouassida, M.; Zribi, S.; Krimi, B.; Laamiri, G.; Mroua, B.; Slama, H.; Mighri, M.M.; M’saddak Azzouz, M.; Hamzaoui, L.; Touinsi, H. C-reactive Protein Is the Best Biomarker to Predict Advanced Acute Cholecystitis and Conversion to Open Surgery. A Prospective Cohort Study of 556 Cases. J. Gastrointest. Surg. 2020, 24, 2766–2772. [Google Scholar] [CrossRef]
- Turhan, V.B.; Gök, H.F.; Ünsal, A.; Akpınar, M.; Güler Şimşek, G.; Buluş, H. Pre-operative neutrophil/lymphocyte and platelet/lymphocyte ratios are effective in predicting complicated acute cholecystitis. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Díez Ares, J.Á.; Martínez García, R.; Estellés Vidagany, N.; Peris Tomás, N.; Planells Roig, M.; Valenzuela Gras, M.; Ripollés González, T. Can inflammatory biomarkers help in the diagnosis and prognosis of gangrenous acute cholecystitis? A prospective study. Rev. Esp. Enfermedades Dig. 2021, 113, 41–44. [Google Scholar] [CrossRef]
- Yokoe, M.; Takada, T.; Strasberg, S.M.; Solomkin, J.S.; Mayumi, T.; Gomi, H.; Pitt, H.A.; Garden, O.J.; Kiriyama, S.; Hata, J.; et al. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2013, 20, 35–46. [Google Scholar] [CrossRef]
- Yokoe, M.; Hata, J.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Wakabayashi, G.; Kozaka, K.; Endo, I.; Deziel, D.J.; Miura, F.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepato-Biliary-Pancreat. Sci. 2018, 25, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Jessica Mok, K.W.; Goh, Y.L.; Howell, L.E.; Date, R.S. Is C-reactive protein the single most useful predictor of difficult laparoscopic cholecystectomy or its conversion? A pilot study. J. Minimal Access Surg. 2016, 12, 26–32. [Google Scholar] [CrossRef]
- Wevers, K.P.; van Westreenen, H.L.; Patijn, G.A. Laparoscopic cholecystectomy in acute cholecystitis: C-reactive protein level combined with age predicts conversion. Surg. Laparosc. Endosc. Percutan. Technol. 2013, 23, 163–166. [Google Scholar] [CrossRef]
- Díaz-Flores, A.; Cárdenas-Lailson, E.; Cuendis-Velázquez, A.; Rodríguez-Parra, A.; Trejo-Ávila, M.E. C-Reactive Protein as a Predictor of Difficult Laparoscopic Cholecystectomy in Patients with Acute Calculous Cholecystitis: A Multivariate Analysis. J. Laparoendosc. Adv. Surg. Technol. Part A 2017, 27, 1263–1268. [Google Scholar] [CrossRef]
- Vural, S.; Aydin, I.; Kesicioglu, T. Association of Serum C-Reactive Protein Level and Treatment Duration in Acute Cholecystitis Patients Treated Conservatively. Cureus 2022, 14, e22146. [Google Scholar] [CrossRef]
- Çeliktürk, E.; Salt, Ö.; Sayhan, M.B.; Dıbırdık, İ. A novel biomarker in acute cholecystitis: YKL-40. Asian J. Surg. 2023, 46, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kim, O.H.; Lee, S.C.; Kim, K.H.; Hong, H.E.; Seo, H.; Choi, H.J.; Kim, S.J. Serum level of visfatin can reflect the severity of inflammation in patients with acute cholecystitis. Ann. Surg. Treat. Res. 2020, 99, 26–36. [Google Scholar] [CrossRef]
- Yaow, C.Y.L.; Chong, R.I.H.; Chan, K.S.; Chia, C.T.W.; Shelat, V.G. Should Procalcitonin Be Included in Acute Cholecystitis Guidelines? A Systematic Review. Medicina 2023, 59, 805. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yue, Z.; Weng, Y.; Zhen, G. Diagnostic value of ROC curve evaluation of serum markers in acute cholecystitis with bacterial infection. J. Pak. Med. Assoc. 2022, 72, 1133–1136. [Google Scholar] [CrossRef]
- Nechita, V.I.; Hajjar, N.A.; Drugan, C.; Cătană, C.-S.; Moiş, E.; Nechita, M.-A.; Graur, F. Chitotriosidase and Neopterin as Potential Biomarkers for the Evaluation of Complicated Cholecystitis—A Pilot Study. J. Clin. Med. 2023, 12, 1641. [Google Scholar] [CrossRef]
- Modica, R.; Minotta, R.; Liccardi, A.; Cannavale, G.; Benevento, E.; Colao, A. Evaluation of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR) and Systemic Immune–Inflammation Index (SII) as Potential Biomarkers in Patients with Sporadic Medullary Thyroid Cancer (MTC). J. Pers. Med. 2023, 13, 953. [Google Scholar] [CrossRef]
- Xia, W.; Tan, Y.; Hu, S.; Li, C.; Jiang, T. Predictive Value of Systemic Immune-Inflammation index and Neutrophil-to-Lymphocyte Ratio in Patients with Severe COVID-19. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221111391. [Google Scholar] [CrossRef]
- Serban, D.; Papanas, N.; Dascalu, A.M.; Kempler, P.; Raz, I.; Rizvi, A.A.; Rizzo, M.; Tudor, C.; Silviu Tudosie, M.; Tanasescu, D.; et al. Significance of Neutrophil to Lymphocyte Ratio (NLR) and Platelet Lymphocyte Ratio (PLR) in Diabetic Foot Ulcer and Potential New Therapeutic Targets. Int. J. Low Extrem. Wounds 2021, 15347346211057742. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, S.A.; Nabipoorashrafi, S.A.; Hernandez, J.; Nguyen, A.; Lucke-Wold, B.; Nourigheimasi, S.; Khanzadeh, S. Neutrophil to Lymphocyte Ratio and Spontaneous Bacterial Peritonitis among Cirrhotic Patients: A Systematic Review and Meta-analysis. Can. J. Gastroenterol. Hepatol. 2022, 2022, 8604060. [Google Scholar] [CrossRef] [PubMed]
- Hajibandeh, S.; Hajibandeh, S.; Hobbs, N.; Mansour, M. Neutrophil-to-lymphocyte ratio predicts acute appendicitis and distinguishes between complicated and uncomplicated appendicitis: A systematic review and meta-analysis. Am. J. Surg. 2020, 219, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Ertao, Z.; Zhimei, Z.; Weigang, D.; Jianjun, P.; Jianhui, C.; Chuangqi, C. Systemic immune-inflammation index (SII): A More Promising Inflammation-Based Prognostic Marker for Patients with synchronic colorectal peritoneal carcinomatosis. J. Cancer 2020, 11, 5264–5272. [Google Scholar] [CrossRef] [PubMed]
- Micić, D.; Stanković, S.; Lalić, N.; Đukić, V.; Polovina, S. Prognostic Value of Preoperative Neutrophil-to-lymphocyte Ratio for Prediction of Severe Cholecystitis. J. Med. Biochem. 2018, 37, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Safari, S.; Baratloo, A.; Elfil, M.; Negida, A. Evidence Based Emergency Medicine; Part 5 Receiver Operating Curve and Area under the Curve. Emergency 2016, 4, 111–113. [Google Scholar]
- Er, S.; Ozden, S.; Celik, C.; Yuksel, B.C. Can we predict severity of acute cholecystitis at admission? Pak. J. Med. Sci. 2018, 34, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Aykota, M.R.; Ozgen, U.; Birsen, O.; Simsek, S.; Kabay, B. Might simple peripheral blood parameters be an early indicator in the prediction of severity and morbidity of cholecystitis? Ann. Surg. Treat. Res. 2023, 104, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.H.; Liu, Y.H.; Chen, W.K.; Huang, F.W.; Hsu, T.Y.; Cheng, H.T.; Hsueh, P.R.; Hsiao, C.T.; Wu, S.Y.; Shih, H.M. Value of monocyte distribution width for predicting severe cholecystitis: A retrospective cohort study. Clin. Chem. Lab. Med. 2023, 61, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Khataniar, H.; Ck, A.; Rao, H.K. Preoperative scoring system validation and analysis of associated risk factors in predicting difficult laparoscopic cholecystectomy in patients with acute calculous cholecystitis: A prospective observational study. Turk. J. Surg. 2022, 38, 375–381. [Google Scholar] [CrossRef]
- Önder, A.; Kapan, M.; Ülger, B.V.; Oğuz, A.; Türkoğlu, A.; Uslukaya, Ö. Gangrenous cholecystitis: Mortality and risk factors. Int. Surg. 2015, 100, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.; Hasan, M. The Accuracy of Neutrophil-to-Lymphocyte Ratio and Abdominal Computed Tomography to Predict the Severity of Acute Cholecystitis. Cureus 2022, 14, e32243. [Google Scholar] [CrossRef]
- Gojayev, A.; Karakaya, E.; Erkent, M.; Yücebaş, S.C.; Aydin, H.O.; Kavasoğlu, L.; Aydoğan, C.; Yildirim, S. A novel approach to distinguish complicated and non-complicated acute cholecystitis: Decision tree method. Medicine 2023, 102, e33749. [Google Scholar] [CrossRef] [PubMed]
- Ünal, Y.; Tuncal, S.; Küçük, B.; Barlas, A.M.; Altıner, S.; Balık, R.; Aydın, S.M.; Senlikci, A.; Pekcici, M.R. An effective and reliable marker in gradıng the severity of acute cholecystitis: Increased immature granulocyte percentage. Akut kolesistitin şiddetini derecelendirmede etkili ve güvenilir bir belirteç: Artmış immatür granülosit yüzdesi. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 1716–1722. [Google Scholar] [CrossRef]
- Sato, N.; Kinoshita, A.; Imai, N.; Akasu, T.; Yokota, T.; Iwaku, A.; Koike, K.; Saruta, M. Inflammation-based prognostic scores predict disease severity in patients with acute cholecystitis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 484–489. [Google Scholar] [CrossRef]
- Cakcak, İ.E.; Kula, O. Predictive evaluation of SIRI, SII, PNI, and GPS in cholecystostomy application in patients with acute cholecystitis. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 940–946. [Google Scholar] [CrossRef]
- Beliaev, A.M.; Angelo, N.; Booth, M.; Bergin, C. Evaluation of neutrophil-to-lymphocyte ratio as a potential biomarker for acute cholecystitis. J. Surg. Res. 2017, 209, 93–101. [Google Scholar] [CrossRef]
- Kartal, M.; Kalaycı, T. Can neutrophil-lymphocyte ratio, platelet-lymphocyte ratio, prognostic nutrition index, and albumin be used to predict cholecystectomy morbidity in super-elderly patients? Ulus. Travma Acil Cerrahi Derg. 2023, 29, 890–896. [Google Scholar] [CrossRef]
- Manuel-Vázquez, A.; Latorre-Fragua, R.; Alcázar, C.; Requena, P.M.; de la Plaza, R.; Blanco Fernández, G.; Serradilla-Martín, M.; Ramia, J.M.; SCORELAP Group. Reaching a consensus on the definition of “difficult” cholecystectomy among Spanish experts. A Delphi project. A qualitative study. Int. J. Surg. 2022, 102, 106649. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Savlovschi, C.; Borcan, R.; Pantu, H.; Serban, D.; Gradinaru, S.; Smarandache, G.; Trotea, T.; Branescu, C.; Musat, L.; et al. Clinical case—voluminous diaphragmatic hernia—surgically acute abdomen: Diagnostic and therapeutical challenges. Chirurgia 2011, 106, 657–660. [Google Scholar] [PubMed]
- Savlovschi, C.; Serban, D.; Trotea, T.; Borcan, R.; Dumitrescu, D. Post-surgery morbidity and mortality in colorectal cancer in elderly subjects. Chirurgia 2013, 108, 177–179. [Google Scholar] [PubMed]
- Serban, D.; Badiu, D.C.; Davitoiu, D.; Tanasescu, C.; Tudosie, M.S.; Sabau, A.D.; Dascalu, A.M.; Tudor, C.; Balasescu, S.A.; Socea, B.; et al. Systematic review of the role of indocyanine green near-infrared fluorescence in safe laparoscopic cholecystectomy (Review). Exp. Ther. Med. 2022, 23, 187. [Google Scholar] [CrossRef]
- Pisano, M.; Allievi, N.; Gurusamy, K.; Borzellino, G.; Cimbanassi, S.; Boerna, D.; Coccolini, F.; Tufo, A.; Di Martino, M.; Leung, J.; et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World J. Emerg. Surg. 2020, 15, 61. [Google Scholar] [CrossRef]
- Karamouzos, V.; Paraskevas, T.; Mulita, F.; Karteri, S.; Oikonomou, E.; Ntoulias, N.; Pantzaris, N.D.; Bourganou, V.; Velissaris, D. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Percentage Ratio as Predictors of In-hospital Mortality in Sepsis. An Observational Cohort Study. Mater. Socio-Medica 2022, 34, 33–36. [Google Scholar] [CrossRef]
- Boicean, A.; Neamtu, B.; Birsan, S.; Batar, F.; Tanasescu, C.; Dura, H.; Roman, M.D.; Hașegan, A.; Bratu, D.; Mihetiu, A.; et al. Fecal Microbiota Transplantation in Patients Co-Infected with SARS-CoV2 and Clostridioides difficile. Biomedicines 2023, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Huang, X.; Zhang, W. Platelet-to-lymphocyte ratio as a prognostic predictor of mortality for sepsis: Interaction effect with disease severity-a retrospective study. BMJ Open 2019, 9, e022896. [Google Scholar] [CrossRef]
- Mansour, N.O.; Boraii, S.; Elnaem, M.H.; Elrggal, M.E.; Omar, T.; Abdelraouf, A.; Abdelaziz, D.H. Evaluation of preoperative duloxetine use for postoperative analgesia following laparoscopic cholecystectomy: A randomized controlled trial. Front. Pharmacol. 2022, 13, 944392. [Google Scholar] [CrossRef]
- Chicea, R.; Bratu, D.; Chicea, A.L.; Mihetiu, A.; Preluca, V.; Tantar, C.; Sava, M. A comparative Histologic and Immunohistochemistry Evaluation Between Normal Aponeurotic Tissue, Fibrotic Aponeurotic Scars and Polypropylene Embedded Aponeurotic Scars. Mater. Plast. 2017, 54, 510–512. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, L.; Zhao, H.; Fu, H.; Li, J.; Yu, A. The enhanced recovery after surgery (ERAS) protocol in elderly patients with acute cholecystitis: A retrospective study. Medicine 2023, 102, e32942. [Google Scholar] [CrossRef]
- Mulita, F.; Karpetas, G.; Liolis, E.; Vailas, M.; Tchabashvili, L.; Maroulis, I. Comparison of analgesic efficacy of acetaminophen monotherapy versus acetaminophen combinations with either pethidine or parecoxib in patients undergoing laparoscopic cholecystectomy: A randomized prospective study. Med. Glas. 2021, 18, 27–32. [Google Scholar] [CrossRef]
- Kouroukli, I.; Zompolas, V.; Tsekoura, V.; Papazoglou, I.; Louizos, A.; Panaretou, V. Comparison between lornoxicam quick-release and parecoxib for post-operative analgesia after laparoscopic cholecystectomy: A prospective randomized, placebo-controlled trial. J. Anaesthesiol. Clin. Pharmacol. 2013, 29, 485–490. [Google Scholar] [CrossRef]


| Parameter | Total | Mild AC | Advanced AC | p Value |
| No. of patients | 235 | 166 (70.6%) | 69 (29.4%) | |
| Females | 168 (71.4%) | 127 (76.5%) | 41 (59.4%) | 0.008 1 |
| Age | 54.6 ± 16.3 | 52 ± 15.9 | 61 ± 15.6 | <0.001 1 |
| Comorbidities (No.): Obesity Arterial hypertension Cardiac ischemic disease Chronic hepatic diseases Chronic respiratory diseases Chronic renal diseases Cardiac failure/shock Diabetes Others | 2 ± 1.4 | 1.8 ± 1.3 | 2.6 ± 1.6 | <0.001 2 |
| 116 (49.4%) | 75 (45.1%) | 41 (59.4%) | 0.047 2 | |
| 107 (45.5%) | 57 (53.3%) | 50 (46.7%) | 0.093 2 | |
| 28 (11.9%) | 14 (8.4%) | 14 (20.2%) | 0.01 2 | |
| 67 (28.5%) | 40 (24%) | 27 (39.1%) | 0.02 2 | |
| 31 (13.2%) | 18 (10.8%) | 13 (18.8%) | 0.099 2 | |
| 39 (16.6%) | 25 (15%) | 14 (20.2%) | 0.327 2 | |
| 11 (4.7%) | 4 (2.4%) | 7 (10.1%) | 0.01 2 | |
| 32 (13.6%) | 15 (9%) | 17 (24.6%) | 0.001 2 | |
| 84 (35.7%) | 62 (37.3%) | 22 (31.8%) | 0.426 2 | |
| ASA PS risk scale I II III IV V | 0.008 2 (0.0003 3 for trend) | |||
| 16 (6.8%) | 14 (8.4%) | 2 (2.8%) | ||
| 124 (52.8%) | 96 (57.8%) | 28 (40.5%) | ||
| 78 (33.2%) | 48 (28.9%) | 30 (43.4%) | ||
| 16 (6.8%) | 8 (4.8%) | 8 (11.5%) | ||
| 1 (0.4%) | 0 | 1 (1.4%) | ||
| TG 13/18 severity grading I II III | <0.0001 2 (<0.0001 3 for trend) | |||
| 145 (61.7%) | 121 (72.9%) | 24 (37.4%) | ||
| 73 (31.1%) | 40 (24%) | 33 (47.8%) | ||
| 17 (7.2%) | 5 (3%) | 12 (17.4%) | ||
| Angiocholitis/CBD stones | 18 (7.6%) | 7 (4.2%) | 11 (15.9%) | 0.013 2 |
| Leukocytes (/μL) | 10,441 ± 4895.3 | 9187.6 ± 3787.4 | 13,456.2 ± 5882 | <0.0001 1 |
| Neutrophils (/μL) | 7796 ± 4867.5 | 6413.4 ± 3728.6 | 11,124.9 ± 5646.6 | 0.001 1 |
| Platelets (/μL) | 239,767.1 ± 82,016.7 | 245,341.4 ± 77,532.9 | 226,356.5 ± 91,122 | 0.053 1 |
| Fibrinogen (mg/dL) | 450.1 ± 186.2 | 389.1 ± 119.1 | 596.8 ± 232.3 | <0.001 1 |
| INR | 1.3 ± 1.1 | 1.2 ± 0.9 | 1.3 ± 1.2 | 0.327 1 |
| Bilirubin | 1.3 ± 2.0 | 0.95 ± 1.1 | 2.3 ± 3.1 | <0.001 1 |
| AST | 68.9 ± 116 | 63.2 ± 116.2 | 125 ± 297.1 | 0.063 1 |
| ALT | 107.4 ± 165.9 | 85.8 ± 136.9 | 120 ± 179 | 0.056 1 |
| Creatinine | 1.3 ± 0.5 | 1.2 ± 0.3 | 1.5 ± 1.4 | 0.341 1 |
| NLR | 7.29 ± 12.2 | 4.3 ± 5.2 | 14.3 ± 19.4 | <0.001 1 |
| PLR | 181.2 ± 229.4 | 143.8 ± 68.7 | 273.5 ± 397 | <0.001 1 |
| SII | 1701.6 ± 3416.4 | 1009.5 ± 993.8 | 3366.8 ± 5812.4 | <0.001 1 |
| TG13/18 Grade I (1) | TG13/18 Grade II (2) | TG13/18 Grade III (3) | p Value (Chi-Squared Test) | Scheffe Test for Pairwise Comparison | |
|---|---|---|---|---|---|
| NLR | 3.6 ± 3 | 11.7 ± 14.6 | 18.8 ± 28 | <0.001 | (1) differs from (2) and (3) |
| PLR | 147.8 ± 80.3 | 191.2 ± 123.6 | 432.1 ± 752 | <0.001 | (1) and (2) differ from (3) |
| SII | 879.9 ± 726.5 | 2393.6 ± 2477 | 5738.6 ± 10,617 | <0.001 | Each group differs significantly from the others. |
| Sensitivity | Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 85.5 | 66.9 | >4.19 | 0.824 | <0.001 |
| PLR | 49.3 | 83.1 | >189.3 | 0.679 | <0.001 |
| SII | 63.8 | 80.7 | >1442.4 | 0.787 | <0.001 |
| TG13/18 | 65.22 | 72.8 | >1(mild) | 0.704 | <0.001 |
| Leukocytes | 60.87 | 75.9 | >11,300 | 0.741 | <0.001 |
| Total (n = 235) | Mild AC (n = 166) | Advanced AC (n = 69) | p Value | |
|---|---|---|---|---|
| Types of surgery: LC LC-conversion CC Cholecystostomy | <0.0001 1 (<0.0001 2 for trend) | |||
| 208 (88.6%) | 162 (97.6%) | 46 (66.6%) | ||
| 16 (6.8%) | 1 (0.6%) | 15 (21.7%) | ||
| 10 (4.2%) | 3 (1.8%) | 7 (10.2%) | ||
| 1 (0.4%) | 0 | 1 (1.5%) | ||
| Kehr drainage | 5 (2.1%) | 1 (0.6%) | 4 (5.8%) | 0.012 1 |
| ERCP + calculi removal (pre or postop) | 17 (7.2%) | 7 (4.2%) | 10 (14.5%) | 0.005 1 |
| Postoperative hospital stay (days) | 3.6 ± 3.4 | 2.9 ± 2.8 | 5.1 ± 4 | <0.001 3 |
| Length of stay (days) | 7.1 ± 4.5 | 6.1 ± 3.9 | 9.3 ± 5.2 | <0.001 3 |
| Total (n = 235) | Mild AC (n = 166) | Advanced AC (n = 69) | p-Value * | |
|---|---|---|---|---|
| I (surgical site infections) | 4 (1.7%) | 1 (0.6%) | 3 (4.3%) | 0.043 |
| II (requiring pharmacological treatment) surgical-related complications, treated conservatory Nosocomial infections | ||||
| 11 (4.6%) | 5 (3%) | 6 (8.6%) | 0.064 | |
| 15 (6.4%) | 6 (3.6%) | 9 (13%) | 0.007 | |
| III (surgical-related complications requiring endoscopic/surgical/Rx approach) | 2 (0.8%) | 1 (0.6%) | 1 (1.4%) | 1.00 |
| IV (general complications requiring intensive care) Malign hypertension Hemodynamic instability Sepsis Pulmonary edema/pleurisy | 16 (6.8%) | 4 (2.4%) | 12 (17.3%) | 0.002 |
| 4 (1.7%) | 1 (0.6%) | 3 (4.3%) | 0.043 | |
| 1 (0.4%) | 0 | 1 (1.4%) | 0.12 | |
| 8 (3.4%) | 2 (1.2%) | 6 (8.6%) | 0.004 | |
| 3 (1.3%) | 1 (0.6%) | 2 (2.8%) | 0.15 | |
| V (deceased) | 5 (2.1%) | 2 (1.2%) | 3 (4.3%) | 0.129 |
| LC (1) | LC-Conversion (2) | CC/Cholecystostomy (3) | p-Value | Scheffe Test for Pairwise Comparison | |
|---|---|---|---|---|---|
| NLR | 6.2 ± 11.8 | 12.1 ± 7 | 20.2 ± 17.3 | 0.001 1 | (1) differs from (3) |
| PLR | 178.5 ± 241.9 | 182 ± 82.7 | 248.1 ± 73.4 | 0.823 1 | NS |
| SII | 1583.8 ± 3572.4 | 2323.2 ± 1474.2 | 3014.5 ± 1779.5 | 0.49 1 | NS |
| TG 13/18 grading | <0.0001 2 | Each group differs significantly from the others | |||
| I | 139 (66.9%) | 4 (25%) | 2 (18.2%) | ||
| II | 58 (27.9%) | 10 (62.5%) | 5 (45.4%) | ||
| III | 11 (5.2%) | 2 (12.5%) | 4 (36.4%) |
| PDR Sensitivity | PDR Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
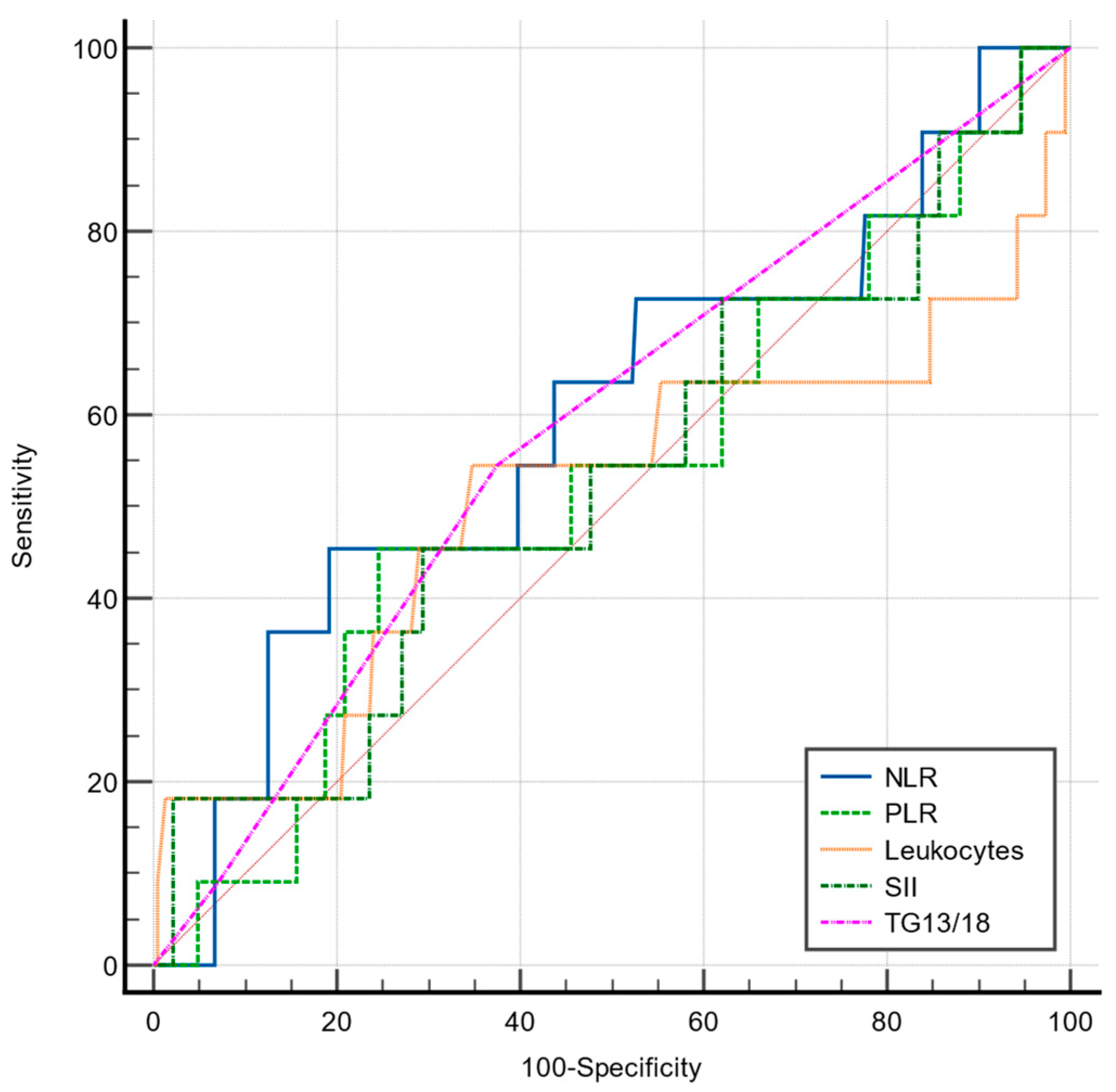
| NLR | 93.7 | 55.2 | >4.24 | 0.802 | <0.001 |
| PLR | 68.7 | 53 | >141.8 | 0.582 | 0.246 |
| SII | 87.5 | 53 | >949.6 | 0.734 | <0.001 |
| TG13/18 | 75 | 64.3 | >1 | 0.690 | 0.001 |
| Leukocytes | 75 | 75.3 | >12,200 | 0.755 | <0.001 |
| PDR Sensitivity | PDR Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 45.45 | 80.8 | >8.88 | 0.595 | 0.33 |
| PLR | 45.45 | 75.45 | >194.6 | 0.528 | 0.776 |
| SII | 45.45 | 70.54 | >1525.9 | 0.530 | 0.3 |
| TG13/18 | 54.5 | 62.5 | >1 | 0.583 | 0.31 |
| Leukocytes | 72.73 | 5 | <17,800 | 0.510 | 0.935 |
| Sensitivity | Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
| NLR | 66.7 | 80.6 | >7.67 | 0.758 | <0.001 |
| PLR | 45.8 | 83.9 | >221.3 | 0.640 | 0.02 |
| SII | 83.3 | 51.7 | >858.3 | 0.697 | 0.001 |
| TG13/18 | 70.83 | 65.4 | >1 | 0.715 | <0.001 |
| Leukocytes | 79.2 | 54.4 | >9100 | 0.668 | 0.006 |
| PDR Sensitivity | PDR Specificity | Cut-Off Value | AUC | p | |
|---|---|---|---|---|---|
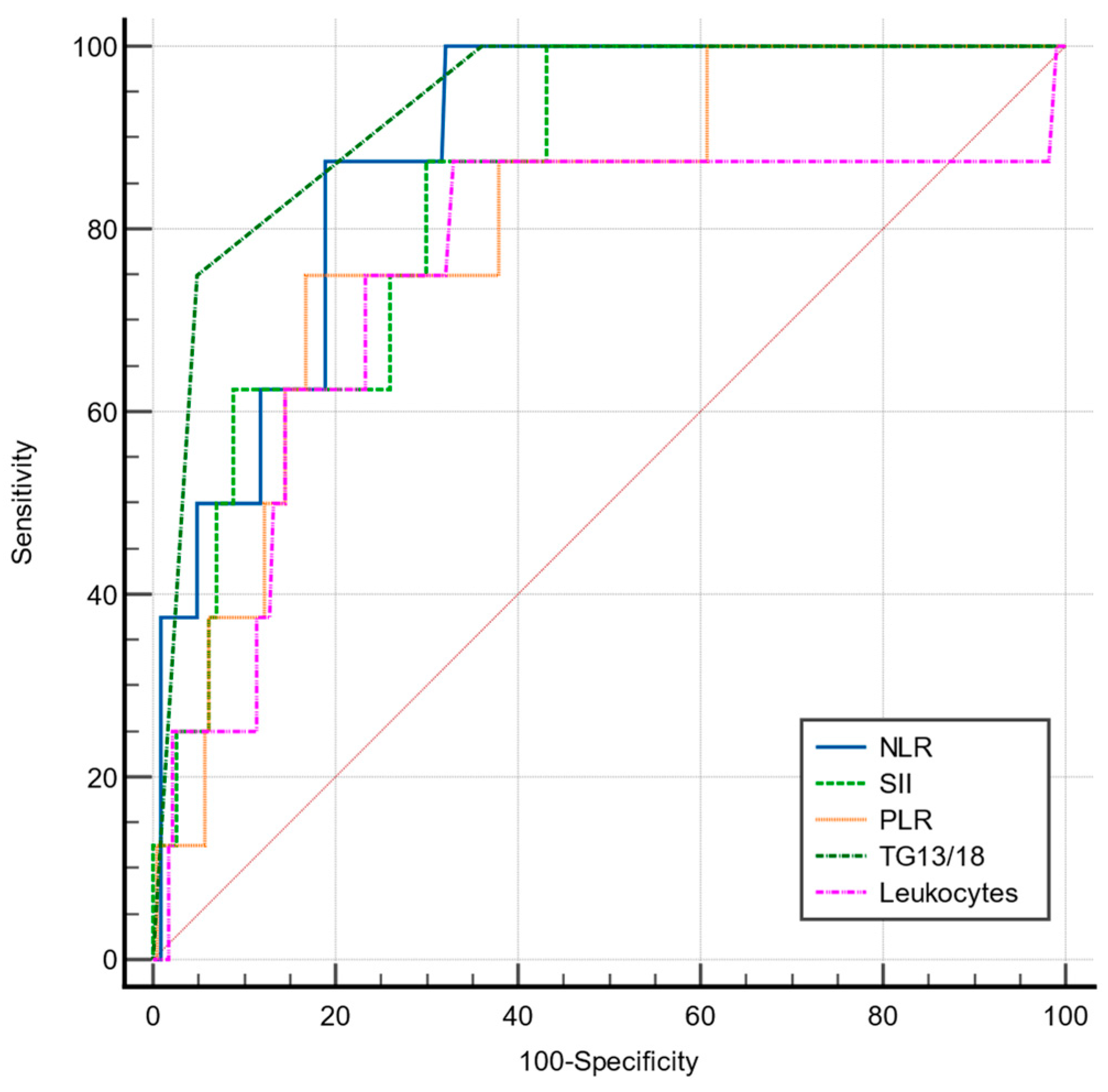
| NLR | 87.5 | 81 | >8.54 | 0.888 | <0.001 |
| PLR | 75 | 83.2 | >222.46 | 0.807 | <0.001 |
| SII | 87.5 | 70.04 | >1447.68 | 0.845 | <0.001 |
| TG13/18 | 75 | 92.1 | >2 | 0.931 | <0.0001 |
| Leukocytes | 87.5 | 66.9 | >11,300 | 0.753 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serban, D.; Stoica, P.L.; Dascalu, A.M.; Bratu, D.G.; Cristea, B.M.; Alius, C.; Motofei, I.; Tudor, C.; Tribus, L.C.; Serboiu, C.; et al. The Significance of Preoperative Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Systemic Inflammatory Index (SII) in Predicting Severity and Adverse Outcomes in Acute Calculous Cholecystitis. J. Clin. Med. 2023, 12, 6946. https://doi.org/10.3390/jcm12216946
Serban D, Stoica PL, Dascalu AM, Bratu DG, Cristea BM, Alius C, Motofei I, Tudor C, Tribus LC, Serboiu C, et al. The Significance of Preoperative Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Systemic Inflammatory Index (SII) in Predicting Severity and Adverse Outcomes in Acute Calculous Cholecystitis. Journal of Clinical Medicine. 2023; 12(21):6946. https://doi.org/10.3390/jcm12216946
Chicago/Turabian StyleSerban, Dragos, Paul Lorin Stoica, Ana Maria Dascalu, Dan Georgian Bratu, Bogdan Mihai Cristea, Catalin Alius, Ion Motofei, Corneliu Tudor, Laura Carina Tribus, Crenguta Serboiu, and et al. 2023. "The Significance of Preoperative Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Systemic Inflammatory Index (SII) in Predicting Severity and Adverse Outcomes in Acute Calculous Cholecystitis" Journal of Clinical Medicine 12, no. 21: 6946. https://doi.org/10.3390/jcm12216946
APA StyleSerban, D., Stoica, P. L., Dascalu, A. M., Bratu, D. G., Cristea, B. M., Alius, C., Motofei, I., Tudor, C., Tribus, L. C., Serboiu, C., Tudosie, M. S., Tanasescu, D., Vancea, G., & Costea, D. O. (2023). The Significance of Preoperative Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Systemic Inflammatory Index (SII) in Predicting Severity and Adverse Outcomes in Acute Calculous Cholecystitis. Journal of Clinical Medicine, 12(21), 6946. https://doi.org/10.3390/jcm12216946






