Prognosis of Adrenal Oncocytic Neoplasms (AONs): Literature Review of 287 Cases and Presentation of the Oldest Patient
Abstract
1. Introduction
2. Materials and Methods
3. Case Presentation
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamperl, H. Oncocytes and the so-called hurthle cell tumor. Arch. Pathol. 1950, 49, 563–570. [Google Scholar]
- Jaffe, R.H. Adenolymphoma [oncocytoma] of parotid gland. Am. J. Cancer 1932, 16, 1415–1423. [Google Scholar]
- Saygin, I.; Cakir, E.; Ercin, M.E.; Eyüboğlu, I. Incidental retroperitoneal oncocytoma (Ectopic oncocytic adrenocortical adenoma): Case report and review of the literature. Indian J. Pathol. Microbiol. 2019, 62, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.; Ma, L.; Kowalski, D. Pathologic quiz case: A 55-yearold woman with an adrenal mass. Arch. Pathol. Lab. Med. 2003, 127, e167–e168. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, S.; Yushita, Y.; Sanefuji, T.; Kondo, A.; Fujishima, N.; Kishikawa, M.; Matsumoto, K. Non-hormonal adrenocortical adenoma with oncocytoma-like appearances. Hinyokika Kiyo 1986, 32, 757–763. [Google Scholar] [PubMed]
- Bisceglia, M.; Ludovico, O.; Di Mattia, A.; Ben-Dor, D.; Sandbank, J.; Pasquinelli, G.; Lau, S.K.; Weiss, L.M. Adrenocortical oncocytic tumors: Report of 10 cases and review of the literature. Int. J. Surg. Pathol. 2004, 12, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Panizzo, V.; Rubino, B.; Piozzi, G.N.; Ubiali, P.; Morandi, A.; Nencioni, M.; Micheletto, G. Laparoscopic Trans-Abdominal Right Adrenalectomy for a Large Primitive Adrenal Oncocytic Carcinoma: A Case Report and Review of Literature. Am. J. Case Rep. 2018, 19, 1096–1102. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2014; Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 17 February 2014).
- Lin, B.T.; Bonsib, S.M.; Mierau, G.W.; Weiss, L.M.; Medeiro, L.J. Oncocytic adrenocortical neoplasms: A report of seven cases and review of the literature. Am. J. Surg. Pathol. 1998, 22, 603–614. [Google Scholar] [CrossRef]
- Kim, E.I.; Mamedova, E.O.; Selivanova, L.S.; Buryakina, S.A.; Gorbunova, N.P.; Latkina, N.V.; Kuznetsov, N.S.; Belaya, Z.E. Cushing’s syndrome due to bilateral oncocytic adrenal tumor. Probl. Endokrinol. 2020, 66, 47–55. [Google Scholar] [CrossRef]
- Peynirci, H.; Taskıran, B.; Dik, N.; Saraydaroğlu, Ö.; Ersoy, C. Oncocytic neoplasms; rare adrenocortical tumours—A report of eleven patients. Endokrynol. Pol. 2018, 69, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Shastri, C.; Rana, C.; Kumari, N.; Agarwal, G.; Krishnani, N. Bilateral adrenocortical oncocytoma with bilateral myelolipomatous metaplasia. Endocr. Pathol. 2012, 23, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Park, W.Y.; Seo, H.I.; Choi, K.U.; Kim, A.; Kim, Y.K.; Lee, S.J.; Lee, C.H.; Huh, G.Y.; Park, D.Y. Three cases of adrenocortical tumors mistaken for hepatocellular carcinomas/diagnostic pitfalls and differential diagnosis. Ann. Diagn. Pathol. 2017, 31, 9–13. [Google Scholar] [CrossRef]
- Godin, K.; Bang, N.; Tolkach, Y. Case report: Heterotopic intrarenally located adrenocortical oncocytoma. F1000Research 2014, 3, 73. [Google Scholar] [CrossRef]
- Surrey, L.F.; Thaker, A.A.; Zhang, P.J.; Karakousis, G.; Feldman, M.D. Ectopic functioning adrenocortical oncocytic adenoma (oncocytoma) with myelolipoma causing virilization. Case Rep. Pathol. 2012, 2012, 326418. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.A.; Jameson, C.; Christmas, T.J.; Sohaib, S.A. Retroperitoneal oncocytoma: Case report and review of the imaging features. Br. J. Radiol. 2011, 84, e161–e163. [Google Scholar] [CrossRef]
- Schittenhelm, J.; Ebner, F.H.; Harter, P.; Bornemann, A. Symptomatic intraspinal oncocytic adrenocortical adenoma. Endocr. Pathol. 2009, 20, 73–77. [Google Scholar] [CrossRef]
- Minamimoto, R.; Yamanaka, S.; Kawamoto, M.; Endoh, M.; Nishito, R.; Yoshida, K.; Nakaigawa, N.; Yao, M.; Kubota, Y.; Inoue, T. High FDG uptake on oncocytoma located in the retroperitoneum mimicking malignancy. Clin. Nucl. Med. 2007, 32, 582–583. [Google Scholar] [CrossRef]
- Modi, P.; Goel, R.; Kadam, G. Retroperitoneoscopic partial adrenalectomy for large adrenocortical oncocytoma. J. Endourol. 2007, 21, 419–422. [Google Scholar] [CrossRef]
- Nguyen, G.-K.; Vriend, R.; Ronaghan, D.; Lakey, W.H. Heterotopic adrenocortical oncocytoma: A case report with light and electron microscopic studies. Cancer 1992, 70, 2681–2684. [Google Scholar] [CrossRef]
- Wong, D.D.; Spagnolo, D.V.; Bisceglia, M.; Havlat, M.; McCallum, D.; Platten, M.A. Oncocytic adrenocortical neoplasms--a clinicopathologic study of 13 new cases emphasizing the importance of their recognition. Hum. Pathol. 2011, 42, 489–499. [Google Scholar] [CrossRef]
- Thway, K.; Olmos, D.; Shah, C.; Flora, R.; Shipley, J.; Fisher, C. Oncocytic adrenal cortical carcinosarcoma with pleomorphic rhabdomyosarcomatous metastases. Am. J. Surg. Pathol. 2012, 36, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Goel, T.; Garg, S.; Rao, A.C.; Reddy, S. Adrenal oncocytoma masquerading as a functional tumor. Indian J. Urol. 2007, 23, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Kasem, K.; Lam, A.K. Adrenal oncocytic phaeochromocytoma with putative adverse histologic features: A unique case report and review of the literature. Endocr. Pathol. 2014, 25, 416–421. [Google Scholar] [CrossRef]
- Roma, A.A. Adrenal cortical oncocytoma mimicking pheochromocytoma. Hormones 2012, 11, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Kiriakopoulos, A.; Papaioannou, D.; Linos, D. Adrenal cortical oncocytoma mimicking pheochromocytoma. Hormones 2011, 10, 76–79. [Google Scholar] [CrossRef]
- Sharma, N.; Dogra, P.N.; Mathur, S. Functional adrenal oncocytoma: A rare neoplasm. Indian J. Pathol. Microbiol. 2008, 51, 531–533. [Google Scholar] [CrossRef]
- Kawahara, Y.; Morimoto, A.; Onoue, A.; Kashii, Y.; Fukushima, N.; Gunji, Y. Persistent fever and weight loss due to an interleukin-6-producing adrenocortical oncocytoma in a girl—Review of the literature. Eur. J. Pediatr. 2014, 173, 1107–1110. [Google Scholar] [CrossRef]
- Akatsu, T.; Kameyama, K.; Araki, K.; Ashizawa, T.; Wakabayashi, G.; Kitajima, M. Functioning adrenocortical oncocytoma: The first documented case producing interleukin-6 and review of the literature. J. Endocrinol. Investig. 2008, 31, 68–73. [Google Scholar] [CrossRef]
- Dong, D.; Liu, X.; Ji, Z.; Li, H. Diagnosis and Treatment of Adrenocortical Oncocytoma: Case Report of Five Cases and Review of the Literature. Front. Oncol. 2019, 9, 338. [Google Scholar] [CrossRef]
- Son, S.H.; Lee, S.-W.; Song, B.-I.; Jang, Y.-J.; Park, J.-Y.; Jeong, S.Y.; Ahn, B.-C.; Lee, J. Recurrence of a functional adrenocortical oncocytoma of borderline malignant potential showing high FDG uptake on 18F-FDG PET/CT. Ann. Nucl. Med. 2014, 28, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Duregon, E.; Volante, M.; Cappia, S.; Cuccurullo, A.; Bisceglia, M.; Wong, D.D.; Spagnolo, D.V.; Szpak-Ulczok, S.; Bollito, E.; Daffara, F.; et al. Oncocytic Adrenocortical Tumors: Diagnostic Algorithm and Mitochondrial DNA Profile in 27 Cases. Am. J. Surg. Pathol. 2011, 35, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.P.; Ayala, A.G.; Albores-Saavedra, J. Oncocytic adrenocortical carcinoma: A morphologic, immunohistochemical and ultrastructural study of four cases. Mod. Pathol. 2002, 15, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Seo, I.S.; Henley, J.D.; Min, K.W. Peculiar cytoplasmic inclusions in oncocytic adrenal cortical tumors: An electron microscopic observation. Ultrastruct. Pathol. 2002, 26, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Behaeghe, O.; Geurde, B.; Jourdan, J.-L.; Bodson, C.; Seydel, B.; Lacremans, D. A case of a borderline adrenal oncocytoma in a 62-year old female. Acta Chir. Belg. 2021, 122, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Chisté, M.; Poppiti, R.J.; Bianco, F.J. Oncocytoma of the adrenal gland medulla. Ann. Diagn. Pathol. 2013, 17, 123–126. [Google Scholar] [CrossRef]
- Pereira, B.D.; Rios, E.S.; Cabrera, R.A.; Portugal, J.; Raimundo, L. Adrenocortical oncocytoma presenting as Cushing’s syndrome in pregnancy with spontaneous postpartum uterine rupture. Ceska Gynekol. 2016, 81, 228–232. [Google Scholar]
- Segal, S.; Cytron, S.; Shenhav, S.; Gemer, O. Adrenocortical oncocytoma in pregnancy. Obstet. Gynecol. 2001, 98 Pt 2, 916–918. [Google Scholar]
- Subbiah, S.; Nahar, U.; Samujh, R.; Bhansalia, A. Heterosexual precocity: Rare manifestation of virilizing adrenocortical oncocytoma. Ann. Saudi Med. 2013, 33, 294–297. [Google Scholar] [CrossRef]
- Sahin, S.B.; Yucel, A.F.; Bedir, R.; Ogullar, S.; Ayaz, T.; Algun, E. Testosterone- and cortisol-secreting adrenocortical oncocytoma: An unusual cause of hirsutism. Case Rep. Endocrinol. 2014, 2014, 206890. [Google Scholar] [CrossRef]
- Logasundaram, R.; Parkinson, C.; Donaldson, P.; Coode, P.E. Co-secretion of testosterone and cortisol by a functional adrenocortical oncocytoma. Histopathology 2007, 51, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Terui, K.; Sakihara, S.; Kageyama, K.; Nigawara, T.; Takayasu, S.; Matsuhashi, Y.; Kon, A.; Yamamoto, H.; Ohyama, C.; Sasano, H.; et al. A case of adrenocortical oncocytoma occurring with aldosteronoma. J. Clin. Endocrinol. Metab. 2010, 95, 3597–3598. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.E.; Raphael, S.J. Functional oncocytic adrenocortical carcinoma. Endocr. Pathol. 2007, 18, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO classification of tumours of endocrine organs. In World Health Organization Classification of Tumours; International Agency for Research on Cancer: Lyon, France, 2017; Volume 10, pp. 1–355. [Google Scholar]
- Weiss, L.M. Comparative histologic study of 43 metastasizing andnonmetastasizing adrenocortical tumors. Am. J. Surg. Pathol. 1984, 8, 163–169. [Google Scholar] [CrossRef]
- Juliano, J.J.; Cody, R.L.; Suh, J.H. Metastatic adrenocortical oncocytoma: A case report. Urol. Oncol. 2008, 26, 198–201. [Google Scholar] [CrossRef]
- Renaudin, K.; Smati, S.; Wargny, M.; Al Ghuzlan, A.; Aubert, S.; Leteurtre, E.; Patey, M.; Sibony, M.; Sturm, N.; Tissier, F.; et al. Clinicopathological description of 43 oncocytic adrenocortical tumors: Importance of Ki-67 in histoprognostic evaluation. Mod. Pathol. 2018, 31, 1708–1716. [Google Scholar] [CrossRef]
- Coppola, M.; Romeo, V.; Verde, F.; Raia, G.; Mainolfi, C.G.; Aprea, G.; Guadagno, E.; Cavaliere, C.; Baldi, D.; Soricelli, A.; et al. Integrated imaging of adrenal oncocytoma: A case of diagnostic challenge. Quant. Imaging Med. Surg. 2019, 9, 1896–1901. [Google Scholar] [CrossRef]
- Gilardi, L.; Vadrucci, M.; Pirola, S.; Grana, C.M. Oncocytoma as a growing FDG-avid adrenal mass on serial (18)F-FDG PET/CT imaging: A potential pitfall in oncological evaluation. Rev. Española Med. Nucl. Imagen Mol. 2017, 36, 201–203. [Google Scholar] [CrossRef]
- Sato, N.; Nakamura, Y.; Takanami, K.; Ono, Y.; Omata, K.; Morimoto, R.; Satoh, F.; Ise, K.; Yamada, S.; Kasajima, A.; et al. Case report: Adrenal oncocytoma associated with markedly increased FDG uptake and immunohistochemically positive for GLUT1. Endocr. Pathol. 2014, 25, 410–415. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Shelton, T.O.; Ayala, A.G.; Sneige, N.; Ordóñez, N.G. Fine-needle aspiration cytology of a case of oncocytic adrenocortical carcinoma. Diagn Cytopathol. 1999, 22, 299–303. [Google Scholar] [CrossRef]
- Conzo, G.; Patrone, R.; Flagiello, L.; Catauro, A.; Conzo, A.; Cacciatore, C.; Mongardini, F.M.; Cozzolino, G.; Esposito, R.; Pasquali, D.; et al. Impact of Current Technology in Laparoscopic Adrenalectomy: 20 Years of Experience in the Treatment of 254 Consecutive Clinical Cases. J. Clin. Med. 2023, 12, 4384. [Google Scholar] [CrossRef] [PubMed]
- Mearini, L.; Del Sordo, R.; Costantini, E.; Nunzi, E.; Porena, M. Adrenal oncocytic neoplasm: A systematic review. Urol. Int. 2013, 91, 125–133. [Google Scholar] [CrossRef] [PubMed]
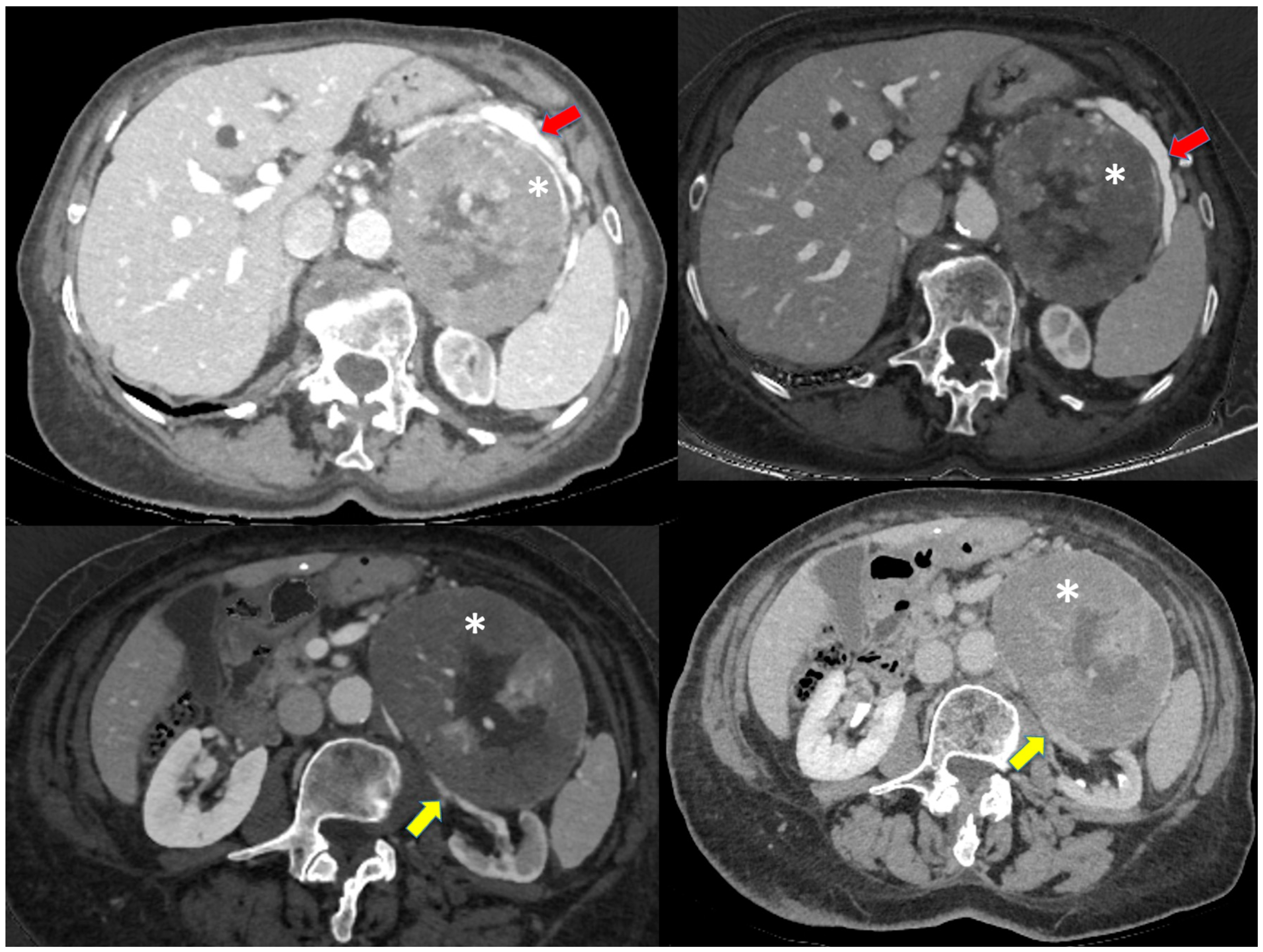
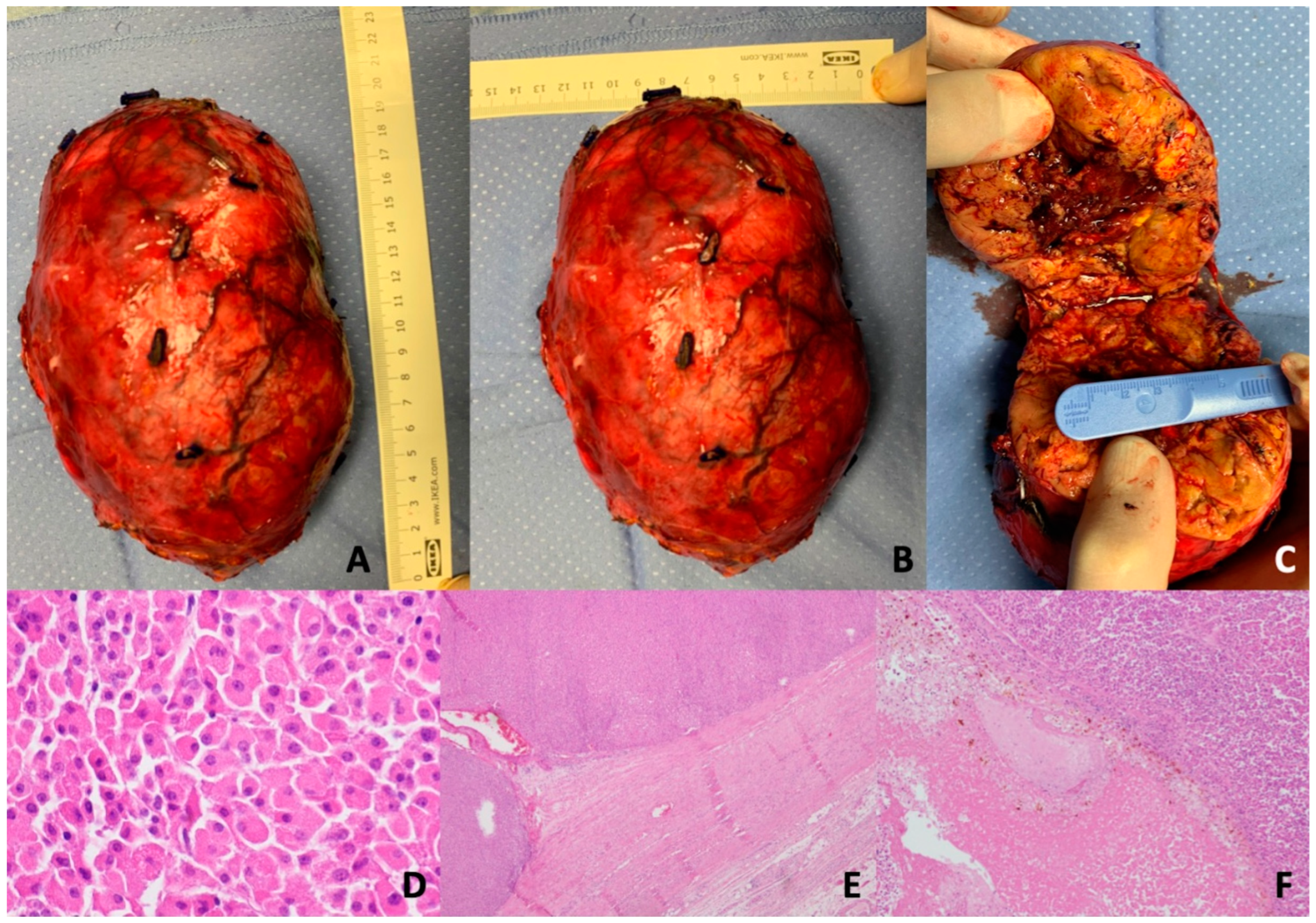
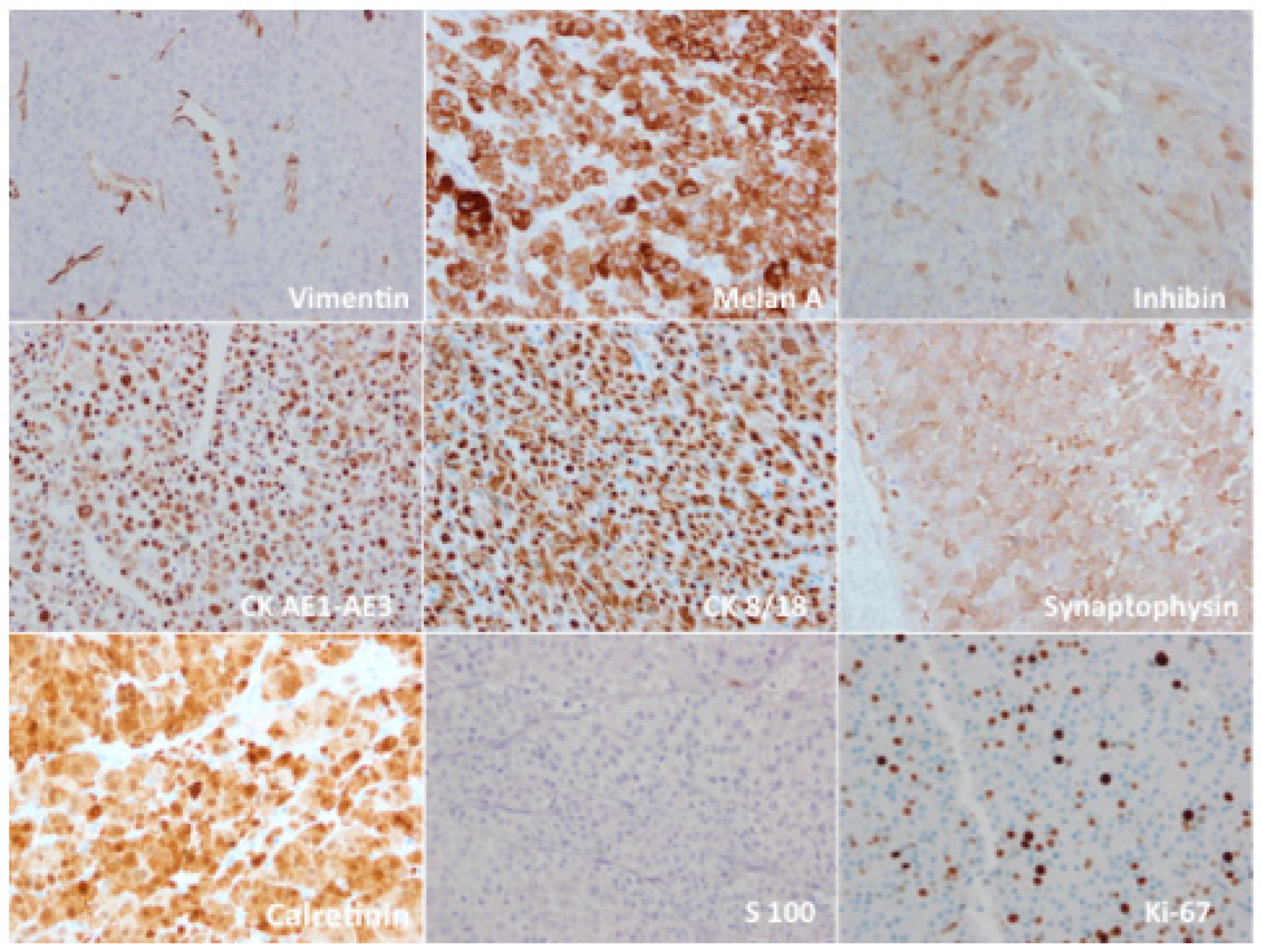
| Characteristics | AONs (n = 287) | A/NA |
|---|---|---|
| Gender | 282/5 | |
| Females | 185 (65.6%) | |
| Males | 97 (34.3%) | |
| Age (years) | 44.2 (3.5–87) | 285/2 |
| Location | 246/41 | |
| Left | 143 (58.1%) | |
| Right | 100 (40.6%) | |
| Bilateral | 3 (1.2%) | |
| Functionality | 272/15 | |
| Non-functional | 185 (68%) | |
| Functional | 87 (32%) | |
| Size (mm) | 8.9 (2–28) | 244/43 |
| Weight (g) | 467 (8–5720) | 54/133 |
| LWB system | 278/9 | |
| AO | 117 (42%) | |
| AONUMP | 78 (28%) | |
| AOC | 83 (30%) |
| Functional AONs (n = 87) | % (n = 272) | |
|---|---|---|
| Hyperandrogenism | 36 | 13.2 |
| Cushing syndrome | 22 | 8 |
| Hypercortisolism | 9 | 3.3 |
| Suppressed ACTH levels | 3 | 1.1 |
| Hypercortisolism + Hyperandrogenism | 8 | 2.9 |
| Mimicking Pheochromocytoma | 5 | 1.8 |
| Conn syndrome | 2 | 0.7 |
| IL6 secretion | 2 | 0.7 |
| Classification | n (%) | Median Follow-Up (m) | Range of Follow-Up (m) | Local Recurrence [n (%)] | Metastasis [n (%)] | Tumor-Related Deaths [n (%)] |
|---|---|---|---|---|---|---|
| AO | 58 (43) | 41.3 | 3–180 | 2 (1.5) | 0 | 0 |
| AONUMP | 47 (35) | 34.8 | 5–180 | 1 (0.7) | 1 (0.7) | 2 (4.2) |
| AOC | 30 (22) | 32.5 | 1–180 | 7 (5.1) | 5 (3.7) | 9 (30) |
| Total | 135 | 37.1 | 1–180 | 10 (7.4) | 6 (4.4) | 11 (8.1) |
| Unclassifiable | 152 | NA | NA | NA | NA | NA |
| Authors | Age | Sex | Location | Sizec m | Weight g | Functional | LWB System | Tumor-Related Deaths (m) | Recurrence/Metastasis | |
|---|---|---|---|---|---|---|---|---|---|---|
| Son SH (2013) [32] | 53 | m | left | 9.8 | NA | Cushing | AONUMP | Necrosis | 15 | Local and hepatic |
| Wong DD (2011) [22] | 36 | w | left | 14 | 885 | NO | AOC | NA | 1 | |
| 47 | w | left | 10 | 552 | NO | AOC | NA | 9 | ||
| 41 | w | left | 28 | 5720 | Virilization | AOC | NA | 16 | Hepatic | |
| 68 | w | Right | 8 | 70 | NO | AOC | NA | 21 | Local | |
| Duregon (2011) [33] | 42 | w | left | 16 | 530 | NO | AONUMP | Dimension + Necrosis | 15 | |
| 32 | m | Right | 23 | NO | AOC | >5 mitotic figures per 50 HPFs atypical mitoses | 24 | |||
| Bisceglia M (2004) [6] | 46 | m | Right | 17 | 190 | NO | AOC | >5 mitotic figures per 50 HPFs atypical mitoses | 58 | Local |
| 62 | w | left | 8 | 260 | NO | AOC | >5 mitotic figures per 50 HPFs atypical mitoses | 6 | ||
| Hoang MP (2002) [34] | 53 | w | left | 17 | 1200 | NO | AOC | Venous invasion | 24 | Bone |
| Seo IS (2002) [35] | 49 | w | left | 8,5 | 200 | MO | AOC | NA | 72 | Carcinosis |
| MEAN | 48 | 8/3 | 8/3 | 14.5 | 1067 | 9/2 | 9/2 | 23.7 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola Bottazzi, E.; Gambardella, C.; Mongardini, F.M.; Vanella, S.; Noviello, A.; Palma, T.; Murano, R.; De Chiara, G.; Conzo, G.; Docimo, L.; et al. Prognosis of Adrenal Oncocytic Neoplasms (AONs): Literature Review of 287 Cases and Presentation of the Oldest Patient. J. Clin. Med. 2023, 12, 6925. https://doi.org/10.3390/jcm12216925
Coppola Bottazzi E, Gambardella C, Mongardini FM, Vanella S, Noviello A, Palma T, Murano R, De Chiara G, Conzo G, Docimo L, et al. Prognosis of Adrenal Oncocytic Neoplasms (AONs): Literature Review of 287 Cases and Presentation of the Oldest Patient. Journal of Clinical Medicine. 2023; 12(21):6925. https://doi.org/10.3390/jcm12216925
Chicago/Turabian StyleCoppola Bottazzi, Enrico, Claudio Gambardella, Federico Maria Mongardini, Serafino Vanella, Adele Noviello, Tommaso Palma, Rosa Murano, Giovanni De Chiara, Giovanni Conzo, Ludovico Docimo, and et al. 2023. "Prognosis of Adrenal Oncocytic Neoplasms (AONs): Literature Review of 287 Cases and Presentation of the Oldest Patient" Journal of Clinical Medicine 12, no. 21: 6925. https://doi.org/10.3390/jcm12216925
APA StyleCoppola Bottazzi, E., Gambardella, C., Mongardini, F. M., Vanella, S., Noviello, A., Palma, T., Murano, R., De Chiara, G., Conzo, G., Docimo, L., & Crafa, F. (2023). Prognosis of Adrenal Oncocytic Neoplasms (AONs): Literature Review of 287 Cases and Presentation of the Oldest Patient. Journal of Clinical Medicine, 12(21), 6925. https://doi.org/10.3390/jcm12216925





