Face the Future—Artificial Intelligence in Oral and Maxillofacial Surgery
Abstract
:1. Introduction
2. Materials and Methods
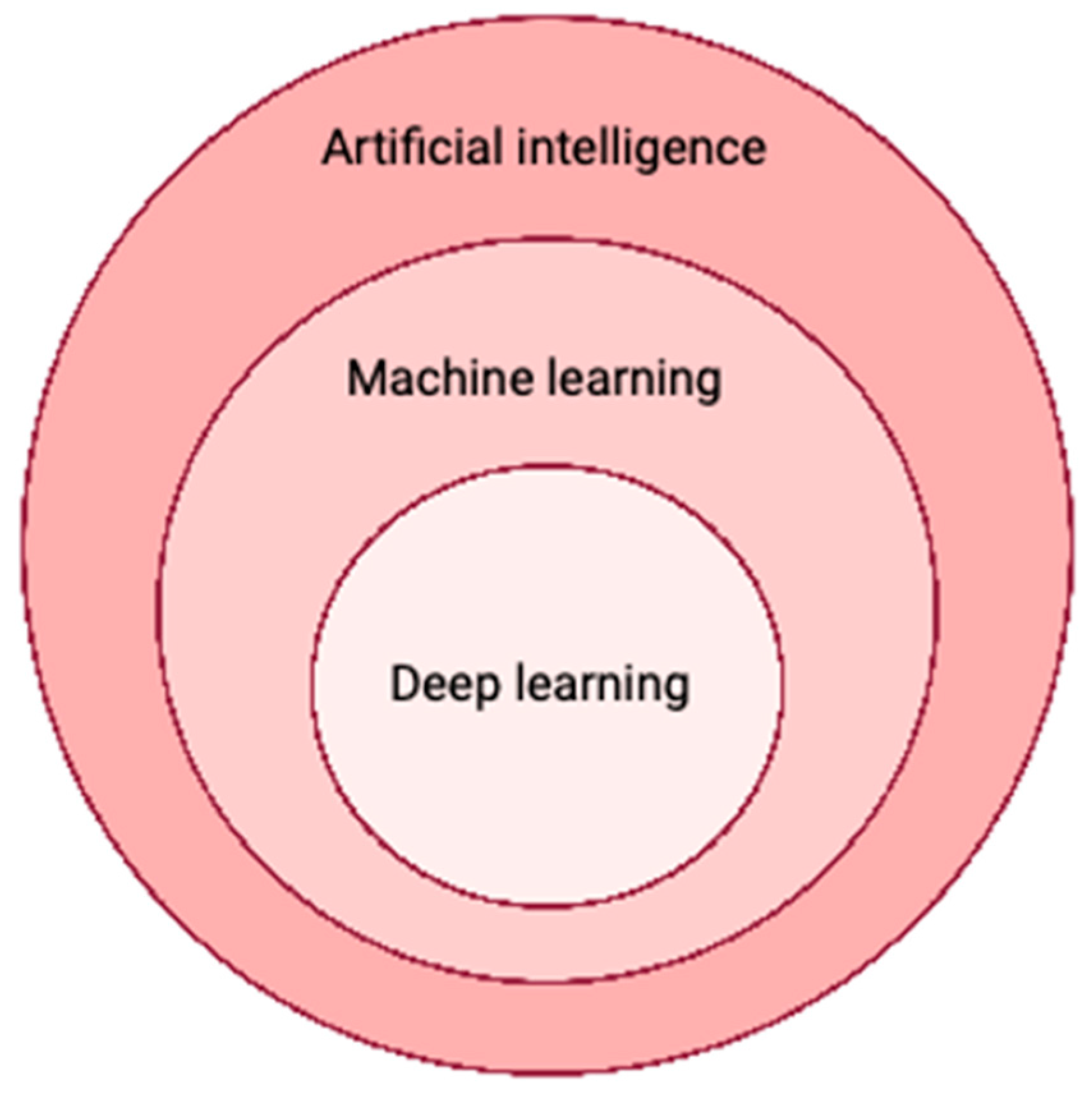
3. Artificial Intelligence in Medicine
4. Artificial Intelligence for Clinical Scenarios
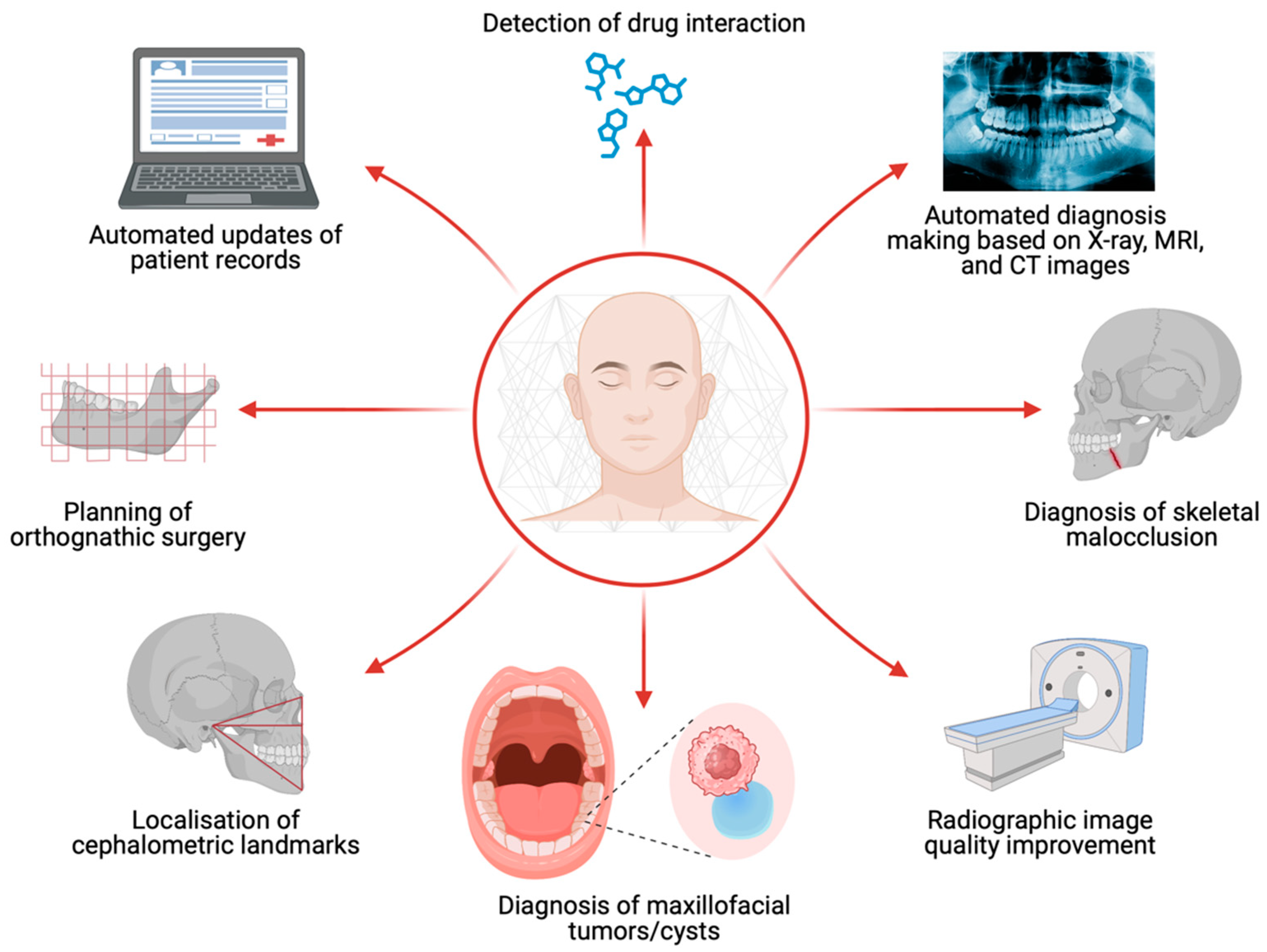
5. Artificial Intelligence in Oral and Maxillofacial Surgery
5.1. Radiographic Image Quality Improvement
5.2. Diagnosis of Maxillofacial Cysts and Tumors
5.3. Localisation of Cephalometric Landmarks and Diagnosis of Skeletal Malocclusion
5.4. Planning of Orthognathic Surgery
5.5. Additional Benefits
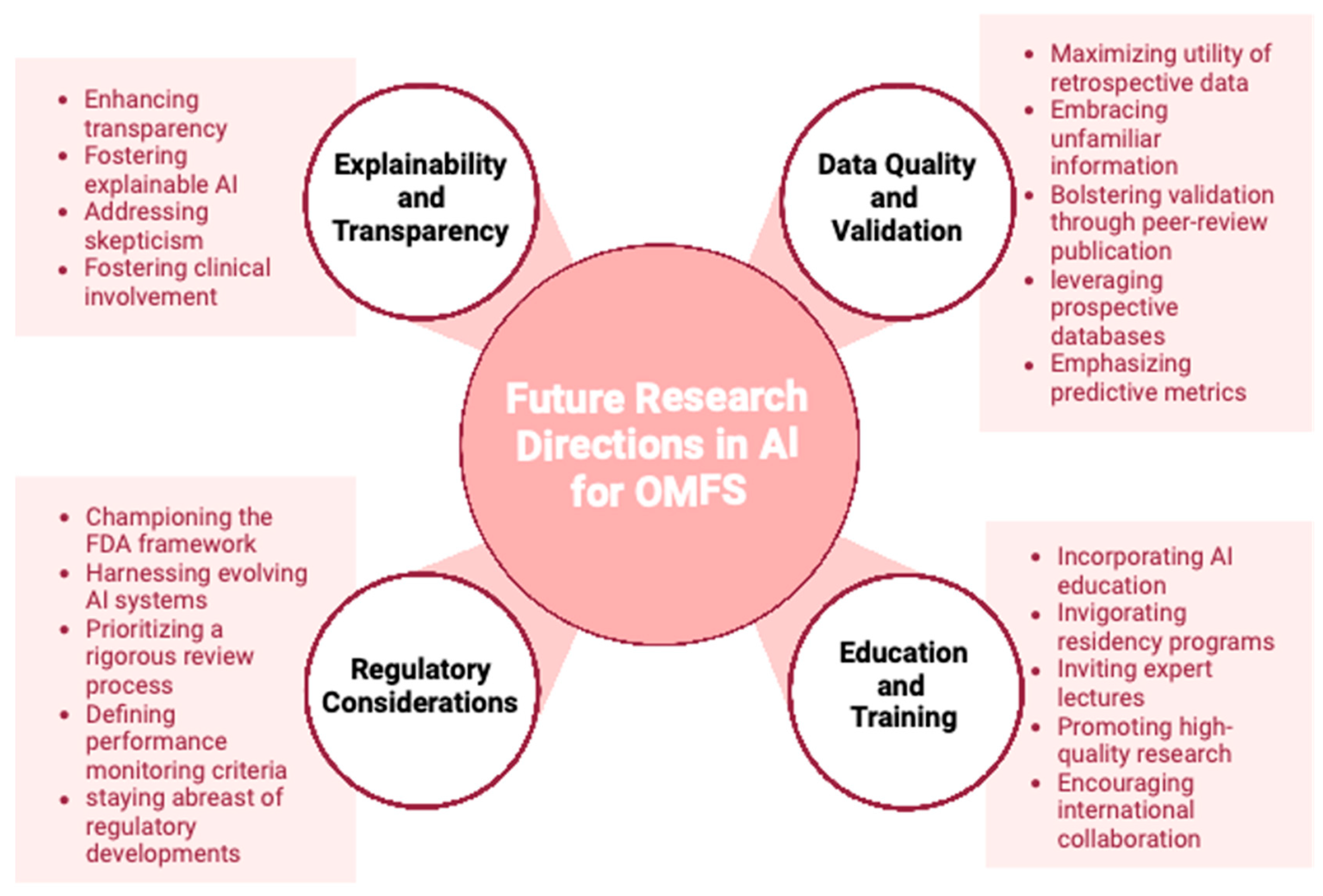
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Limketkai, B.N.; Mauldin, K.; Manitius, N.; Jalilian, L.; Salonen, B.R. The Age of Artificial Intelligence: Use of Digital Technology in Clinical Nutrition. Curr. Surg. Rep. 2021, 9, 20. [Google Scholar] [CrossRef]
- Clarke, S.L.; Parmesar, K.; Saleem, M.A.; Ramanan, A.V. Future of machine learning in paediatrics. Arch. Dis. Child. 2022, 107, 223–228. [Google Scholar] [CrossRef]
- Orth, M.; Averina, M.; Chatzipanagiotou, S.; Faure, G.; Haushofer, A.; Kusec, V.; Machado, A.; Misbah, S.A.; Oosterhuis, W.; Pulkki, K.; et al. Opinion: Redefining the role of the physician in laboratory medicine in the context of emerging technologies, personalised medicine and patient autonomy (‘4P medicine’). J. Clin. Pathol. 2019, 72, 191–197. [Google Scholar] [CrossRef]
- Abdulnabi, M.; Al-Haiqi, A.; Kiah, M.L.M.; Zaidan, A.A.; Zaidan, B.B.; Hussain, M. A distributed framework for health information exchange using smartphone technologies. J. Biomed. Inform. 2017, 69, 230–250. [Google Scholar] [CrossRef]
- Topol, E.J. A decade of digital medicine innovation. Sci. Transl. Med. 2019, 11, eaaw7610. [Google Scholar] [CrossRef]
- Morawski, K.; Ghazinouri, R.; Krumme, A.; Lauffenburger, J.C.; Lu, Z.; Durfee, E.; Oley, L.; Lee, J.; Mohta, N.; Haff, N.; et al. Association of a Smartphone Application with Medication Adherence and Blood Pressure Control: The MedISAFE-BP Randomized Clinical Trial. JAMA Intern. Med. 2018, 178, 802–809. [Google Scholar] [CrossRef]
- Rokhshad, R.; Keyhan, S.O.; Yousefi, P. Artificial intelligence applications and ethical challenges in oral and maxillo-facial cosmetic surgery: A narrative review. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 14. [Google Scholar] [CrossRef]
- Rasteau, S.; Ernenwein, D.; Savoldelli, C.; Bouletreau, P. Artificial intelligence for oral and maxillo-facial surgery: A narrative review. J. Stomatol. Oral. Maxillofac. Surg. 2022, 123, 276–282. [Google Scholar] [CrossRef]
- Apell, P.; Eriksson, H. Artificial intelligence (AI) healthcare technology innovations: The current state and challenges from a life science industry perspective. Technol. Anal. Strateg. Manag. 2023, 35, 179–193. [Google Scholar] [CrossRef]
- Fakoor, R.; Ladhak, F.; Nazi, A.; Huber, M. Using deep learning to enhance cancer diagnosis and classication. In Proceedings of the International Conference on Machine Learning, Atlanta, GA, USA, 17–19 June 2013; ACM: New York, NY, USA, 2013. [Google Scholar]
- Vial, A.; Stirling, D.; Field, M.; Ros, M.; Ritz, C.; Carolan, M.; Holloway, L.; Miller, A.A. The role of deep learning and radiomic feature extraction in cancer-specific predictive modelling: A review. Transl. Cancer Res. 2018, 7, 803–816. [Google Scholar] [CrossRef]
- Güneş, E.D.; Yaman, H.; Çekyay, B.; Verter, V. Matching patient and physician preferences in designing a primary care facility network. J. Oper. Res. Soc. 2014, 65, 483–496. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S. The Next Era: Deep Learning in Pharmaceutical Research. Pharm. Res. 2016, 33, 2594–2603. [Google Scholar] [CrossRef]
- Jing, Y.; Bian, Y.; Hu, Z.; Wang, L.; Xie, X.Q. Deep Learning for Drug Design: An Artificial Intelligence Paradigm for Drug Discovery in the Big Data Era. Aaps J. 2018, 20, 58. [Google Scholar] [CrossRef]
- Caffery, L.J.; Clunie, D.; Curiel-Lewandrowski, C.; Malvehy, J.; Soyer, H.P.; Halpern, A.C. Transforming Dermatologic Imaging for the Digital Era: Metadata and Standards. J. Digit. Imaging 2018, 31, 568–577. [Google Scholar] [CrossRef]
- Li, C.X.; Shen, C.B.; Xue, K.; Shen, X.; Jing, Y.; Wang, Z.Y.; Xu, F.; Meng, R.S.; Yu, J.B.; Cui, Y. Artificial intelligence in dermatology: Past, present, and future. Chin. Med. J. 2019, 132, 2017–2020. [Google Scholar] [CrossRef]
- Siddiqui, T.A.; Sukhia, R.H.; Ghandhi, D. Artificial intelligence in dentistry, orthodontics and Orthognathic surgery: A literature review. J. Pak. Med. Assoc. 2022, 72 (Suppl. S1), S91–S96. [Google Scholar]
- Vranckx, M.; Van Gerven, A.; Willems, H.; Vandemeulebroucke, A.; Ferreira Leite, A.; Politis, C.; Jacobs, R. Artificial Intelligence (AI)-Driven Molar Angulation Measurements to Predict Third Molar Eruption on Panoramic Radiographs. Int. J. Environ. Res. Public. Health 2020, 17, 3716. [Google Scholar] [CrossRef]
- Yoo, J.H.; Yeom, H.G.; Shin, W.; Yun, J.P.; Lee, J.H.; Jeong, S.H.; Lim, H.J.; Lee, J.; Kim, B.C. Deep learning based prediction of extraction difficulty for mandibular third molars. Sci. Rep. 2021, 11, 1954. [Google Scholar] [CrossRef]
- Yin, C.; Qian, B.; Wei, J.; Li, X.; Zhang, X.; Li, Y.; Zheng, Q. Automatic Generation of Medical Imaging Diagnostic Report with Hierarchical Recurrent Neural Network. In Proceedings of the 2019 IEEE International Conference on Data Mining (ICDM), Beijing, China, 8–11 November 2019; pp. 728–737. [Google Scholar]
- Krishnan, D.G. Artificial Intelligence in Oral and Maxillofacial Surgery Education. Oral. Maxillofac. Surg. Clin. N. Am. 2022, 34, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, L.; Miragall, M.; Kauke-Navarro, M.; Obed, D.; Bauer, M.; Tißler, P.; Prantl, L.; Machens, H.G.; Broer, P.N.; Baecher, H.; et al. A Ready-to-Use Grading Tool for Facial Palsy Examiners-Automated Grading System in Facial Palsy Patients Made Easy. J. Pers. Med. 2022, 12, 1739. [Google Scholar] [CrossRef]
- Knoedler, L.; Baecher, H.; Kauke-Navarro, M.; Prantl, L.; Machens, H.G.; Scheuermann, P.; Palm, C.; Baumann, R.; Kehrer, A.; Panayi, A.C.; et al. Towards a Reliable and Rapid Automated Grading System in Facial Palsy Patients: Facial Palsy Surgery Meets Computer Science. J. Clin. Med. 2022, 11, 4998. [Google Scholar] [CrossRef]
- Jaemsuwan, S.; Arunjaroensuk, S.; Kaboosaya, B.; Subbalekha, K.; Mattheos, N.; Pimkhaokham, A. Comparison of the accuracy of implant position among freehand implant placement, static and dynamic computer-assisted implant surgery in fully edentulous patients: A non-randomized prospective study. Int. J. Oral. Maxillofac. Surg. 2023, 52, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.; Inam, W.; Kennedy, R.; Faiella, R. The Future of Dentistry: How AI is Transforming Dental Practices. Compend. Contin. Educ. Dent. 2021, 42, 14–17. [Google Scholar]
- Peña-Cantillana, F.; Díaz-Pernil, D.; Berciano, A.; Gutiérrez-Naranjo, M.A. A Parallel Implementation of the Thresholding Problem by Using Tissue-Like P Systems. In Proceedings of the Computer Analysis of Images and Patterns, Seville, Spain, 29–31 August 2011; Springer: Berlin/Heidelberg, Germany, 2011; pp. 277–284. [Google Scholar]
- Pauwels, R.; Araki, K.; Siewerdsen, J.H.; Thongvigitmanee, S.S. Technical aspects of dental CBCT: State of the art. Dentomaxillofac. Radiol. 2015, 44, 20140224. [Google Scholar] [CrossRef]
- Tian, C.; Fei, L.; Zheng, W.; Xu, Y.; Zuo, W.; Lin, C.W. Deep learning on image denoising: An overview. Neural. Netw. 2020, 131, 251–275. [Google Scholar] [CrossRef]
- Zhang, K.; Zuo, W.; Chen, Y.; Meng, D.; Zhang, L. Beyond a Gaussian Denoiser: Residual Learning of Deep CNN for Image Denoising. IEEE Trans. Image Process 2017, 26, 3142–3155. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Y.; Kalra, M.K.; Lin, F.; Chen, Y.; Liao, P.; Zhou, J.; Wang, G. Low-Dose CT with a Residual Encoder-Decoder Convolutional Neural Network. IEEE Trans. Med. Imaging 2017, 36, 2524–2535. [Google Scholar] [CrossRef]
- Park, J.; Hwang, D.; Kim, K.Y.; Kang, S.K.; Kim, Y.K.; Lee, J.S. Computed tomography super-resolution using deep convolutional neural network. Phys. Med. Biol. 2018, 63, 145011. [Google Scholar] [CrossRef]
- De Man, B.; Nuyts, J.; Dupont, P.; Marchal, G.; Suetens, P. Metal streak artifacts in X-ray computed tomography: A simulation study. In Proceedings of the 1998 IEEE Nuclear Science Symposium Conference Record (Cat No98CH36255), Toronto, ON, Canada, 8–14 November 1998; Volume 1863, pp. 1860–1865. [Google Scholar]
- Hyun, C.M.; Bayaraa, T.; Yun, H.S.; Jang, T.J.; Park, H.S.; Seo, J.K. Deep learning method for reducing metal artifacts in dental cone-beam CT using supplementary information from intra-oral scan. Phys. Med. Biol. 2022, 67, 175007. [Google Scholar] [CrossRef]
- Abdolali, F.; Zoroofi, R.A.; Otake, Y.; Sato, Y. Automatic segmentation of maxillofacial cysts in cone beam CT images. Comput. Biol. Med. 2016, 72, 108–119. [Google Scholar] [CrossRef]
- Rana, M.; Modrow, D.; Keuchel, J.; Chui, C.; Rana, M.; Wagner, M.; Gellrich, N.C. Development and evaluation of an automatic tumor segmentation tool: A comparison between automatic, semi-automatic and manual segmentation of mandibular odontogenic cysts and tumors. J. Craniomaxillofac. Surg. 2015, 43, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Mikulka, J.; Gescheidtová, E.; Kabrda, M.; Peřina, V. Classification of Jaw Bone Cysts and Necrosis via the Processing of Orthopantomograms. Radioengineering 2013, 22, 114–122. [Google Scholar]
- Yilmaz, E.; Kayikcioglu, T.; Kayipmaz, S. Computer-aided diagnosis of periapical cyst and keratocystic odontogenic tumor on cone beam computed tomography. Comput. Methods Programs Biomed. 2017, 146, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Santer, M.; Kloppenburg, M.; Gottfried, T.M.; Runge, A.; Schmutzhard, J.; Vorbach, S.M.; Mangesius, J.; Riedl, D.; Mangesius, S.; Widmann, G.; et al. Current Applications of Artificial Intelligence to Classify Cervical Lymph Nodes in Patients with Head and Neck Squamous Cell Carcinoma—A Systematic Review. Cancers 2022, 14, 5397. [Google Scholar]
- Wang, C.W.; Huang, C.T.; Lee, J.H.; Li, C.H.; Chang, S.W.; Siao, M.J.; Lai, T.M.; Ibragimov, B.; Vrtovec, T.; Ronneberger, O.; et al. A benchmark for comparison of dental radiography analysis algorithms. Med. Image Anal. 2016, 31, 63–76. [Google Scholar] [CrossRef]
- Grau, V.; Alcañiz, M.; Juan, M.C.; Monserrat, C.; Knoll, C. Automatic localization of cephalometric Landmarks. J. Biomed. Inform. 2001, 34, 146–156. [Google Scholar] [CrossRef]
- Lindner, C.; Wang, C.W.; Huang, C.T.; Li, C.H.; Chang, S.W.; Cootes, T.F. Fully Automatic System for Accurate Localisation and Analysis of Cephalometric Landmarks in Lateral Cephalograms. Sci. Rep. 2016, 6, 33581. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Li, J.; Zhang, Y.; Zou, B. Automatic Analysis of Lateral Cephalograms Based on Multiresolution Decision Tree Regression Voting. J. Healthc. Eng. 2018, 2018, 1797502. [Google Scholar] [CrossRef]
- Ibragimov, B.; Likar, B.; Pernuš, F.; Vrtovec, T. Shape representation for efficient landmark-based segmentation in 3-d. IEEE Trans. Med. Imaging 2014, 33, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Huang, C.T.; Hsieh, M.C.; Li, C.H.; Chang, S.W.; Li, W.C.; Vandaele, R.; Marée, R.; Jodogne, S.; Geurts, P.; et al. Evaluation and Comparison of Anatomical Landmark Detection Methods for Cephalometric X-ray Images: A Grand Challenge. IEEE Trans. Med. Imaging 2015, 34, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Ibragimov, B. Automatic Cephalometric X-ray Landmark Detection by Applying Game Theory and Random Forests; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Arık, S.; Ibragimov, B.; Xing, L. Fully automated quantitative cephalometry using convolutional neural networks. J. Med. Imaging 2017, 4, 014501. [Google Scholar] [CrossRef] [PubMed]
- Olivetti, E.C.; Nicotera, S.; Marcolin, F.; Vezzetti, E.; Sotong, J.P.; Zavattero, E.; Ramieri, G. 3D soft-tissue prediction methodologies for orthognathic surgery—A literature review. Appl. Sci. 2019, 9, 4550. [Google Scholar] [CrossRef]
- Park, J.C.; Lee, J.; Lim, H.J.; Kim, B.C. Rotation tendency of the posteriorly displaced proximal segment after vertical ramus osteotomy. J. Craniomaxillofac Surg. 2018, 46, 2096–2102. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, H.P.; Jeon, K.; Lee, S.H.; Seo, J.K. Automatic 3D cephalometric annotation system using shadowed 2D image-based machine learning. Phys. Med. Biol. 2019, 64, 055002. [Google Scholar] [CrossRef]
- Yun, H.S.; Jang, T.J.; Lee, S.M.; Lee, S.H.; Seo, J.K. Learning-based local-to-global landmark annotation for automatic 3D cephalometry. Phys. Med. Biol. 2020, 65, 085018. [Google Scholar] [CrossRef]
- Kim, I.; Misra, D.; Rodriguez, L.; Gill, M.; Liberton, D.K.; Almpani, K.; Lee, J.S.; Antani, S. Malocclusion Classification on 3D Cone-Beam CT Craniofacial Images Using Multi-Channel Deep Learning Models. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 2020, 1294–1298. [Google Scholar]
- Lin, G.; Kim, P.J.; Baek, S.H.; Kim, H.G.; Kim, S.W.; Chung, J.H. Early Prediction of the Need for Orthognathic Surgery in Patients with Repaired Unilateral Cleft Lip and Palate Using Machine Learning and Longitudinal Lateral Cephalometric Analysis Data. J. Craniofac Surg. 2021, 32, 616–620. [Google Scholar] [CrossRef]
- Murata, S.; Lee, C.; Tanikawa, C.; Date, S. Towards a Fully Automated Diagnostic System for Orthodontic Treatment in Dentistry. In Proceedings of the 2017 IEEE 13th International Conference on e-Science (e-Science), Auckland, New Zealand, 24–27 October 2017; pp. 1–8. [Google Scholar]
- Ho, C.T.; Lin, H.H.; Liou, E.J.; Lo, L.J. Three-dimensional surgical simulation improves the planning for correction of facial prognathism and asymmetry: A qualitative and quantitative study. Sci. Rep. 2017, 7, 40423. [Google Scholar] [CrossRef]
- Xia, J.J.; Gateno, J.; Teichgraeber, J.F. New clinical protocol to evaluate craniomaxillofacial deformity and plan surgical correction. J. Oral. Maxillofac. Surg. 2009, 67, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Lin, H.H.; Lo, L.J.; Ho, C.T. Postoperative outcomes of two- and three-dimensional planning in orthognathic surgery: A comparative study. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Lonic, D.; Lo, L.J. 3D printing in orthognathic surgery—A literature review. J. Formos. Med. Assoc. 2018, 117, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Alkhayer, A.; Piffkó, J.; Lippold, C.; Segatto, E. Accuracy of virtual planning in orthognathic surgery: A systematic review. Head. Face Med. 2020, 16, 34. [Google Scholar] [CrossRef]
- Joshi, G.; Jain, A.; Adhikari, S.; Garg, H.; Bhandari, M. FDA approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: An updated 2022 landscape. medRxiv 2022. [Google Scholar] [CrossRef]
| AI Use | Description |
|---|---|
| Diagnosis and Treatment in OMFS | AI aids in diagnosis, cephalometrics, preoperative planning, intraoperative measurements, outcome evaluation, and postoperative follow-up. Also assists in managing impacted teeth, predicting tooth eruption risks, assessing surgical difficulty, predicting osteointegration success, and designing dental implants. |
| Robotic Surgery in OMFS | AI assists in cranial surgical procedures such as dental implants, tumor resection, biopsies, and temporomandibular joint surgery. AI enhances accuracy, reduces need for revisions, and ensures precise surgical resections. |
| Radiographic Image Enhancement | AI and deep learning improve radiographic image quality by reducing noise and deblocking, especially in low-dose CT images. Also, AI reduces motion artifacts and metal artifacts in dental CT and CBCT images. |
| Diagnostic AI Programs | Commercial programs such as dentalXr and Dentomo aid in diagnosing maxillofacial issues. AI is utilized to automatically segment and diagnose various cysts and tumors, and measure their volume. |
| Cephalometric Landmark Detection | AI and machine learning techniques automate landmark detection in cephalometric radiographs. Deep learning assists in this field, and AI algorithms help in detecting skeletal malocclusions using 3D craniofacial scans. |
| Surgical Planning | While current standard programs are non-AI, advancements in 3D imaging have enabled better surgical simulation. Computer-aided planning simplifies the surgical planning process and provides enhanced visualization. There is potential for AI growth in this area. |
| Efficiency and Safety in OMFS | AI can improve efficiency among surgeons by aiding in automated diagnosis based on radiology and updating patient records. It can also enhance patient safety by detecting drug interactions and correcting potential prescribing errors. |
| AI-powered apps | Promote optimal therapeutic compliance, ensuring adherence to treatment plans. |
| Smartphones with embedded biosensors | Continuous monitoring of physiological metrics for health prognostication. |
| Advanced AI algorithms | Analyze complex medical imaging data like CT scans and 3D models for preoperative assessments, surgical simulations, and personalized treatment plans. |
| Deep Learning Models | Identification of tumors, especially in oncology-oriented image analysis. |
| AI-assisted diagnosis-making | Match patient symptoms with appropriate physicians, medical diagnosis, prognosis, drug discovery, language translation, and organizing medical records. |
| Deep Convolutional Neural Network (DCNN) model | Diagnosis of skin conditions such as malignant melanoma, benign nevus, keratinocyte carcinoma, and seborrheic keratosis. |
| AI Assistants (e.g., BotMD) | Provide around-the-clock clinical support, bridging the gap between patients and healthcare providers, assisting in clinical decision-making. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miragall, M.F.; Knoedler, S.; Kauke-Navarro, M.; Saadoun, R.; Grabenhorst, A.; Grill, F.D.; Ritschl, L.M.; Fichter, A.M.; Safi, A.-F.; Knoedler, L. Face the Future—Artificial Intelligence in Oral and Maxillofacial Surgery. J. Clin. Med. 2023, 12, 6843. https://doi.org/10.3390/jcm12216843
Miragall MF, Knoedler S, Kauke-Navarro M, Saadoun R, Grabenhorst A, Grill FD, Ritschl LM, Fichter AM, Safi A-F, Knoedler L. Face the Future—Artificial Intelligence in Oral and Maxillofacial Surgery. Journal of Clinical Medicine. 2023; 12(21):6843. https://doi.org/10.3390/jcm12216843
Chicago/Turabian StyleMiragall, Maximilian F., Samuel Knoedler, Martin Kauke-Navarro, Rakan Saadoun, Alex Grabenhorst, Florian D. Grill, Lucas M. Ritschl, Andreas M. Fichter, Ali-Farid Safi, and Leonard Knoedler. 2023. "Face the Future—Artificial Intelligence in Oral and Maxillofacial Surgery" Journal of Clinical Medicine 12, no. 21: 6843. https://doi.org/10.3390/jcm12216843
APA StyleMiragall, M. F., Knoedler, S., Kauke-Navarro, M., Saadoun, R., Grabenhorst, A., Grill, F. D., Ritschl, L. M., Fichter, A. M., Safi, A.-F., & Knoedler, L. (2023). Face the Future—Artificial Intelligence in Oral and Maxillofacial Surgery. Journal of Clinical Medicine, 12(21), 6843. https://doi.org/10.3390/jcm12216843






