Abstract
Background: Exercise has been shown to improve quality of life (QoL) and even treatment outcomes in cancer patients. However, the evidence to support the benefits of exercise in patients with high-grade glioma (HGG) is limited. Therefore, we performed a randomized clinical trial (RCT) to examine the effect of augmented-reality-based rehabilitation exercises on physical and functional fitness, cognitive function, fatigue, mood, QoL, selected blood parameters, brain derived neurotrophic factor (BDNF), and S100 protein in patients with HGG. Methods: Adult patients with HGG scheduled to undergo radiotherapy after tumor resection were randomized to participate in an exercise program (experimental group, n = 25) or to receive usual care (controls, n = 22). Physical and mental fitness was measured at baseline, after the completion of radiotherapy, and at 3 months. The following tests were administered: Handgrip Strength Test; 6-Minute Walk Test; Time Up and Go test; Functional Independent Measure scale; Addenbrooke’s Cognitive Examination III (ACE III); Hospital Anxiety and Depression Scale; Functional Cancer Therapy Assessment—Brain; and Functional Assessment of Chronic Illness Therapy—Fatigue. We also measured blood parameters, BDNF, and S100 protein levels. Results: No significant changes were observed in the exercise group. However, the controls experienced a significant decrease in HGS and in the ACE III attention domain. No significant changes were observed in QoL, fatigue, BDNF, or S100 levels in either group. Conclusions: Augmented-reality-based exercise during radiation therapy may prevent loss of muscle strength and attention in patients with HGG.
1. Introduction
High-grade glioma (HGG) is an aggressive type of brain tumor. Although effective treatment options remain limited, standard care includes surgical resection, chemotherapy, radiotherapy (RT), and immunotherapy [1]. However, most patients develop recurrent disease. Although complete recovery is generally not possible, treatment can prolong the progression-free survival time [2], but with important treatment-related side effects, which may include motor dysfunction, neurocognitive disorders, pain, and fatigue [3,4], Fatigue is one of the most widespread and distressing long-term effects of cancer, particularly in brain tumor patients (in whom rates are 40–50% higher than in patients with other types of cancer) [5]. The effects of the disease and/or the treatment can result in mobility limitations, the impairment of activities of daily living (ADL), depression, loss of functional independence, and a decrease in quality of life (QoL) [3,6]. For this reason, maintaining or even improving QoL has increasingly become one of the main treatment objectives for these patients [7]. Although QoL in patients with glioblastoma is often already impaired at diagnosis [8], the side effects of treatment may further worsen QoL [9].
In recent years, several literature reviews have shown that physical activity and rehabilitation (both physical and cognitive) can improve motor function, cognitive function, the performance of ADLs, and QoL in patients with HGG [10,11], which is why the European Society of Neuro-Oncology recommends rehabilitation for patients with brain tumors [12]. Research has shown that, during oncological treatment, many patients reduce their physical activity levels [11]. Moreover, patients with brain tumors show lower levels of physical activity (only 22–41% meet recommended levels) than patients with other types of cancer (approximately 50%) [11,13]. Given these data, it could be advantageous to initiate exercise at the hospital during oncological treatment and to continue it after treatment has been completed.
At present, there is little information on the effectiveness of rehabilitation programs specifically designed for people with glioblastoma. However, some studies have been performed to assess the feasibility and effectiveness of resistance and aerobic training in different settings, including outpatient, hospital, home, and virtual reality environments [10,14,15]. To the best of our knowledge, only two studies [16,17] using virtual reality in the treatment of patients with brain tumors have been published so far. However, the results of a meta-analysis [18] indicate the usefulness of physiotherapy using augmented reality in improving balance and gait, upper limb functionality, muscle mass, physical performance, and exercise self-efficacy and in reducing the risk of falls in other patient groups. Augmented reality is increasingly used to support patients in physical rehabilitation, enabling them to perform virtual exercises and track progress in real time [19]. Virtual and augmented reality technology has great potential in the field of neurorehabilitation, providing patients with an engaging and safe environment in which to improve their motor and cognitive functions. This technology offers a more engaging and personalized approach to rehabilitation [19]. In turn, the most successful interventions in the rehabilitation of brain tumor patients reported to date are those that include personalized exercise recommendations, individualized training, and strategies designed to increase adherence, such as close monitoring of training data and regular guidance from a physiotherapist [10]. Given these findings, it seems that exercise and training programs supported by augmented reality could be highly beneficial, as they would allow physiotherapists to personalize the exercise program and to monitor the patient’s performance in the hospital ward and at home [20,21].
Another key aspect of rehabilitation in this patient population (apart from physical exercise) is cognitive function therapy. At diagnosis, a substantial proportion of patients report cognitive impairment (31–81%), at a rate that is higher than in any other type of cancer [22]. The incidence of cognitive dysfunction may vary over the course of the disease. Although some reports suggest that cognitive function improves 3 to 6 months after tumor resection [22], other studies have found that cognitive function remains impaired (or even worsens further) due to radiotherapy and chemotherapy and/or tumor progression. Not surprisingly, this dysfunction also negatively impacts health-related quality of life (HRQoL) for these patients [22,23].
The effectiveness of rehabilitation in patients with primary brain tumors can be assessed through the use of functional assessment scales and fitness and cognitive tests [14]. However, the impact of physical exercise on biochemical parameters in this patient population has not yet been evaluated. In this regard, serum levels of brain-derived neurotrophic factor (BDNF) may fluctuate depending on the level of exercise and may affect cognitive performance in patients with HGG. BDNF is a key molecule involved in brain plasticity, and it also plays an essential role in neurocognitive function [24,25]. Exercise has been shown to increase BDNF expression under both normal and pathological conditions [25]. However, the role of BDNF in HGG remains unclear. One study evaluated the association between BDNF and physical activity in HGG [26], finding that BDNF produced by glioblastoma-differentiated cells acts on glioblastoma stem cells, fostering their growth through paracrine signaling. A recent preliminary report suggested that BDNF, acting on different cells, can reorganize the brain microenvironment in such a way that it becomes resilient to neurodegeneration [24].
Proteins from the S100 family have been associated with the progression, diagnosis, and prognosis of glioma [27]. Changes in S100 protein levels induced by physical exercise have been studied in patients with multiple sclerosis [28] and in older people with vascular cognitive impairment [29]. However, studies on the relationship between the S100 protein and exercise in patients with HGG have not been undertaken.
Therefore, we hypothesized that training using augmented reality will have a beneficial effect on the physical and cognitive performance of patients with brain tumors treated with radiotherapy and will improve their quality of life. We also assume that exercise can have a positive impact on the selected blood parameters. In this context, we conducted a randomized clinical trial (RCT) to examine the effect of augmented-reality-based rehabilitation exercises on physical and functional fitness, cognitive function, fatigue, mood, QoL, selected blood parameters, BDNF, and S100 protein in patients with HGG.
2. Materials and Methods
2.1. Study Design
This was a randomized clinical trial involving patients scheduled to undergo radiotherapy after the surgical removal of a brain tumor. Patients were randomized to the active exercise group or to the control group. The study was conducted from October 2021 to April 2023 at the Radiotherapy Department at the Greater Poland Cancer Center in Poznan, Poland. The study protocol was approved by the local Bioethics Committee of the Poznan University of Medical Sciences (No. 703/18). The study was registered at clinicaltrials.gov (accessed on 9 September 2023) (identifier: NCT05192447) and was created as a result of the research project No. 2020/37/B/NZ7/01122 supported by the National Science Center.
2.2. Participants
The inclusion criteria were as follows: age 18–70 years; confirmed diagnosis of stage III or IV glioma (according to the 2021 World Health Organization Classification of Tumors of the CNS [30]); eligibility for radiotherapy; and good general physical condition (score of 0–2 on the Eastern Cooperative Oncology Group fitness scale). The exclusion criteria were: ≥2 brain lesions; psychological or psychiatric illness under pharmacological treatment; presence of other neurological disorders (e.g., multiple sclerosis, Parkinson’s disease); and/or significant clinical circulatory failure (New York Heart Association scale, stage III or IV).
Patients meeting the above criteria were given the opportunity to participate in the trial by their treating radiation oncologist. All participants were required to provide written informed consent to participate in the study. Participation was completely voluntary, and participants could withdraw at any time.
2.3. Sample Size
To determine the sample size, we performed a power analysis using G*Power [31] with the following assumptions: Cohen’s f for repeated measures ANOVA: 0.25; alpha: 0.05; power: 0.80; number of groups: 2; number of time points: 3. Based on these assumptions, the calculated minimum sample size was N = 44. To allow for dropouts, we recruited a total of 72 participants.
2.4. Radiotherapy Procedure
All patients received intensity-modulated radiotherapy (IMRT), delivered over 30 days under a conventional fractionation regimen (2 Gy per dose, total dose = 60 Gy) following the schedule described by Scaringi et al. [32].
2.5. Randomization
Patients were divided into the two study groups by simple randomization using a computer-generated list of random numbers.
2.6. Exercise Program
Patients allocated to the exercise group undertook regular physical exercise according to an in-house protocol. The physical training schedule (duration, frequency, and intensity) was designed to meet the American Cancer Society’s recommendations for cancer patients [33]. Exercises were carried out at the hospital under the supervision of a qualified physiotherapist throughout the 30-day RT treatment period. Physical training was conducted before each RT session (23 h after the previous dose). Upon completion of the full 30-day RT program, the patients performed a set of exercises in the morning at home according to the same rules, using the Neuroforma remote program https://www.neuro-forma.com/science/ (accessed on 9 September 2023). Once a month, stationary in-person consultations with a physiotherapist were held in order to verify the effects and introduce any corrections to the training plan. Physical activity was moderate, with a maximum heart rate (HRmax) of 70% (as calculated by HRmax = 220−age) during training. Exercises were performed five times a week. The duration of the training session was 60 min, distributed as follows: 10 min of warm-up, 40 min of training, and 10 min of relaxation exercises. “Proper” training included 40 min of exercises using the Neuroforma neurorehabilitation device. This device allows users to perform exercise in an augmented reality environment using a posturographic platform with visual biofeedback. This device consists of a large display (20-inch monitor, which provides interaction feedback), a computer system, a 3D optical system enabling precise observation of patients’ activity, a balance platform for assessing balance, and a safety barrier. During the exercises, the patient stands or sits in front of the monitor screen. The camera system records his figure and movements. The exerciser sees a real, mirror image on the screen, with virtual objects appearing around it. The patient’s task is to direct his reflection in such a way as to catch, move, or hit the appearing objects. Figure 1 shows a patient performing exercises using an augmented reality device. The program allows users to choose from 20 games to improve the joint range of motion and muscle endurance of the upper limbs, eye–hand coordination, balance, reaction speed, and cognitive functions (including attention, memory, reading, and counting). During balance exercises, the patient stands on a wireless posturography platform. Static posturography is performed, recording the displacement of the center of gravity projection relative to the plane of the device platform by measuring the directions of foot pressure forces. During balance exercises, the patient sees an object on the screen, which he controls by balancing on the platform. Some of the exercises also require performing tasks with the upper limbs while controlling balance. In addition to the exercise function, monitoring of important posturographic parameters is also available: measurement of the ellipse area and center of pressure path. Using augmented reality technology, the patient receives immediate feedback on the correctness and level of exercise performance (i.e., biofeedback). For each exercise, the level of difficulty can be graduated as appropriate. The training was progressive. The physiotherapist selected the initial level of difficulty and increased or decreased it during the examination depending on the condition of the exerciser.
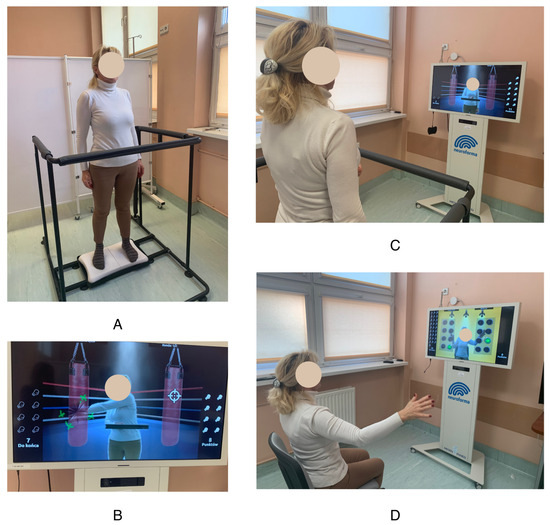
Figure 1.
Exercises using augmented reality. (A) Standing position on the posturographic platform; (B,C) screen with the figure of the patient during exercise; (D) sitting position during the exercise.
2.7. Control Group
The control group performed normal activities during the day. However, they were asked to record their physical activity using daily activity notes. Patients allocated to this group received the standard recommendations regarding the minimum level of physical activity [34].
2.8. Study Scheme
Study participants were examined at three different time points: (1) before the start of radiotherapy (T0), (2) the day after completion of the full RT program (T1), and (3) three months after completion of RT (T2).
2.9. Measurements
To assess the effectiveness of patients’ rehabilitation, questionnaires and research tools were used that are validated and widely used in the assessment of oncological patients, in particular patients with brain tumors. Both subjective and objective assessment tools were used. The Hand Grip Strength (HGS) test, 6-Minute Walk Test (6MWT), and Timed Up and Go (TUG) test are indicated as the most common cancer objective outcome measures [35].
A qualified physiotherapist conducted the fitness tests and assessed QoL and fatigue levels. A neuropsychologist was responsible for assessing cognitive function, depression, and anxiety. The following tests were administered.
2.9.1. Hand Grip Strength (HGS) Test
The HGS is an indicator of overall muscle strength, physical fitness, and overall health and nutrition. HGS is a predictor of mortality and length of hospital stay [36]. This measurement is widely used to assess older individuals and cancer patients [37,38]. The HGS test was performed according to the guidelines established by the American Society of Hand Therapists [39] using a hand hydraulic dynamometer (JAMAR, Sammons Preston Rolyan, Bolingbrook, IL, USA). During this process, the participant was asked to sit in a chair without armrests or a backrest, with his/her feet located parallel on the floor. The knees and hips were flexed at 90 degrees. The arm of the tested hand was adducted to the trunk, the elbow was flexed at a right angle, the forearm was in a neutral position. Then, the participants were asked to grip a level with as much force as possible for 6 s. Three repetitions were performed, with a 1 min interval between them. The highest value achieved was used for the study.
2.9.2. 6-Minute Walk Test (6MWT)
The 6MWT was used to assess functional capacity. This test is commonly used in clinical trials on cancer patients to estimate aerobic capacity [40]. The test was conducted following the guidelines of the American Thoracic Society [41]. A total distance of 30 m was divided into 3 m sections marked with tape on the floor. A 10 min rest was required before starting the test. During the test, the patient was asked to walk at a natural pace for 6 min. The parameter of interest was the total distance walked.
2.9.3. Timed Up and Go (TUG) Test
The TUG test was used to assess functional mobility. This is a reliable, validated tool originally developed to assess mobility in older populations, but it is also widely used for younger populations. It is used to measure response to treatment in terms of improvement in function and QoL. During the test, the patient sits in a chair, with his/her back against the backrest with the forearms on the armrests. The patient is then asked to stand up and walk a total of 3 m (marked with tape on the floor) at normal speed. The patient then turns around, returns to the chair, and sits down. The task was performed three times, and the times were averaged for the statistical analysis [42].
2.9.4. The Functional Independence Measure (FIM)
The FIM is an 18-item scale recommended for use on patients with neurological illnesses. This scale is designed to evaluate physical, psychological, and social function [43]. It is also used in the evaluation of oncological patients with both primary and metastatic brain tumors [44]. It assesses performance in six areas: self-care, continence, mobility, transfers, communication, and cognition [43]. The scale assesses the patient’s degree of dependence on the help of others in everyday activities. This tool is used to assess a patient’s level of disability and changes in response to rehabilitation or a medical intervention [43,44].
2.9.5. Quality of Life
The Polish language version of the FACT-Br (Functional Cancer Therapy Assessment—Brain) questionnaire, originally developed for use in clinical trials, was used to assess QoL [45]. This questionnaire was developed specifically for the assessment of patients with primary brain tumors and is widely used for this purpose [46]. This scale contains 51 questions. The scale is considered highly accurate, has good psychometric properties, and is an effective measure of QoL in patients with brain tumors. The instrument includes physical, functional, familial, social, and emotional domains and other items specific to the problems commonly encountered by patients with brain tumors. Respondents answer questions on a five-point Likert scale, ranging from “0” (“not at all”) to “4” (“very much”) [47].
2.9.6. Fatigue
The Functional Assessment of Chronic Illness Therapy—Fatigue (FACIT-F) scale was used to assess symptoms of fatigue. This tool is widely used to assess fatigue in patients with cancer and has good internal consistency and test–retest reliability. This scale consists of 13 items to measure an individual’s level of fatigue during ADLs over the past week. Responses are given on a 5-point Likert scale and range from 0 to 4. Total scores range from 0 to 52, with higher scores indicating less fatigue. Scores < 30 are considered to indicate severe fatigue [48].
2.9.7. Depression and Anxiety
The Hospital Anxiety and Depression Scale (HADS) was used to assess depressive and anxiety symptoms. This self-report questionnaire is commonly used to assess emotional stress, including for cancer patients. HADS consists of two subscales to evaluate anxiety and depression over the past week. Each subscale consists of seven items with four possible response options. Total scores on each subscale range from 0 to 21, with higher total scores indicating greater levels of anxiety or depression [49].
2.9.8. Addenbrooke’s Cognitive Examination III (ACE III)
ACE III was used to assess cognitive function. This screening tool can differentiate between participants with and without cognitive impairment. The questionnaire consists of 21 questions divided into five sections (attention, memory, fluency, language, and visuospatial processing). The maximum test score is 100, which should be interpreted in the context of the patient’s overall history and clinical examination [50]. ACE III shows its accuracy in assessing cancer patients and is indicated as an important tool for quick and easy neurocognitive function assessment in patients with glioma [51].
2.9.9. Laboratory Tests
Blood was drawn via a venous puncture after overnight fasting. Patients were asked to avoid intense physical activity in the 24 h before blood sampling. The serum and plasma samples were stored at −80 °C until final analysis.
Venous blood samples were taken to measure hemoglobin (Hb), white blood cells, red blood cells, neutrophils, lymphocytes, and monocytes. Platelets were also obtained and processed by a centralized laboratory. Biochemical markers were measured using the Cobas 6000TM clinical chemistry analyzer (Roche; Mannheim, Germany). Hematological indices (complete blood count, hemoglobin) were analyzed in EDTA-blood with the XT-2000iTM (Sysmex Corporation; Kobe, Japan).
We also measured alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase (CK), sodium, creatinine, and bilirubin. These parameters were determined quantitatively in serum using the COBAS 6000 analyzer. AST, CK, and CKMB tests were performed using kinetic methods with absorbance measurement. High-sensitivity C-reactive protein (hsCRP) was determined using an immunoturbidimetric method enhanced with latex particles.
BDNF was evaluated in sera and in peripheral blood mononuclear cell lysates using the enzyme-linked immunoassay (ELISA) method. The astrocytic protein S-100 was estimated using ELISA. To improve the quality of the results and to shorten the reading time, we used the Synergy HTX system to automate the reading of ELISA tests.
2.10. Statistical Analysis
Statistical analysis was performed using the Statistica 13.3 software (TIBCO Software, Poland). The threshold for statistical significance was set at p < 0.05. The Shapiro–Wilk test was used to check distribution normality. The ANOVA test for repeated measures was used to examine differences over time. The assumption of sphericity was checked using the Mauchley test; if sphericity was violated, the Greenhaus–Geisser correction was applied. Tukey’s test was used for post hoc analyses. For variables with a non-normal distribution, and in comparisons of ordinal variables, we applied Friedman’s test with Dunn’s post-hoc test. Student’s t test was used for the comparison of groups with normally distributed variables; the Mann–Whitney test was used to compare groups with a non-normal distribution and for ordinal variables.
3. Results
A total of 100 patients were assessed for study eligibility. Of these, 28 were excluded for the following reasons: lack of consent (n = 13); age > 70 years (n = 4); more than two tumors (n = 4); neurological disorders (n = 3); NYHA III or IV (n = 3). During the first assessment (T0), a total of fourteen patients (six in the experimental group and eight in the controls) withdrew consent. At the second assessment (T1), a recurrence was detected in seven patients (three in the experimental group and four in controls), one patient in the exercise group died, and six patients (two and four in the exercise and control groups, respectively) withdrew. Figure 2 shows the study flow diagram.
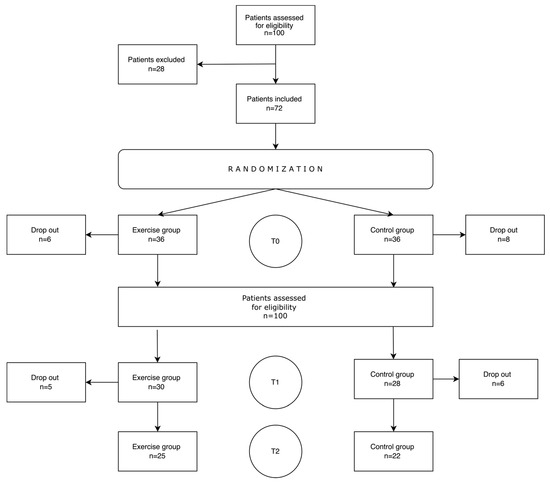
Figure 2.
Flow of participants through the study.
Overall, the mean age of the study participants was 52.58 ± 14.21 years. Patients in the exercise group were, on average, significantly younger than controls (Table 1). For this reason, we initially compared the two groups in terms of independence on the FIM scale and cognitive functioning, finding no differences. Most patients in both groups were men. All participants underwent surgical resection, and all received radiotherapy and chemotherapy.

Table 1.
Characteristics of the study groups.
The characteristics of the participants according to treatment allocation are presented in Table 1.
3.1. Physical Fitness, Mental Health, and Quality of Life Results
In the controls, we observed a statistically significant decrease in HGS between T0 and T2 (p = 0.017). By contrast, HGS values in the experimental group increased, although not significantly. Table 2 and Table 3 show changes in results over time by group. No significant changes in the results of the other fitness tests were observed in either group. QoL decreased in both groups between T0 and T2, but this change was not statistically significant.

Table 2.
Physical fitness, mental health, and quality of life results at the three study time points in the exercise group.

Table 3.
Physical fitness, mental health, and quality of life results at the three study time points in the control group.
The ACE III domain attention score decreased significantly in the controls (p = 0.047) but remained unchanged in the exercise group.
Table 4 shows the individual measurements of the two groups (Table 4). There were statistically significant between-group differences in changes in HGS between T0 and T1 (p = 0.015) and between T0 and T2 (p = 0.006). In the experimental group, HGS increased by 2.59 ± 5.14 kg between T0 and T1 and by 2.91 ± 4.87 kg between T0 and T2. By contrast, in the control group, these values decreased by 1.82 ± 2.75 kg and 8.00 ± 9.54 kg, respectively. The changes in the time taken to perform the TUG test between the T0 and T2 were statistically significantly in both groups (p = 0.029), decreasing by 0.02 ± 1.08 s in the exercise group and increasing by 1.75 ± 2.31 s in the control group.

Table 4.
Mean differences between measurements of physical fitness, mental health, and quality of life at different time points for the exercise and control groups.
Significant differences were observed between the groups regarding the change in the HADS depression results between T0 and T1(p = 0.022), with an increase of 2.09 ± 5.86 points in the control group and a decrease of 1.69 ± 3.42 in the experimental group.
Selected results are presented in Figure 3.
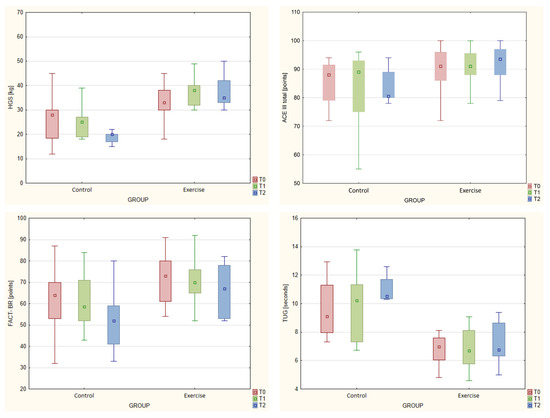
Figure 3.
Box and whisker charts showing changes over time in selected variables across the two groups.
3.2. Laboratory Test Results
Regarding the laboratory tests, statistically significant changes were only observed for mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH), with significant increases in both the experimental (MCV, p = 0.029 and MCH, p = 0.023) and control group (p = 0.014 and p = 0.029, respectively). Bilirubin levels increased by 0.30 ± 0.41 in the experimental group and decreased by 0.07 ± 0.10 mg/dL in the control group, presenting a significant between-group difference (p = 0.019). Detailed laboratory test results for both groups are presented in the Appendix (Table A1, Table A2 and Table A3).
4. Discussion
The main aim of this RCT was to determine the effects of an augmented-reality-based physical and cognitive rehabilitation program on physical and cognitive function, mental health, laboratory parameters, and QoL in patients with HGG undergoing radiotherapy. Our main finding was that the exercise group experienced no significant changes in any of the parameters. By contrast, the controls experienced a significant decrease in hand grip strength and a decline in attention (ACE III). These findings suggest that exercise during radiation therapy may prevent loss of muscle strength and attention in this patient population.
Much is already known about the positive impact of physical exercise on oncological patients. Nearly 700 clinical trials involving more than 50,000 cancer patients have demonstrated the positive effects of exercise during the treatment and recovery phases, especially in ameliorating side effects such as fatigue, mental stress, and physical limitations [52]. However, in patients with HGG, less attention has been paid to physical and cognitive rehabilitation. Studies have shown that most patients with a primary brain tumor are open to exercise suggestions and that most patients are able to participate in an exercise program during and after cancer treatment [53,54]. Our research was one of the few studies that used augmented reality in the exercise program. It is worth noting that the study used non-wearable equipment that did not employ full immersion. In previous studies in other patient groups, head-mounted display caused some patients to experience nausea and headaches [55].
At present, there is no standardized exercise protocol for patients with brain glioma. A mini review published in 2023 [10] found that the most successful interventions included personalized exercise recommendations, individualized training, and adherence strategies such as training data monitoring and regular guidance from a physiotherapist. Based on the findings of that review, we decided to offer patients augmented reality training because it allows the physiotherapists to personalize and regularly monitor the exercise program, both during the stay in the ward and at home.
One of the key aims of our study was to assess the impact of exercise on physical fitness (measured by the HGS, TUG, and 6MWT tests). Previous studies on patients with low-grade glioma have found reduced muscle strength and cardiorespiratory capacity during cancer treatment [56]. For this reason, we wanted to see if exercise could prevent this process in HGG patients. Based on the result of the HSG test—in which the exercise group maintained their strength level while the controls lost some strength—it seems clear that exercise can help patients maintain their strength over time. In terms of functional mobility, the results of the TUG tests also suggest the benefit of exercise, as evidenced by the significant between-group differences in the TUG test results between T0 and T2 (a 1.75 s increase in controls vs. no change in the exercise group). Regarding the 6MWT, there were no significant changes over time in either group, and no significant differences in mean scores between the groups. Nowak et al. [57] recently evaluated the impact of resistance and aerobic training on HGG patients during radiochemotherapy. Those researchers observed a significant increase in lower limb muscle strength before and after the intervention (6 weeks). Unfortunately, that study did not include a control group. Eisenhut et al. [58] compared the effect of two types of physical training (endurance and strength) on the physical fitness of patients with glioma (WHO grades III and IV). Interestingly, while muscle strength (measured by HGS) improved in both groups, 6MWT scores improved only in the active control group (but not in the endurance training group). Given the findings described above, it seems clear that more research is needed to better determine the effects of different types of training on muscle strength during oncological treatment in patients with glioblastoma. Our findings suggest that while exercise may not significantly improve strength, it could prevent a decline during and after oncological treatment, which would have a direct positive impact on the functional efficiency of these patients.
The only significant change regarding the cognitive tests was a decrease in the attention domain of the ACE III test in the control group. Importantly, attention did not decrease in the exercise group, suggesting that exercise may be preventative. In the control group, we observed a decrease in mean total ACE III scores, but this was not statistically significant. Attention is one of the cognitive domains most often disturbed in patients with glioma [59]. However, we have not found any studies that have assessed the impact of physical exercise on cognitive function in patients with HGG during radiochemotherapy using validated neuropsychological tests. Gehring et al. [60] found that aerobic exercise had a positive impact on cognitive function, but this study was performed on patients who had completed oncological treatment at least 6 months before enrolment. Similarly, in other types of cancer, the relationship between exercise and cognitive function during cancer treatment is not well understood. A national cohort study (in the United States) of 580 breast cancer patients and 363 age-matched controls showed that patients who met the physical activity guidelines (defined as 150 min per week of moderate-to-vigorous physical activity) during chemotherapy had significantly better cognitive function than those who did not meet the recommended amount of exercise [61]. A protocol was recently published describing a new study [62] to determine the impact of intensive interval training on cognitive function in breast cancer patients. That study will undoubtedly shed more light on this topic when the results are published.
The positive benefits of physical activity on cognitive function are generally believed to be attributable to the exercise-induced expression of neurotrophic and neuroprotective markers (including BDNF), which promote neurogenesis in certain areas of the brain [63]. This is important given that chemoradiation may contribute to hippocampal degeneration [64,65] while exercise has been shown to improve hippocampal-dependent cognition and to increase hippocampal volume [66,67,68]. Exercise also reduces inflammatory markers, which are commonly elevated in response to aggressive cancer treatment [69]. To our knowledge, our study is the first to determine changes in BDNF levels in response to training in patients with HGG during oncological treatment. However, we observed no significant changes in BDNF levels over time in either group, suggesting that exercise may not impact BDNF, a finding that is consistent with a study by Miklja et al. [26], who found that the level of exercise in adults with glioblastoma had no effect on the circulating secretion of BDNF. Nonetheless, those findings must be interpreted cautiously given that physical activity was self-reported in that study. In a study involving women with ovarian cancer [70], Cartmel et al. found no differences in BDNF levels between exercisers and non-exercisers. By contrast, a meta-analysis of randomized clinical trials found that exercise increases BDNF concentration in plasma in healthy people [71]. Similarly, one study found that exercise increased BDNF in patients with neurodegenerative disorders [72]. However, the impact of physical activity on BDNF levels in patients with cancer (including HGG), remains unclear at present. In this regard, we believe that the study by Miklja et al. [26], together with our study, may serve as a basis for further research into this question.
We found no significant changes over time in either group in terms of QoL or fatigue levels, nor did we observe any between-group differences in these variables. In both groups, QoL worsened over time, but not significantly. In the control group, the level of fatigue was similar at all three time points. By contrast, perceived fatigue increased in the experimental group, but not significantly. Nowak et al. examined the effects of physical training on patients with glioblastoma [57], finding no improvement in fatigue or QoL, but—importantly—no deterioration in either of these variables over time. Eisenhut et al. [58] carried out of a similar study, which showed an increase in fatigue in the exercise groups and a decrease in the active control group. Hansen et al. also found that physiotherapy based on an exercise intervention and occupational therapy did not positively impact HRQOL [12]. By contrast, other researchers have found that exercise has a positive impact on fatigue and QoL [73]. The findings of the aforementioned studies suggest that, unlike other types of cancer [74,75], it may be more difficult to alleviate fatigue in patients with glioblastoma; alternatively, perhaps the dose and type of training were not appropriate. In any case, it is clear that more research is warranted.
Although we found no significant changes in either group in anxiety or depression, the control group presented worse HADS depression scores between T0 and T1 compared to the exercise group (Table 4). This finding suggests that physical exercise during radiochemotherapy may help to prevent depression during hospitalization. Other researchers [58] found that the endurance training and control groups had a positive change in depressive symptoms while the strength training group did not.
To our knowledge, this is the first study to examine the effects of exercise on blood chemistry and the permeability of the blood–brain barrier (BBB) (S100 protein) in patients with HGG during radiotherapy. However, we found no statistically significant changes in S100 protein levels in either group and no differences between the groups. In controls, S100 levels decreased slightly, while these levels remained unchanged in the exercise group. In studies involving older women, physical exercise appears to seal the BBB [76]. However, this effect was not observed in our sample of patients with HGG.
Bilirubin levels increased in the exercise group but decreased in controls. This finding may or may not be relevant given that many factors, including chemotherapy, can influence bilirubin levels. However, this finding is consistent with other studies showing that physical activity increases plasma bilirubin levels [77], which can cause certain adaptations and beneficial metabolic changes in people who exercise [78].
This study has several limitations. First, the study design did not consider the interaction between the studied variables. Further research focused on this topic should be based on multivariate analyses. Another study limitation is that we did not directly assess the level of physical activity in the control group, but rather we relied on the patients’ self-reported declaration of daily activity. By contrast, an important strength of our study is that it is the first to determine and compare BDNF and S100 protein levels in exercising and non-exercising HGG patients during radiotherapy. Another strength is that we measured a large range of parameters at three distinct time points (pre- and post-RT, and at 3 months of follow-up), thus providing a clear picture of how these parameters change over time. These novel data complement the findings of other studies, thus broadening our understanding of the effects of exercise in this patient population.
5. Conclusions
In conclusion, the results of this trial suggest that augmented reality training in HGG patients during and after radiotherapy can prevent the decline of muscle strength and attention. The effects of physical training on blood parameters, BDNF, and S100 protein levels remain unclear.
Author Contributions
Conceptualization, A.P. (Anna Pieczyńska), E.Z. and K.H.; methodology, A.P. (Anna Pieczyńska), D.P. and K.H.; formal analysis, K.H., K.A. and A.P. (Agnieszka Pilarska); investigation, A.P. (Anna Pieczyńska), D.P. and A.P. (Agnieszka Pilarska); resources, A.P. (Anna Pieczyńska) and E.Z.; data curation, A.P. (Anna Pieczyńska), K.A., D.P. and A.P. (Agnieszka Pilarska); writing—original draft preparation, A.P. (Anna Pieczyńska) and E.Z.; writing—review and editing, K.H., D.P., K.A. and E.Z.; visualization, A.P. (Anna Pieczyńska) and K.H.; supervision, K.H.; project administration, A.P. (Anna Pieczyńska) and K.H.; funding acquisition, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was funded by the National Science Center, grant number UMO2020/37/B/NZ7/01122.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Poznan University of Medical Sciences (No 703/18) in Poland.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated for this study are available upon request to the corresponding author.
Acknowledgments
The authors would like to thank the patients who participated in the study for their cooperation, as well as the head and staff of the radiotherapy department for helping to organize the examination of the patients.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Laboratory results at three time points in the exercise group.
Table A1.
Laboratory results at three time points in the exercise group.
| Parameters (Mean ± SD) | Baseline | After RT | After 3 Months | p-Value |
|---|---|---|---|---|
| S-100 µg/L | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.223 |
| Sodium mmol/L | 139.88 ± 2.06 | 142.63 ± 4.37 | 139.17 ± 2.48 | 0.459 |
| Potassium mmol/L | 4.25 ± 0.36 | 4.35 ± 0.57 | 4.00 ± 0.15 | 0.789 |
| Glucose mg/dL | 94.69 ± 7.85 | 95.45 ± 15.58 | 99.50 ± 14.34 | 0.534 |
| Creatinine mg/dL | 0.87 ± 0.17 | 0.92 ± 0.18 | 0.97± 0.22 | 0.496 |
| AST U/L | 18.44 ± 5.34 | 18.90 ± 9.61 | 20.83 ± 4.36 | 0.311 |
| ALT U/L | 26.69 ± 12.20 | 34.80 ± 32.50 | 33.67± 18.77 | 0.401 |
| Bilirubin mg/dL | 0.50 ± 0.37 | 0.66 ± 0.41 | 0.76 ± 0.50 | 0.105 |
| WBC G/L | 7.95 ± 2.46 | 8.25 ± 3.54 | 8.55 ± 3.76 | 0.937 |
| LYM G/L | 2.01 ± 0.67 | 1.79 ± 0.90 | 1.73 ± 0.84 | 0.148 |
| NEU G/L | 5.14 ± 1.89 | 5.69 ± 2.93 | 5.97 ± 3.35 | 0.913 |
| MON G/L | 0.60 ± 0.26 | 0.62 ± 0.27 | 0.65 ± 0.26 | 0.803 |
| EOS G/L | 0.12 ± 0.10 | 0.14 ± 0.17 | 0.18 ± 0.25 | 0.661 |
| IG G/L | 0.08 ± 0.12 | 0.07 ± 0.07 | 0.12 ± 0.20 | 0.704 |
| BASO G/L | 0.04 ± 0.03 | 0.01 ± 0.02 | 0.02 ± 0.02 | 0.423 |
| RBC T/L | 4.71 ± 0.38 | 4.67 ± 0.56 | 4.68 ± 0.53 | 0.523 |
| HBG mmol/L | 8.59 ± 0.74 | 8.85 ± 1.07 | 8.69 ± 0.71 | 0.289 |
| HCT L/L | 0.42 ± 0.03 | 0.43 ± 0.05 | 0.42 ± 0.03 | 0.307 |
| MCV fL | 88.82 ± 4.89 | 91.34 ± 4.15 | 90.98 ± 7.24 | 0.029 b |
| MCH fmol | 1.83 ± 0.13 | 1.90 ± 0.10 | 1.88 ± 0.19 | 0.023 b |
| MCHC mmol/L | 20.54 ± 0.55 | 20.79 ± 0.43 | 20.57 ± 0.53 | 0.305 |
| PLT G/L | 267.94 ± 81.01 | 187.60 ± 51.45 | 229.17 ± 55.62 | 0.148 |
| BDNF pg/mL | 233.89 ± 222.25 | 242.94 ± 188.68 | 330.67 ± 297.57 | 0.847 |
b. difference in post-hoc test between baseline and after 3 months. Abbreviations: RT, radiotherapy; SD, standard deviation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; WBC, white blood count; LYM, lymphocytes; NEU, neutrophils; MON, monocytes; EOS, eosinophils; IG, immunoglobulins; BASO, basophils; RBC; red blood cell; HBG; hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet count; BDNF, brain-derived neurotrophic factor.

Table A2.
Laboratory results at three time points in the control group.
Table A2.
Laboratory results at three time points in the control group.
| Parameters (Mean ± SD) | Baseline | After RT | After 3 Months | p-Value |
|---|---|---|---|---|
| S-100 µg/L | 0.13 ± 0.17 | 0.06 ± 0.05 | 0.07 ± 0.04 | 0.105 |
| Sodium mmol/L | 139.50 ± 2.99 | 139.17 ± 3.06 | 140.25 ± 2.19 | 0.09 |
| Potassium mmol/L | 4.12 ± 0.22 | 4.05 ± 0.33 | 4.07 ± 0.22 | 0.441 |
| Glucose mg/dL | 110.63 ± 27.50 | 98.86 ± 26.28 | 108.88 ± 26.71 | 0.779 |
| Creatinine mg/dL | 0.79 ± 0.10 | 0.87 ± 0.15 | 0.77 ± 0.05 | 0.075 |
| AST U/L | 18.93 ± 5.93 | 23.33 ± 16.00 | 20.00 ± 9.01 | 0.738 |
| ALT U/L | 31.53 ± 23.51 | 43.44 ± 17.52 | 39.75 ± 29.60 | 0.513 |
| Bilirubin mg/dL | 0.39 ± 0.12 | 0.44 ± 0.12 | 0.30 ± 0.09 | 0.094 |
| WBC G/L | 8.76 ± 3.88 | 6.90 ± 2.01 | 6.83 ± 2.60 | 0.399 |
| LYM G/L | 1.71 ± 0.76 | 1.34 ± 0.54 | 1.23 ± 0.65 | 0.186 |
| NEU G/L | 6.34 ± 3.32 | 5.14 ± 1.72 | 5.22 ± 2.36 | 0.686 |
| MON G/L | 0.56 ± 0.23 | 0.53 ± 0.21 | 0.38 ± 0.14 | 0.206 |
| EOS G/L | 0.07 ± 0.07 | 0.07 ± 0.11 | 0.04 ± 0.06 | 0.513 |
| IG G/L | 0.14 ± 0.24 | 0.06 ± 0.10 | 0.11 ± 0.18 | 0.216 |
| BASO G/L | 0.03 ± 0.04 | 0.01 ± 0.01 | 0.02 ± 0.03 | 0.129 |
| RBC T/L | 4.34 ± 0.40 | 4.30 ± 0.36 | 4.08 ± 0.43 | 0.149 |
| HBG mmol/L | 8.31 ± 0.77 | 8.34 ± 0.71 | 8.08 ± 0.80 | 0.658 |
| HCT L/L | 0.40 ± 0.03 | 0.40 ± 0.04 | 0.39 ± 0.03 | 0.760 |
| MCV fL | 92.31 ± 3.05 | 93.26 ± 2.31 | 96.37 ± 5.64 | 0.014 b |
| MCH fmol | 1.91 ± 0.07 | 1.94 ± 0.06 | 1.99 ± 0.10 | 0.0285 a,c |
| MCHC mmol/L | 20.75 ± 0.47 | 20.84 ± 0.45 | 20.65 ± 0.45 | 0.559 |
| PLT G/L | 253.75 ± 77.37 | 211.86 ± 71.76 | 187.10 ± 52.20 | 0.202 |
| BDNF pg/mL | 386.02 ± 369.26 | 227.69 ± 149.58 | 143.24 ± 99.94 | 0.234 |
a. difference in post-hoc test between baseline and after RT; b. difference in post-hoc test between baseline and after 3 months; c. difference in post-hoc test between after RT and after 3 months. Abbreviations: RT, radiotherapy; SD, standard deviation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; WBC, white blood count; LYM, lymphocytes; NEU, neutrophils; MON, monocytes; EOS, eosinophils; IG, immunoglobulins; BASO, basophils; RBC; red blood cell; HBG; hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet count; BDNF, brain-derived neurotrophic factor.

Table A3.
Mean differences between laboratory tests in both groups at three time points.
Table A3.
Mean differences between laboratory tests in both groups at three time points.
| Parameters | T0 and T1 | T1 and T2 | T0 and T2 | |||
|---|---|---|---|---|---|---|
| EG | CG | EG | CG | EG | CG | |
| S-100 µg/L | 0.00 ± 0.02 | −0.07 ± 0.17 | −0.01 ± 0.02 | −0.01 ± 0.07 | −0.01 ± 0.02 | −0.11 ± 0.19 |
| Sodium mmol/L | 2.63 ± 4.60 | 0.17 ± 2.48 | −3.50 ± 7.90 | 1.80 ± 1.10 | 0.00 ± 3.10 | 1.75 ± 2.25 |
| Potassium mmol/L | 0.18 ± 0.67 | −0.04 ± 0.33 | 0.16 ± 0.15 | 0.10 ± 0.29 | −0.01 ± 0.35 | −0.06 ± 0.28 |
| Glucose mg/dL | 2.82 ± 11.49 | −6.14 ± 22.16 | 5.80 ± 22.16 | −14.75 ± 35.69 | 3.17 ± 16.99 | −0.50 ± 39.83 |
| Creatinine mg/dL | 0.07 ± 0.13 | 0.11 ± 0.12 | −0.01 ± 0.12 | −0.09 ± 0.14 | −0.01 ± 0.12 | −0.09 ± 0.14 |
| AST U/L | −0.60 ± 11.17 | 3.44 ± 10.86 | 3.33 ± 5.20 | 0.50 ± 5.47 | −0.83 ± 5.78 | 0.88 ± 7.51 |
| ALT U/L | 6.90 ± 35.31 | 9.78 ± 12.53 | 6.67 ± 20.11 | 5.50 ± 23.67 | 2.50 ± 13.53 | 4.25 ± 33.88 |
| Bilirubin mg/dL | 0.24 ± 0.26 | 0.08 ± 0.17 | −0.04 ± 0.07 | −0.15 ± 0.10 | 0.30 ± 0.41 * | −0.07 ± 0.10 * |
| WBC G/L | 0.37 ± 3.12 | −1.57 ± 4.37 | −0.37 ± 3.39 | 0.66 ± 1.97 | −0.02 ± 3.65 | −0.88 ± 4.11 |
| LYM G/L | −0.11 ± 0.90 | −0.33 ± 0.57 | −0.36 ± 0.96 | −0.05 ± 0.42 | −0.33 ± 0.68 | −0.29 ± 0.53 |
| NEU G/L | 0.51 ± 2.77 | −0.95 ± 3.51 | −0.01 ± 3.48 | 0.60 ± 2.16 | 0.29 ± 3.13 | −0.29 ± 3.88 |
| MON G/L | 0.02 ± 0.21 | −0.02 ± 0.29 | −0.02 ± 0.32 | −0.09 ± 0.19 | 0.03 ± 0.35 | −0.13 ± 0.15 |
| EOS G/L | 0.02 ± 0.20 | 0.01 ± 0.13 | 0.02 ± 0.18 | −0.01 ± 0.08 | 0.06 ± 0.24 | 0.00 ± 0.09 |
| IG G/L | −0.02 ± 0.10 | −0.09 ± 0.20 | 0.05 ± 0.20 | 0.06 ± 0.22 | 0.02 ± 0.21 | −0.07 ± 0.33 |
| BASO G/L | −0.02 ± 0.04 | −0.02 ± 0.04 | −0.02 ± 0.03 | −0.01 ± 0.03 | −0.02 ± 0.03 | −0.01 ± 0.03 |
| RBC T/L | 0.02 ± 0.42 | −0.09 ± 0.45 | −0.14 ± 0.36 | −0.23 ± 0.35 | 0.00 ± 0.39 | −0.32 ± 0.64 |
| HBG mmol/L | 0.27 ± 0.70 | −0.07 ± 0.73 | −0.23 ± 0.66 | −0.21 ± 0.81 | 0.26 ± 0.62 | −0.24 ± 1.06 |
| HCT L/L | 0.01 ± 0.04 | 0.00 ± 0.04 | −0.01 ± 0.03 | −0.01 ± 0.03 | 0.01 ± 0.03 | −0.01 ± 0.04 |
| MCV fL | 1.75 ± 2.27 | 1.04 ± 1.74 | 0.70 ± 2.69 | 3.85 ± 5.70 | 0.70 ± 2.69 | 3.85 ± 5.70 |
| MCH fmol | 0.05 ± 0.07 | 0.02 ± 0.05 | 0.01 ± 0.06 | 0.06 ± 0.09 | 0.01 ± 0.06 | 0.06 ± 0.09 |
| MCHC mmol/L | 0.22 ± 0.51 | 0.02 ± 0.32 | −0.06 ± 0.34 | −0.18 ± 0.77 | 0.13 ± 0.43 | −0.11 ± 0.61 |
| PLT G/L | −77.73 ± 100.23 | −37.93 ± 101.16 | 22.00 ± 52.23 | −28.30 ± 70.23 | −49.08 ± 72.23 | −54.50 ± 86.43 |
| BDNF pg/mL | 9.05 ± 237.11 | −189.70 ± 409.98 | 87.73 ± 202.68 | −106.78 ± 194.25 | 96.78 ± 407.53 | −285.83 ± 455.51 |
* p < 0.05. Abbreviations: EG, exercise group; CG, control group; AST, aspartate aminotransferase; ALT, alanine aminotransferase; WBC, white blood count; LYM, lymphocytes; NEU, neutrophils; MON, monocytes; EOS, eosinophils; IG, immunoglobulins; BASO, basophils; RBC; red blood cell; HBG; hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet count; BDNF, brain-derived neurotrophic factor.
References
- Kumari, S.; Gupta, R.; Ambasta, R.K.; Kumar, P. Multiple therapeutic approaches of glioblastoma multiforme: From terminal to therapy. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188913. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Kushner, D.S.; Amidei, C. Rehabilitation of motor dysfunction in primary brain tumor patients. Neurooncol. Pract. 2015, 2, 185–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dilalla, V.; Chaput, G.; Williams, T.; Sultanem, K. Radiotherapy side effects: Integrating a survivorship clinical lens to better serve patients. Curr. Oncol. 2020, 27, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Asher, A.; Fu, J.B.; Bailey, C.; Hughes, J.K. Fatigue among patients with brain tumors. CNS Oncol. 2016, 5, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.; Alyahya, M. Neurological and Medical Complications in Brain Tumor Patients. Curr. Neurol. Neurosci. Rep. 2020, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Coomans, M.B.; Dirven, L.; Aaronson, N.; Baumert, B.G.; van den Bent, M.; Bottomley, A.; Brandes, A.A.; Chinot, O.; Coens, C.; Gorlia, T.; et al. Factors associated with health-related quality of life (HRQoL) deterioration in glioma patients during the progression-free survival period. Neuro Oncol. 2022, 24, 2159–2169. [Google Scholar] [CrossRef]
- Ståhl, P.; Henoch, I.; Smits, A.; Rydenhag, B.; Ozanne, A. Quality of life in patients with glioblastoma and their relatives. Acta Neurol. Scand. 2022, 146, 82–91. [Google Scholar] [CrossRef]
- Taphoorn, M.J.B.; Henriksson, R.; Bottomley, A.; Cloughesy, T.; Wick, W.; Mason, W.P.; Saran, F.; Nishikawa, R.; Hilton, M.; Theodore-Oklota, C.; et al. Health-Related Quality of Life in a Randomized Phase III Study of Bevacizumab, Temozolomide, and Radiotherapy in Newly Diagnosed Glioblastoma. J. Clin. Oncol. 2015, 33, 2166–2175. [Google Scholar] [CrossRef]
- Spina, S.; Facciorusso, S.; Cinone, N.; Pellegrino, R.; Fiore, P.; Santamato, A. Rehabilitation interventions for glioma patients: A mini-review. Front. Surg. 2023, 10, 1137516. [Google Scholar] [CrossRef]
- Sandler, C.X.; Matsuyama, M.; Jones, T.L.; Bashford, J.; Langbecker, D.; Hayes, S.C. Physical activity and exercise in adults diagnosed with primary brain cancer: A systematic review. J. Neurooncol. 2021, 153, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Pedersen, C.B.; Jarden, J.O.; Beier, D.; Minet, L.R.; Søgaard, K. Effectiveness of Physical Therapy- and Occupational Therapy-Based Rehabilitation in People Who Have Glioma and Are Undergoing Active Anticancer Treatment: Single-Blind, Randomized Controlled Trial. Phys. Ther. 2020, 100, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Steindorf, K.; Depenbusch, J.; Haussmann, A.; Tsiouris, A.; Schmidt, L.; Hermann, S.; Sieverding, M.; Wiskemann, J.; Ungar, N. Change patterns and determinants of physical activity differ between breast, prostate, and colorectal cancer patients. Support. Care Cancer 2020, 28, 3207–3218. [Google Scholar] [CrossRef] [PubMed]
- Pieczyńska, A.; Pilarska, A.; Hojan, K. Predictors of functional outcomes in adults with brain tumor undergoing rehabilitation treatment: A systematic review. Eur. J. Phys. Rehabil. Med. 2022, 58, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Hojan, K.; Gerreth, K. Can Multidisciplinary Inpatient and Outpatient Rehabilitation Provide Sufficient Prevention of Disability in Patients with a Brain Tumor?-A Case-Series Report of Two Programs and A Prospective, Observational Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 6488. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Chun, M.H.; Lee, S.J.; Kim, B.R. Effect of virtual reality-based rehabilitation on upper-extremity function in patients with brain tumor: Controlled trial. Am. J. Phys. Med. Rehabil. 2015, 94, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chun, M.H.; Son, Y.R. Effect of virtual reality on cognitive dysfunction in patients with brain tumor. Ann. Rehabil. Med. 2014, 38, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.J.V.; Gonzalez-Medina, G.; Lucena-Anton, D.; Perez-Cabezas, V.; Ruiz-Molinero, M.D.C.; Martín-Valero, R. Augmented Reality in Physical Therapy: Systematic Review and Meta-analysis. JMIR Serious Games 2021, 9, e30985. [Google Scholar] [CrossRef]
- Denche-Zamorano, A.; Rodriguez-Redondo, Y.; Barrios-Fernandez, S.; Mendoza-Muñoz, M.; Castillo-Paredes, A.; Rojo-Ramos, J.; Garcia-Gordillo, M.A.; Adsuar, J.C. Rehabilitation Is the Main Topic in Virtual and Augmented Reality and Physical Activity Research: A Bibliometric Analysis. Sensors 2023, 23, 2987. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Du, D.; Wei, X.-Y.; Tong, R.K.-Y. Augmented reality for stroke rehabilitation during COVID-19. J. Neuroeng. Rehabil. 2022, 19, 136. [Google Scholar] [CrossRef]
- Cerdán de Las Heras, J.; Tulppo, M.; Kiviniemi, A.M.; Hilberg, O.; Løkke, A.; Ekholm, S.; Catalán-Matamoros, D.; Bendstrup, E. Augmented reality glasses as a new tele-rehabilitation tool for home use: Patients’ perception and expectations. Disabil. Rehabil. Assist. Technol. 2022, 17, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Weyer-Jamora, C.; Brie, M.S.; Luks, T.L.; Smith, E.M.; Hervey-Jumper, S.L.; Taylor, J.W. Postacute Cognitive Rehabilitation for Adult Brain Tumor Patients. Neurosurgery 2021, 89, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.; Pieczyńska, A.; Hojan, K. Neuropsychological monitoring of cognitive function and ICF-based mental components in patients with malignant brain tumours. Front. Psychol. 2023, 14, 1033185. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Miklja, Z.; Gabel, N.; Altshuler, D.; Wang, L.; Hervey-Jumper, S.L.; Smith, S. Exercise improves health-related quality of life sleep and fatigue domains in adult high- and low-grade glioma patients. Support. Care Cancer 2022, 30, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mao, X.; Ye, L.; Cheng, H.; Dai, X. The Role of the S100 Protein Family in Glioma. J. Cancer 2022, 13, 3022–3030. [Google Scholar] [CrossRef]
- Mokhtarzade, M.; Motl, R.; Negaresh, R.; Zimmer, P.; Khodadoost, M.; Baker, J.S.; Patel, D.; Majdinasab, N.; Ranjbar, R. Exercise-induced changes in neurotrophic factors and markers of blood-brain barrier permeability are moderated by weight status in multiple sclerosis. Neuropeptides 2018, 70, 93–100. [Google Scholar] [CrossRef]
- Barha, C.K.; Hsiung, G.Y.R.; Liu-Ambrose, T. The Role of S100B in Aerobic Training Efficacy in Older Adults with Mild Vascular Cognitive Impairment: Secondary Analysis of a Randomized Controlled Trial. Neuroscience 2019, 410, 176–182. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Scaringi, C.; Agolli, L.; Minniti, G. Technical Advances in Radiation Therapy for Brain Tumors. Anticancer Res. 2018, 38, 6041–6045. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Brawley, O.W.; Wender, R.C. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J. Clin. 2017, 67, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Doyle, C.; Kushi, L.H.; Byers, T.; Courneya, K.S.; Demark-Wahnefried, W.; Grant, B.; McTiernan, A.; Rock, C.L.; Thompson, C.; Gansler, T.; et al. Nutrition and physical activity during and after cancer treatment: An American Cancer Society guide for informed choices. CA Cancer J. Clin. 2006, 56, 323–353. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.; Thalla, N.; Nepaul, S.; Wisotzky, E. Outcome Measures in Cancer Rehabilitation: Pain, Function, and Symptom Assessment. Front. Pain. Res. 2021, 2, 692237. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Muscle strength: Clinical and prognostic value of hand-grip dynamometry. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Simón, A.; Díez-Fernández, D.M.; Artés-Rodríguez, E.; Casimiro-Artés, M.Á.; Rodríguez-Pérez, M.A.; Moreno-Martos, H.; Casimiro-Andújar, A.J.; Soriano-Maldonado, A. Absolute and Relative Handgrip Strength as Indicators of Self-Reported Physical Function and Quality of Life in Breast Cancer Survivors: The EFICAN Study. Cancers 2021, 13, 5292. [Google Scholar] [CrossRef] [PubMed]
- Hadzibegovic, S.; Porthun, J.; Lena, A.; Weinländer, P.; Lück, L.C.; Potthoff, S.K.; Rösnick, L.; Fröhlich, A.-K.; Ramer, L.V.; Sonntag, F.; et al. Hand grip strength in patients with advanced cancer: A prospective study. J. Cachexia Sarcopenia Muscle 2023, 14, 1682–1694. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Solomon, G.S.; Valdes, K.A. Clinical Assessment Recommendations, 3rd ed.; American Society of Hand Therapists: Mount Laurel, NJ, USA, 2015; ISBN 9780692525159. [Google Scholar]
- Michael, C.M.; Lehrer, E.J.; Schmitz, K.H.; Zaorsky, N.G. Prehabilitation exercise therapy for cancer: A systematic review and meta-analysis. Cancer Med. 2021, 10, 4195–4205. [Google Scholar] [CrossRef]
- Agarwala, P.; Salzman, S.H. Six-Minute Walk Test: Clinical Role, Technique, Coding, and Reimbursement. Chest 2020, 157, 603–611. [Google Scholar] [CrossRef]
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed Up and Go (TUG) Test: Normative Reference Values for Ages 20 to 59 Years and Relationships with Physical and Mental Health Risk Factors. J. Prim. Care Community Health 2017, 8, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Noll, K.R.; Bradshaw, M.E.; Weinberg, J.S.; Wefel, J.S. Neurocognitive functioning is associated with functional independence in newly diagnosed patients with temporal lobe glioma. Neurooncol. Pract. 2018, 5, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Byun, Y. Trajectories of Symptom Clusters, Performance Status, and Quality of Life During Concurrent Chemoradiotherapy in Patients with High-Grade Brain Cancers. Cancer Nurs. 2018, 41, E38–E47. [Google Scholar] [CrossRef] [PubMed]
- Addeo, R.; Caraglia, M.; Faiola, V.; Capasso, E.; Vincenzi, B.; Montella, L.; Guarrasi, R.; Caserta, L.; Del Prete, S. Concomitant treatment of brain metastasis with whole brain radiotherapy WBRT and temozolomide TMZ is active and improves quality of life. BMC Cancer 2007, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Thavarajah, N.; Bedard, G.; Zhang, L.; Cella, D.; Beaumont, J.L.; Tsao, M.; Barnes, E.; Danjoux, C.; Sahgal, A.; Soliman, H.; et al. Psychometric validation of the functional assessment of cancer therapy—Brain (FACT-Br) for assessing quality of life in patients with brain metastases. Support. Care Cancer 2014, 22, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Weitzner, M.A.; Meyers, C.A.; Gelke, C.K.; Byrne, K.S.; Levin, V.A.; Cella, D.F. The functional assessment of cancer therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer 1995, 75, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Garg, R.; Minhas, V.; Bhatnagar, S.; Mishra, S.; Kumar, V.; Bharati, S.J.; Gupta, N.; Khan, M.A. To assess the Prevalence and Predictors of Cancer-related Fatigue and its Impact on Quality of Life in Advanced Cancer Patients Receiving Palliative Care in a Tertiary Care Hospital: A Cross-sectional Descriptive Study. Indian J. Palliat. Care 2020, 26, 523–527. [Google Scholar] [CrossRef]
- Annunziata, M.A.; Muzzatti, B.; Bidoli, E.; Flaiban, C.; Bomben, F.; Piccinin, M.; Gipponi, K.M.; Mariutti, G.; Busato, S.; Mella, S. Hospital Anxiety and Depression Scale (HADS) accuracy in cancer patients. Support. Care Cancer 2020, 28, 3921–3926. [Google Scholar] [CrossRef]
- Matías-Guiu, J.A.; Fernández-Bobadilla, R.; Cortés-Martínez, A. Addenbrooke’s Cognitive Examination III: Un test neuropsicológico útil para el cribado y la obtención de perfiles cognitivos. Neurologia (Engl. Ed.) 2018, 33, 140. [Google Scholar] [CrossRef]
- Valiyaveettil, D.G.A.; Malik, M.; Eaga, P.; Ahmed, S.F.; Joseph, D. “A prospective study of assessment of neurocognitive function in illiterate patients with gliomas treated with chemoradiation”: Assessment of neurocognitive function in gliomas. Cancer Treat. Res. Commun. 2021, 26, 100288. [Google Scholar] [CrossRef]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef]
- Cormie, P.; Nowak, A.K.; Chambers, S.K.; Galvão, D.A.; Newton, R.U. The potential role of exercise in neuro-oncology. Front. Oncol. 2015, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Guill, B.; Keir, S.T.; Carter, K.; Friedman, H.S.; Bigner, D.D.; Reardon, D.A. Exercise interest and preferences among patients diagnosed with primary brain cancer. Support. Care Cancer 2007, 15, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Saredakis, D.; Szpak, A.; Birckhead, B.; Keage, H.A.D.; Rizzo, A.; Loetscher, T. Factors Associated with Virtual Reality Sickness in Head-Mounted Displays: A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2020, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- van Coevorden-van Loon, E.M.P.; Horemans, H.H.L.D.; Heijenbrok-Kal, M.H.; van den Berg-Emons, R.J.G.; Rozenberg, R.; Vincent, A.J.P.E.; Ribbers, G.M.; van den Bent, M.J. Physical fitness and its association with fatigue in patients with low-grade glioma. Disabil. Rehabil. 2022, 45, 3323–3329. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.K.; Newton, R.U.; Cruickshank, T.; Cormie, P.; Halkett, G.K.B.; Tsoi, D.; Galvão, D.A. A feasibility, safety, and efficacy evaluation of supervised aerobic and resistance exercise for patients with glioblastoma undertaking adjuvant chemoradiotherapy. Neurooncol. Pract. 2023, 10, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, L.; Sadeghi-Bahmani, D.; Gerber, M.; Saemann, A.; Staub, L.; Brand, S.; Cordier, D. Effects of two types of exercise training on psychological well-being, sleep and physical fitness in patients with high-grade glioma (WHO III and IV). J. Psychiatr. Res. 2022, 151, 354–364. [Google Scholar] [CrossRef]
- Habets, E.J.J.; Hendriks, E.J.; Taphoorn, M.J.B.; Douw, L.; Zwinderman, A.H.; Vandertop, W.P.; Barkhof, F.; de Witt Hamer, P.C.; Klein, M. Association between tumor location and neurocognitive functioning using tumor localization maps. J. Neurooncol. 2019, 144, 573–582. [Google Scholar] [CrossRef]
- Gehring, K.; Stuiver, M.M.; Visser, E.; Kloek, C.; van den Bent, M.; Hanse, M.; Tijssen, C.; Rutten, G.-J.; Taphoorn, M.J.B.; Aaronson, N.K.; et al. A pilot randomized controlled trial of exercise to improve cognitive performance in patients with stable glioma: A proof of concept. Neuro Oncol. 2020, 22, 103–115. [Google Scholar] [CrossRef]
- Salerno, E.A.; Culakova, E.; Kleckner, A.S.; Heckler, C.E.; Lin, P.-J.; Matthews, C.E.; Conlin, A.; Weiselberg, L.; Mitchell, J.; Mustian, K.M.; et al. Physical Activity Patterns and Relationships With Cognitive Function in Patients With Breast Cancer Before, During, and After Chemotherapy in a Prospective, Nationwide Study. J. Clin. Oncol. 2021, 39, 3283–3292. [Google Scholar] [CrossRef]
- Wilson, R.; Kang, D.-W.; Tahbaz, M.; Norris, M.; Uno, H.; Ligibel, J.; Guenette, J.; Christopher, C.; Dieli-Conwright, C. Improving Cognitive Function Through High-Intensity Interval Training in Breast Cancer Patients Undergoing Chemotherapy: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2023, 12, e39740. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Nolen, S.C.; Lee, B.; Shantharam, S.; Yu, H.J.; Su, L.; Billimek, J.; Bota, D.A. The effects of sequential treatments on hippocampal volumes in malignant glioma patients. J. Neurooncol. 2016, 129, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, M.; Dzierma, Y.; Nuesken, F.; Oertel, J.; Rübe, C.; Melchior, P. Automatic Radiotherapy Planning for Glioblastoma Radiotherapy With Sparing of the Hippocampus and nTMS-Defined Motor Cortex. Front. Neurol. 2021, 12, 787140. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Stubbs, B.; Vancampfort, D.; Schuch, F.; Lagopoulos, J.; Rosenbaum, S.; Ward, P.B. Effect of aerobic exercise on hippocampal volume in humans: A systematic review and meta-analysis. Neuroimage 2018, 166, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Niemann, C.; Godde, B.; Voelcker-Rehage, C. Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Front. Aging Neurosci. 2014, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Nauer, R.K.; Dunne, M.F.; Stern, C.E.; Storer, T.W.; Schon, K. Improving fitness increases dentate gyrus/CA3 volume in the hippocampal head and enhances memory in young adults. Hippocampus 2020, 30, 488–504. [Google Scholar] [CrossRef]
- Clifford, B.K.; Kaakoush, N.O.; Tedla, N.; Goldstein, D.; Simar, D. The effect of exercise intensity on the inflammatory profile of cancer survivors: A randomised crossover study. Eur. J. Clin. Investig. 2023, 53, e13984. [Google Scholar] [CrossRef]
- Cartmel, B.; Hughes, M.; Ercolano, E.A.; Gottlieb, L.; Li, F.; Zhou, Y.; Harrigan, M.; Ligibel, J.A.; Von Gruenigen, V.E.; Gogoi, R.; et al. Randomized trial of exercise on depressive symptomatology and brain derived neurotrophic factor (BDNF) in ovarian cancer survivors: The Women’s Activity and Lifestyle Study in Connecticut (WALC). Gynecol. Oncol. 2021, 161, 587–594. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Zhou, H.-H.; Luo, Q.; Cui, S. The effect of physical exercise on circulating brain-derived neurotrophic factor in healthy subjects: A meta-analysis of randomized controlled trials. Brain Behav. 2022, 12, e2544. [Google Scholar] [CrossRef]
- Ruiz-González, D.; Hernández-Martínez, A.; Valenzuela, P.L.; Morales, J.S.; Soriano-Maldonado, A. Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 2021, 128, 394–405. [Google Scholar] [CrossRef]
- Spencer, J.; Staffileno, B.A. Exercise Intervention: A Pilot Study to Assess the Feasibility and Impact on Cancer-Related Fatigue and Quality of Life Among Patients with High-Grade Glioma. Clin. J. Oncol. Nurs. 2021, 25, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Peng, P.; Xu, Z.; Ding, X. The effects of exercise on the quality of life of patients with breast cancer: A systematic review and meta-analysis based on the QLQ-C30 quality of life scale. Gland Surg. 2023, 12, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Roh, H.-T. Effects of Exercise Training on Neurotrophic Factors and Blood-Brain Barrier Permeability in Young-Old and Old-Old Women. Int. J. Environ. Res. Public Health 2022, 19, 16896. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; DelCimmuto, N.R.; Flack, K.D.; Stec, D.E.; Hinds, T.D. Reactive Oxygen Species (ROS) and Antioxidants as Immunomodulators in Exercise: Implications for Heme Oxygenase and Bilirubin. Antioxidants 2022, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D.; Creeden, J.F.; Gordon, D.M.; Stec, D.F.; Donald, M.C.; Stec, D.E. Bilirubin Nanoparticles Reduce Diet-Induced Hepatic Steatosis, Improve Fat Utilization, and Increase Plasma β-Hydroxybutyrate. Front. Pharmacol. 2020, 11, 594574. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).