Effects of Vitamin D Levels on Long-Term Coronary Events in Patients with Proven Coronary Artery Disease: Six-Year Follow-Up
Abstract
:1. Introduction
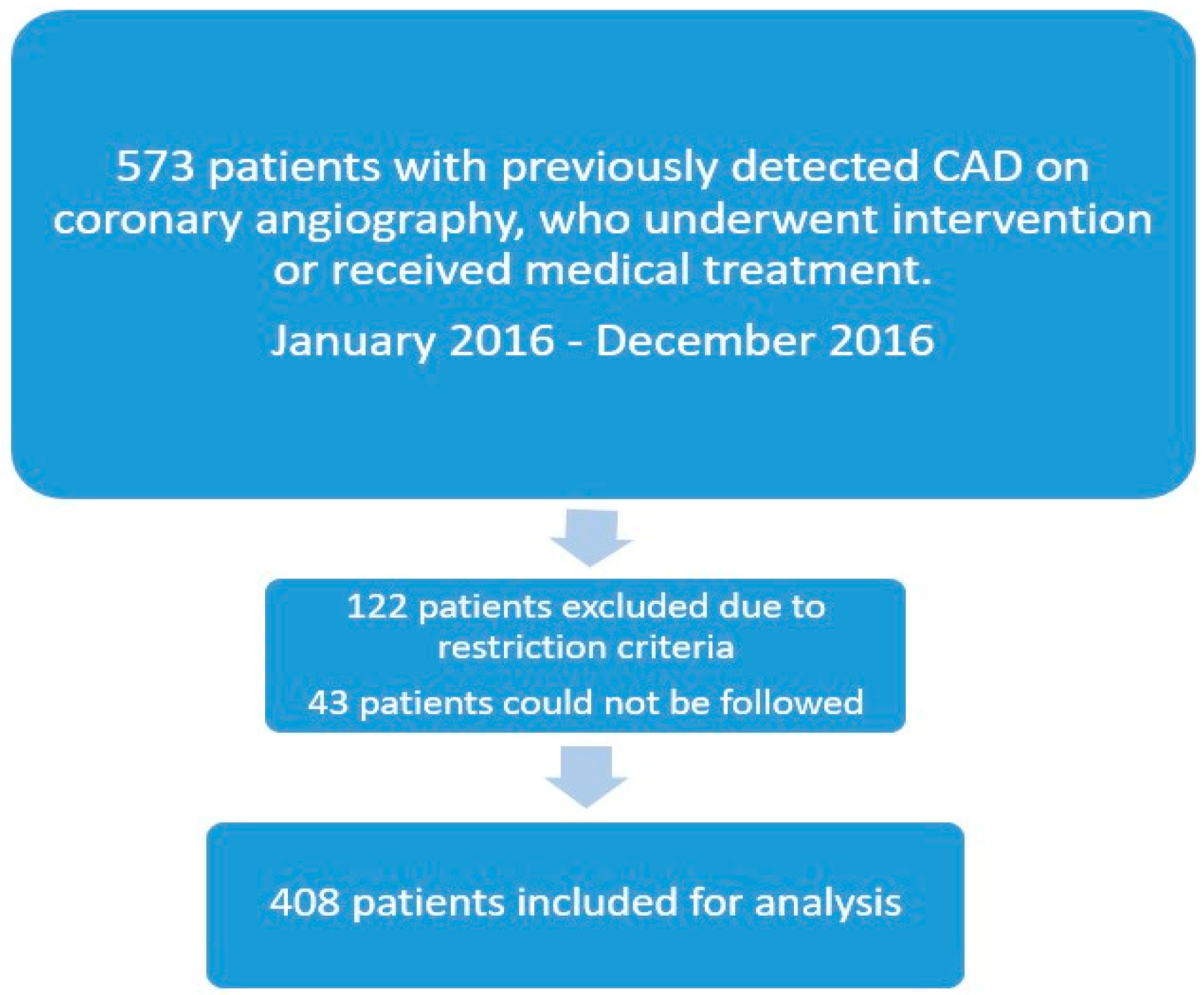
2. Materials and Methods
2.1. The Study Protocol
2.2. Laboratory Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
- Vit-D levels were not related to all-cause mortality.
- A Vit-D level below 20 ng/mL was associated with increased non-STEACS.
- In patients with coronary artery disease, a Vit-D level above 20 ng/mL was associated with the patients remaining more stable throughout the six-year follow-up.
- No association was found between STEMI and Vit-D levels.
- The need for elective revascularization increased as the Vit-D level decreased, but this was not statistically significant.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Husain, K.; Hernandez, W.; Ansari, R.; Ferder, L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J. Biol. Chem. 2015, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, G.; Morello, A.; Conte, S.; Pellegrino, G.; Marra, L.; Golino, P.; Cirillo, P. Vitamin D inhibits Tissue Factor and CAMs expression in oxidized low-density lipoproteins-treated human endothelial cells by modulating NF-KB pathway. Eur. J. Pharmacol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Agrawal, D. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [PubMed]
- Siadat, D.Z.; Kiani, K.; Sadeghi, M.; Shariat, A.; Farajzedegan, Z.; Kheirmand, M. Association of Vitamin D deficiency and coronary artery disease with cardiovascular risk factors. J. Res. Med. Sci. 2012, 17, 1052–1055. [Google Scholar]
- Surdu, A.M.; Pinzariu, O.; Ciobanu, D.M.; Negru, A.G.; Cainap, S.S.; Lazea, C.; Iacob, D.; Saraci, G.; Trinescu, D.; Borda, H.M.; et al. Vitamin D and its role in the lipid metabolism and the development of atherosclerosis. Biomedicines 2021, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, G.; Conte, S.; Morello, M.; Pellegrino, G.; Marra, L.; Morello, A.; Nicoletti, G.; De Rosa, G.; Golino, P.; Cirillo, P. Vitamin D Inhibits IL-6 Pro-Atherothrombotic Effects in Human Endothelial Cells: A Potential Mechanism for Protection against COVID-19 Infection? J. Cardiovasc. Dev. Dis. 2022, 9, 27. [Google Scholar] [CrossRef]
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Disease. Biomed. Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef]
- Mandarino, R.N.; Junior, F.C.; Salgado, J.L.; Lages, J.S.; Filho, N.S. Is Vitamin D deficiency a new risk factor for cardiovascular disease? Open Cardiovasc. Med. J. 2015, 9, 40–49. [Google Scholar] [CrossRef]
- Gardner, G.; Chen, S.; Glenn, D.J. Vitamin D and the heart. Am. J. Physiol. Regul. Comp. Physiol. 2013, 305, RR977. [Google Scholar] [CrossRef]
- Milazzo, V.; Metrio, M.; Cosentino, N.; Marenzi, G.; Tremoli, E. Vitamin D and acute myocardial infarction. World J. Cardiol. 2017, 9, 14–20. [Google Scholar] [CrossRef]
- Ho, J.S.; Cannaday, J.J.; Barlow, C.E.; Reinhardt, D.B.; Wade, W.A.; Ellis, J.R. Low 25-0H Vitamin D levels are not associated with coronary artery calcium or obstructive stenosis. Coron. Artery Dis. 2015, 26, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Excessive Alcohol Use. Available online: www.cdc.gov/chronicdisease/resources/publications/factsheets/alcohol.htm (accessed on 11 July 2022).
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthe, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Elsayed, N.A.; Aleppa, G.; Arada, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hillard, M.E.; Isaacs, D.; Johnson, E.L.; et al. Glycemic targets: Standards of care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), SS110. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Eklund, C.; Elfström, M.; Wagert, P.H.; Söderlund, A.; Gustavsson, A.; Gustavsson, C.; Cederbom, S.; Thunborg, C.; Lööf, H. The meaning of sedentary behavior as experienced by people in the transition from working life to retirement: An empirical phenomenological study. Phys. Ther. 2021, 101, pzab117. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunströmc, M.; Burnierd, M.; Grassie, G.; Januszewiczf, A.; Muiesang, A.L.; Tsioufish, K.; Agabiti-Roseii, E.; Algharablyb, E.A.; et al. 2023 ESH Guidelines for the management of arterial hypertension. J. Hypertens. 2023, 41, 101097. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, B.S.; Braun, L.T.; Ferranti, S.; Tommasino, J.F.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ ABC/ACPM/ADA/AGS/APhA/ASPC/ NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 24, e285–e350. [Google Scholar] [CrossRef]
- Jaiswal, V.; Ishak, A.; Ang, S.P.; Pokhrel, N.B.; Shama, N.; Lnu, K.; Varghese, J.S.; Storozhenko, T.; Chia, J.E.; Naz, S.; et al. Hypovitaminosis D and cardiovascular outcomes: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2022, 40, 101019. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; VITAL Research Group; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, P.; Sun, Y.; Wang, X.; Fan, Y. Predictive value of 25-hydroxy Vitamin D level in patients with coronary artery disease: A meta- analysis. Front. Nutr. 2022, 9, 984487. [Google Scholar] [CrossRef]
- Nudy, M.; Krakowski, G.; Ghahramani, M.; Ruzieh, M.; Joy, A. Vitamin D supplementation, cardiac events and stroke: A systematic review and meta-regression analysis. Int. J. Cardiol. Heart Vasc. 2020, 28, 100537. [Google Scholar] [CrossRef]
- Cosentino, N.; Campadonice, J.; Milazzo, V.; Metrio Mi Brambilla, M.; Camera, M.; Marenzi, G. Vitamin D and cardiovascular disease: Current evidence and future perspectives. Nutrients 2021, 13, 3603. [Google Scholar] [CrossRef]
- Verdoia, M.; Nardin, M.; Rolla, R.; Negro, F.; Gioscia, R.; Afifeh, A.M.S.; Viglione, F.; Suryapranata, H.; Marcolongo, M.; Luca, G. Prognostic impact of Vitamin D deficiency in patients with coronary artery disease undergoing percutaneous coronary intervention. Eur. J. Intern. Med. 2021, 83, 62–67. [Google Scholar] [CrossRef]
- Beska, B.; Chan, D.; Gu, S.; Qiu, W.; Mossop, H.; Neely, D.; Kunadian, V. The association between Vitamin D status and clinical events in high-risk older patients with non- ST elevation acute coronary syndrome undergoing invasive mangement. PLoS ONE 2018, 14, e0217476. [Google Scholar] [CrossRef] [PubMed]
- Cannistraci, C.V.; Nieminen, T.; Nishi, M.; Khachigian, L.M.; Viikila, J.; Laina, M.; Cianflone, D.; Maseri, A.; Yeo, K.K.; Bhindi, R.; et al. Summer Shift: Apotential sunshine on the time onset of ST-elevation acute myocardial infarction. J. Am. Heart Assoc. 2018, 7, e006878. [Google Scholar] [CrossRef] [PubMed]
- Ogbegor, O.; Odugbemi, B.; Maheswaran, R.; Kavya, P. Seasonal variation in mortality secondary to acute myocardial infarction in England and Wales: A secondary data analysis. BMJ Open 2018, 8, e019242. [Google Scholar] [CrossRef] [PubMed]
- Afifeh, A.M.; Verdoia, M.; Nardin, M.; Negro, F.; Viglione, F.; Rolla, R.; Luca, G. Determinants of vitamin D activation in patients with acute coronary syndromes and its correlation with inflammatory markers. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 36–43. [Google Scholar] [CrossRef]
- Byrne, R.; Joner, M.; Kastrati, A. Stent thrombosis and restenosis: What have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur. Heart J. 2015, 36, 3320–3331. [Google Scholar] [CrossRef]
- Yaman, A.E.; Ceylan, U.S. The effect of Vitamin D deficiency on the risk and time of stent restenosis after percutaneous coronary angioplasty: Case-control study. Turk. Klin. J. Cardiovasc. Sci. 2021, 33, 135–141. [Google Scholar] [CrossRef]
- Pravecek, M.K.; Arar, Z.V.; Miskic, B.; Hadzibegovic, I. Vitamin D deficiency in acute coronary syndrome—Clinically relevant or incidental finding? Cent. Eur. J. Public. Health 2017, 25, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Folsom, A.R.; Alanso, A.; Misialek, J.R.; Michos, E.D.; Selvin, E.; Eckfeldt, J.H.; Coresh, J.; Pankow, J.S.; Lutsey, P.L. Parathyroid hormone concentration and risk of cardiovascular diseases: The atherosclerosis risk in communities (ARIC) study. Am. Heart J. 2014, 168, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Gepner, A.D.; Colangelo, L.A.; Blondon, M.; Korcarz, C.E.; Boer, L.H.; Kestenbaum, B.; Siscovick, D.S.; Kaufman, J.D.; Liu, K.; Stein, J.H. 25-hydroxy Vitamin D and parathyroid hormone levels do not predict changes in carotid arterial stiffness: The multi-ethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Landaluce, C.G.; Acena, A.; Pello, A.; Milla, J.M.; Lorenzo, O.G.; Tarin, N.; Cristobal, C.; Blanco-Colio, L.M.; Martin-Ventura, J.L.; Huelmas, A.; et al. Parathormone levels add prognostic ability to N-terminal pro-brain natriuretic peptide in stable coronary patients. ESC Heart Fail. 2021, 8, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
| Vitamin D Levels | p | ||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |||
| (<10 ng/mL) | (10–20 ng/mL) | (>20 ng/mL) (n = 115) | |||
| (n = 98) | (n = 195) | ||||
| Sex (%) | Female | 64.3 | 55.4 | 33.9 | a 0.001 ** |
| Male | 35.7 | 44.6 | 66.1 | ||
| Age | mean ± SD | 62.63 ± 11.30 | 61.96 ± 10.42 | 66.77 ± 10.55 | b 0.001 ** |
| BMI (kg/m2) | mean ± SD | 28.90 ± 5.90 | 27.90 ± 4.50 | 28.20 ± 4.50 | b 0.481 |
| Hypertension (%) | 55.1 | 59 | 58.3 | a 0.810 | |
| Diabetes (%) | 59 | 40.5 | 47 | a 0.011 * | |
| Smokers (%) | 11.2 | 18.5 | 10.4 | a 0.097 | |
| Acetylsalicylic acid (%) | 84.7 | 87.7 | 84.3 | a 0.627 | |
| Beta blocker (%) | 95.9 | 88.7 | 85.2 | a 0.029 * | |
| ACEI, ARB (%) | 77.6 | 80.5 | 68.7 | a 0.062 | |
| Statin (%) | 77.6 | 78.5 | 66.1 | a 0.045 * | |
| PPI (%) | 88.8 | 86.7 | 75.7 | a 0.019 * | |
| Mediterranean type diet (%) | 87 | 86 | 84 | a 0.828 | |
| Sedentary lifestyle (%) | 10 | 9 | 13 | a 0.568 | |
| Group 1 | Group 2 | Group 3 | p | ||
|---|---|---|---|---|---|
| (n = 98) | (n = 195) | (n = 115) | |||
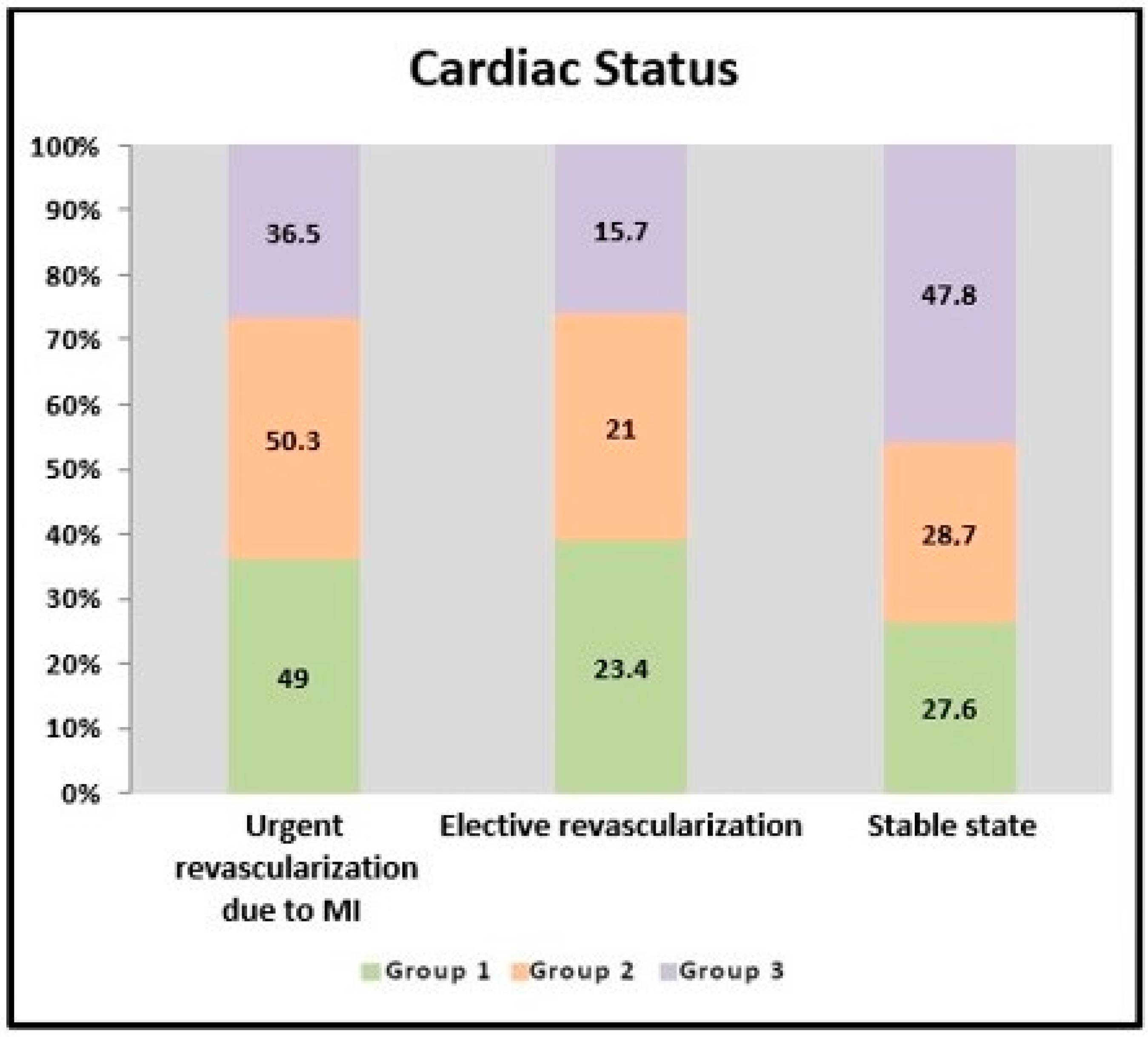
| Cardiac Status (%) | Urgent revascularization due to MI | 49 | 50.3 | 36.5 | a 0.006 ** |
| Elective revascularization | 23.4 | 21 | 15.7 | a 0.331 | |
| Stable state | 27.6 | 28.7 | 47.8 | a 0.006 ** | |
| ACS (%) | STEMI | 23.4 | 34.4 | 26 | a 0.101 |
| Non-STEACS, UA | 49 | 37.9 | 27 | a 0.001 ** | |
| Previous Stent (%) | 58.2 | 55.4 | 43.5 | a 0.059 | |
| Previous Stent Type (%) | BMS | 22.4 | 28.1 | 12.4 | a 0.013 * |
| DES | 25.5 | 22.4 | 21.2 | a 0.719 | |
| Undefined | 10.3 | 4.9 | 9.9 | a 0.126 | |
| Previous CABG (%) | 42.9 | 37.4 | 48.7 | a 0.152 | |
| Mortality (%) | 24.5 | 13.8 | 17.4 | a 0.082 | |
| Follow-up (months) | mean ± SD | 57.04 ± 18.48 | 58,27 ± 18.12 | 53.73 ± 19.10 | c 0.059 |
| Total | Vitamin D Levels | p | ||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | ||||
| <10 ng/mL | 10–20 ng/mL | >20 ng/mL | ||||
| (n = 98) | (n = 195) | (n = 115) | ||||
| Fasting glucose (mg/dL) | mean ± SD | 124.90 ± 53.80 | 132.70 ± 58.60 | 123.40 ± 47.60 | 120.90 ± 59.20 | c 0.135 |
| HbA1c (%) | mean ± SD | 6.60 ± 1.60 | 7.03 ± 2.03 | 6.40 ± 1.60 | 6.40 ± 1.20 | c 0.186 |
| Triglycerides (mg/dL) | mean ± SD | 166.1 ± 111.6 | 176.4 ± 118.2 | 173.2 ± 122.3 | 145.6 ± 80.9 | c 0.038 * |
| HDL (mg/dL) | mean ± SD | 41.50 ± 12.10 | 40.70 ± 10.80 | 41.10 ± 11.60 | 42.80 ± 13.60 | b 0.364 |
| LDL (mg/dL) | mean ± SD | 120.40 ± 53.30 | 120.40 ± 38.70 | 119.60 ± 65.40 | 121.80 ± 39.40 | b 0.941 |
| WBC (103/mm3) | mean ± SD | 8.40 ± 2.30 | 8.60 ± 2.60 | 8.40 ± 2.50 | 8.20 ± 1.90 | b 0.464 |
| Hemoglobin (g/dL) | mean ± SD | 13.30 ± 1.90 | 12.80 ± 2 | 13.50 ± 1.80 | 13.40 ± 1.80 | b 0.012 * |
| Platelet (103/mm3) | mean ± SD | 243 ± 73.40 | 247.70 ± 76.40 | 233.80 ± 72 | 254.60 ± 71.50 | b 0.041 * |
| Neutrophil (103/mm3) | mean ± SD | 5.50 ± 2.60 | 5.70 ± 2.40 | 5.50 ± 3 | 5.20 ± 1.80 | c 0.763 |
| Lymphocyte (103/mm3) | mean ± SD | 2.20 ± 0.80 | 2.20 ± 0.90 | 2.10 ± 0.80 | 2.10 ± 0.60 | b 0.719 |
| MPV (fL) | mean ± SD | 9 ± 1.40 | 8.90 ± 1.40 | 8.90 ± 1.30 | 9.10 ± 1.40 | b 0.468 |
| Platelet/Lymphocyte | mean ± SD | 127.80 ± 78.10 | 128.50 ± 61.40 | 128.20 ± 96.90 | 126.50 ± 50.70 | c 0.694 |
| NLR | mean ± SD | 3.10 ± 3.70 | 3.10 ± 2.10 | 3.50 ± 5.10 | 2.60 ± 1.40 | b 0.186 |
| MPV/Lymphocyte | mean ± SD | 4.90 ± 2.80 | 4.70 ± 2.10 | 5.20 ± 3.50 | 4.60 ± 1.60 | b 0.173 |
| MPV/Neutrophil | mean ± SD | 1.90 ± 0.80 | 1.80 ± 0.70 | 1.90 ± 0.90 | 1.90 ± 0.70 | b 0.421 |
| Monocyte (103/mm3) | mean ± SD | 0.60 ± 0.20 | 0.60 ± 0.20 | 0.60 ± 0.20 | 0.60 ± 0.20 | b 0.463 |
| Eosinophil (103/mm3) | mean ± SD | 0.20 ± 0.10 | 0.10 ± 0.10 | 0.20 ± 0.10 | 0.20 ± 0.10 | b 0.049 * |
| Basophil 103/mm3 | mean ± SD | 0.01 ± 0.10 | 0.10 ± 0.10 | 0.10 ± 0.10 | 0.10 ± 0.10 | c 0.892 |
| Calcium (mg/dL) | mean ± SD | 9.20 ± 0.70 | 9.10 ± 1.10 | 9.20 ± 0.60 | 9.40 ± 0.50 | b 0.002 ** |
| Phosphor (mg/dL) | mean ± SD | 3.70 ± 3 | 3.60 ± 0.90 | 3.80 ± 4.30 | 3.60 ± 0.80 | b 0.865 |
| Uric acid (mg/dL) | mean ± SD | 5 ± 2.40 | 4.90 ± 2.50 | 4.90 ± 2.40 | 5.30 ± 2.40 | b 0.262 |
| CRP (mg/L) | mean ± SD | 1.10 ± 3.10 | 0.90 ± 1.40 | 1.40 ± 4.20 | 0.70 ± 1.20 | c 0.006 ** |
| Creatinine (mg/dL) | mean ± SD | 1 ± 0.50 | 1 ± 0.60 | 1 ± 0.50 | 1.10 ± 0.50 | c 0.017 * |
| BNP (pg/mL) | mean ± SD | 353.2 ± 962.7 | 743.2 ± 1826 | 213.5 ± 287.7 | 245.7 ± 317 | c 0.089 |
| EF (%) | mean ± SD | 50.70 ± 10.50 | 50.70 ± 11.20 | 50.70 ± 10.30 | 50.70 ± 10.40 | b 0.999 |
| Variables | Alive (n = 330) | Dead (n = 78) | p |
|---|---|---|---|
| Age, years | 63 ± 11 | 67 ± 12 | <0.001 |
| Sex, Male, n (%) | 158 (48%) | 40 (51%) | 0.600 |
| Vitamin D level (ng/mL) | 17 ± 11 | 15 ± 9 | 0.212 |
| Vitamin D category, n (%) | 0.077 | ||
| <10 ng/mL | 72 (22%) | 26 (33%) | |
| 10–20 ng/mL | 165 (50%) | 30 (39%) | |
| >20 ng/mL | 93 (28%) | 22 (28%) | |
| Smoking, n (%) | 50 (15%) | 14 (18%) | 0.537 |
| Hypertension, n (%) | 178 (54%) | 58 (74%) | <0.001 |
| BMI (kg/m2) | 28.0 ± 4.6 | 28.9 ± 5.3 | 0.546 |
| Diabetes, n (%) | 142 (43%) | 44 (56%) | 0.037 |
| Fasting glucose (mg/dL) | 122 ± 49 | 139 ± 72 | 0.039 |
| HbA1c (%) | 6.40 ± 1.50 | 7.22 ± 1.92 | <0.001 |
| Triglycerides (mg/dL) | 166 ± 115 | 171 ± 86 | 0.174 |
| HDL (mg/dL) | 42 ± 12 | 39 ± 11 | 0.014 |
| LDL (mg/dL) | 119 ± 41 | 123 ± 89 | 0.463 |
| WBC (103/mm3) | 8.33 ± 2.29 | 8.71 ± 2.70 | 0.179 |
| Hemoglobin (g/dL) | 13.49 ± 1.79 | 12.68 ± 2.19 | 0.003 |
| Platelet (103/mm3) | 241 ± 71 | 252 ± 83 | 0.366 |
| NLR | 3.04 ± 3.92 | 3.45 ± 2.38 | 0.005 |
| CRP (mg/L) | 1.07 ± 3.18 | 1.13 ± 2.07 | 0.115 |
| Creatinine (mg/dL) | 0.99 ± 0.48 | 1.21 ± 0.65 | <0.001 |
| BNP (pg/mL) | 302 ± 1037 | 533 ± 623 | 0.003 |
| LVEF (%) | 52 ± 9 | 46 ± 14 | <0.001 |
| Previous Stent, n (%) | 172 (52%) | 47 (60%) | 0.186 |
| Previous CABG, n (%) | 142 (43%) | 24 (31%) | 0.049 |
| Acetylsalicylic acid, n (%) | 283 (86%) | 71 (91%) | 0.212 |
| Beta blocker, n (%) | 294 (89%) | 73 (94%) | 0.237 |
| ACEI, ARB, n (%) | 251 (76%) | 65 (83%) | 0.162 |
| Statin, n (%) | 251 (76%) | 59 (76%) | 0.989 |
| PPI, n (%) | 274 (83%) | 70 (90%) | 0.163 |
| Hazard Ratio | 95% CI of Hazard Ratio | p | |
|---|---|---|---|
| Age | 1.02 | 1.00, 1.05 | 0.104 |
| Vitamin D level | 1.00 | 0.97, 1.03 | 0.993 |
| Diabetes | 1.41 | 0.83, 2.41 | 0.207 |
| Hypertension | 1.51 | 0.83, 2.74 | 0.176 |
| HDL cholesterol | 0.99 | 0.96, 1.01 | 0.282 |
| Hemoglobin | 0.88 | 0.77, 1.01 | 0.078 |
| Creatinine | 1.27 | 0.90, 1.79 | 0.181 |
| LVEF | 0.97 | 0.95, 0.99 | 0.007 |
| Triglyceride | 1.00 | 1.00, 1.00 | 0.991 |
| WBC | 1.03 | 0.92, 1.15 | 0.596 |
| CRP | 1.02 | 0.95, 1.11 | 0.53 |
| PPI | 1.02 | 0.45, 2.35 | 0.954 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaman, A.E.; Ceylan, U.S. Effects of Vitamin D Levels on Long-Term Coronary Events in Patients with Proven Coronary Artery Disease: Six-Year Follow-Up. J. Clin. Med. 2023, 12, 6835. https://doi.org/10.3390/jcm12216835
Yaman AE, Ceylan US. Effects of Vitamin D Levels on Long-Term Coronary Events in Patients with Proven Coronary Artery Disease: Six-Year Follow-Up. Journal of Clinical Medicine. 2023; 12(21):6835. https://doi.org/10.3390/jcm12216835
Chicago/Turabian StyleYaman, Aysun Erdem, and Ufuk Sadık Ceylan. 2023. "Effects of Vitamin D Levels on Long-Term Coronary Events in Patients with Proven Coronary Artery Disease: Six-Year Follow-Up" Journal of Clinical Medicine 12, no. 21: 6835. https://doi.org/10.3390/jcm12216835
APA StyleYaman, A. E., & Ceylan, U. S. (2023). Effects of Vitamin D Levels on Long-Term Coronary Events in Patients with Proven Coronary Artery Disease: Six-Year Follow-Up. Journal of Clinical Medicine, 12(21), 6835. https://doi.org/10.3390/jcm12216835





