Enhanced Physical Capacity and Gastrointestinal Symptom Improvement in Southern Italian IBS Patients following Three Months of Moderate Aerobic Exercise
Abstract
:1. Introduction
2. Materials and Methods
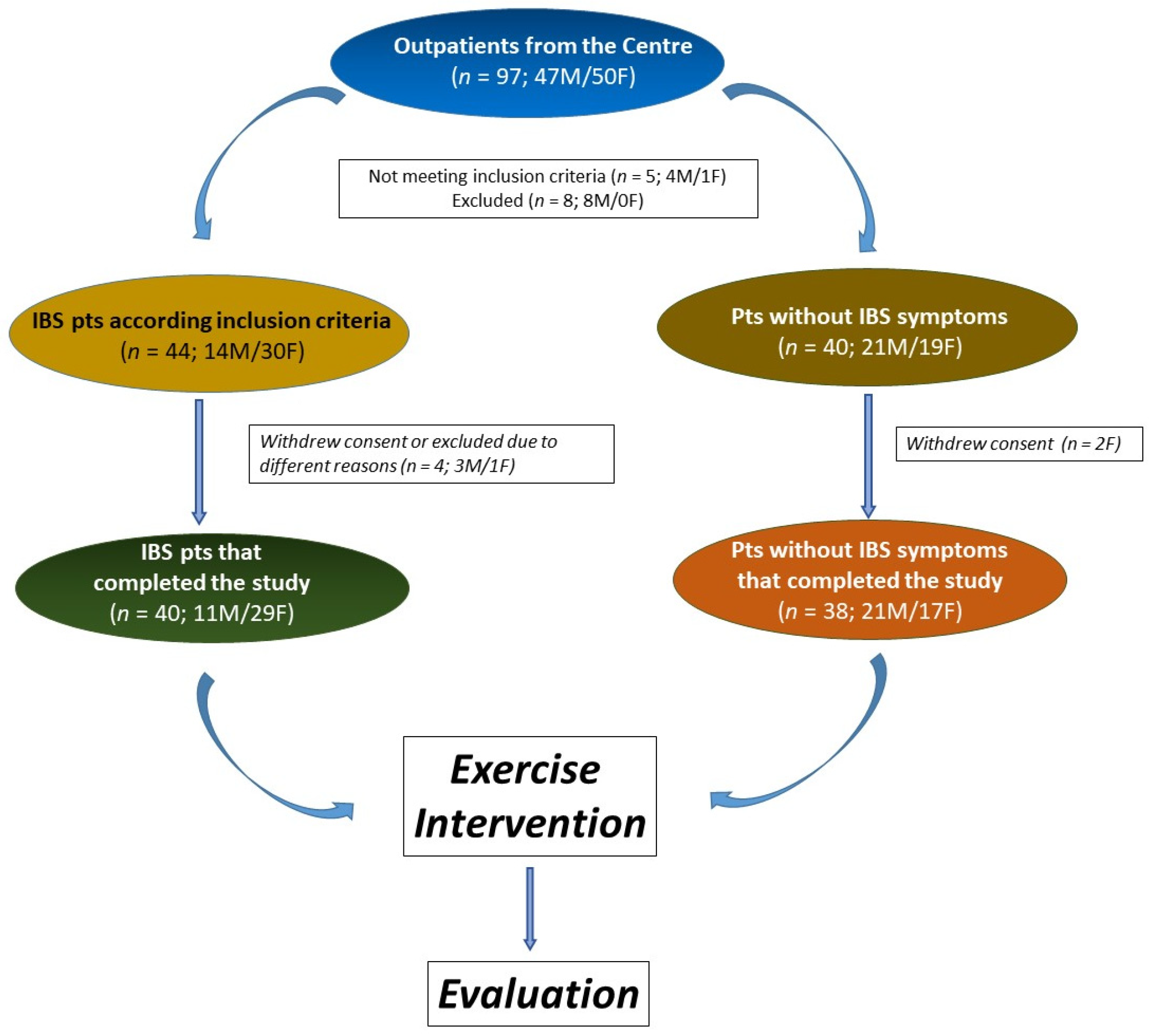
2.1. Participants
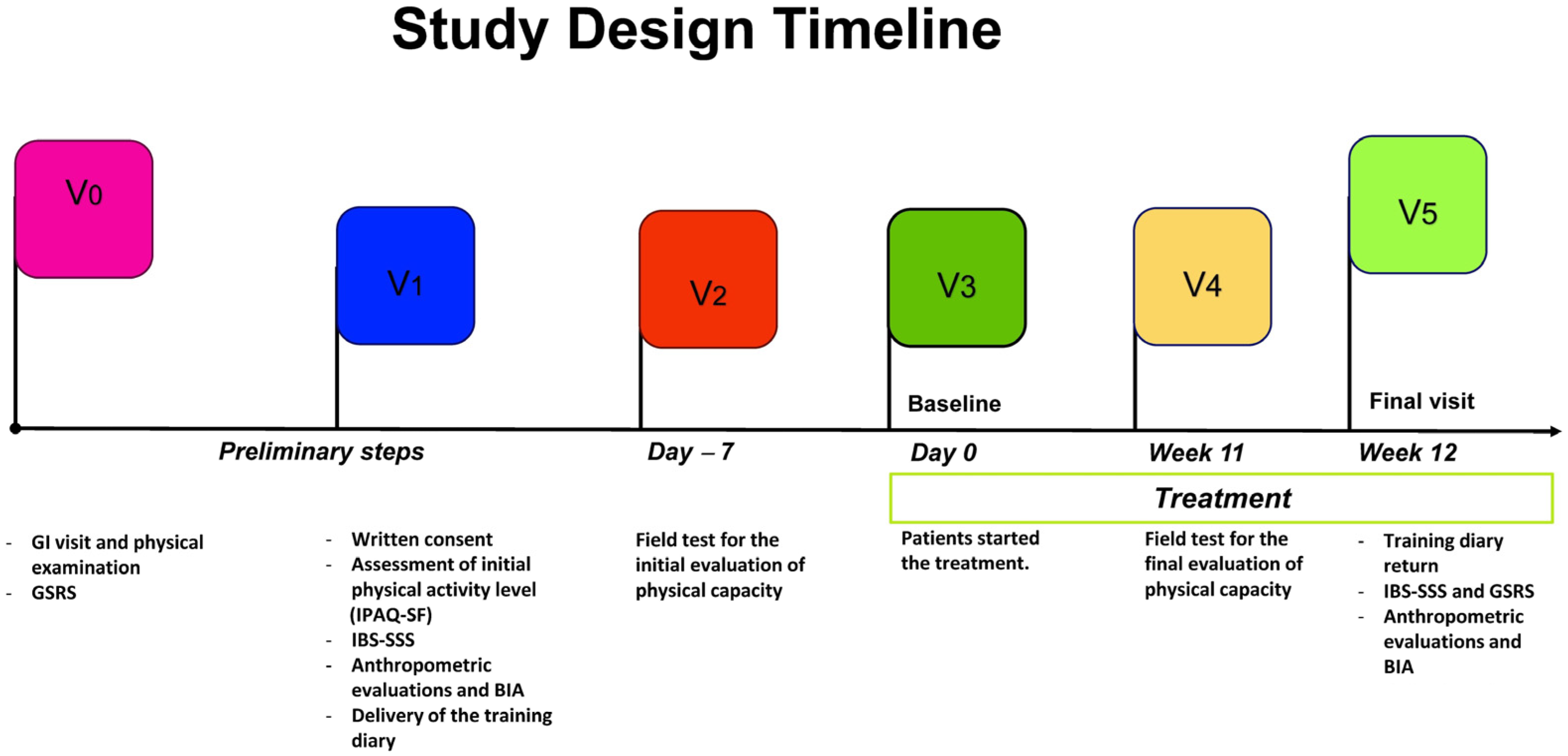
2.2. Study Design
2.3. Data Collection
2.4. Anthropometric and Bioelectrical Impedance Analysis (BIA) Parameters
2.5. Training Diary
2.6. Exercise Protocol
2.6.1. Physical Capacity Assessment Tests
2.6.2. Exercise Intervention
- Frequency. The aerobic exercise was performed outdoors on an urban route thrice a week, on non-consecutive days, for 12 weeks.
- Intensity. The aerobic exercise intensity was moderate (60/75% of HR max); it was monitored through the heart rate monitor and was personalized through Tanaka’s formula [38]. In addition, the Talk Test [39] and the Borg scale [40] were used to measure the rhythm and the perception of fatigue, respectively.
- Type. The aerobic exercise type was walking, ranging from 5 to 10 km/h.
- Time. Each walk had a duration of 60′ for a total of 180′ per week; the single outing lasting 60′ was structured as follows: Warm-up: 5′; Normal walk: 10′; Sustained walking: 30′; Fast walking: 10′; Cool-down: 5′. The entire activity was supervised by experts (Graduates in Preventive and Adapted Physical Activity Science and Techniques), and the presence of the participants at each training session was strictly registered.
2.6.3. Exposure—Global Physical Capacity Score
2.7. Outcome Assessment—IBS Severity Scoring System (IBS-SSS)
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
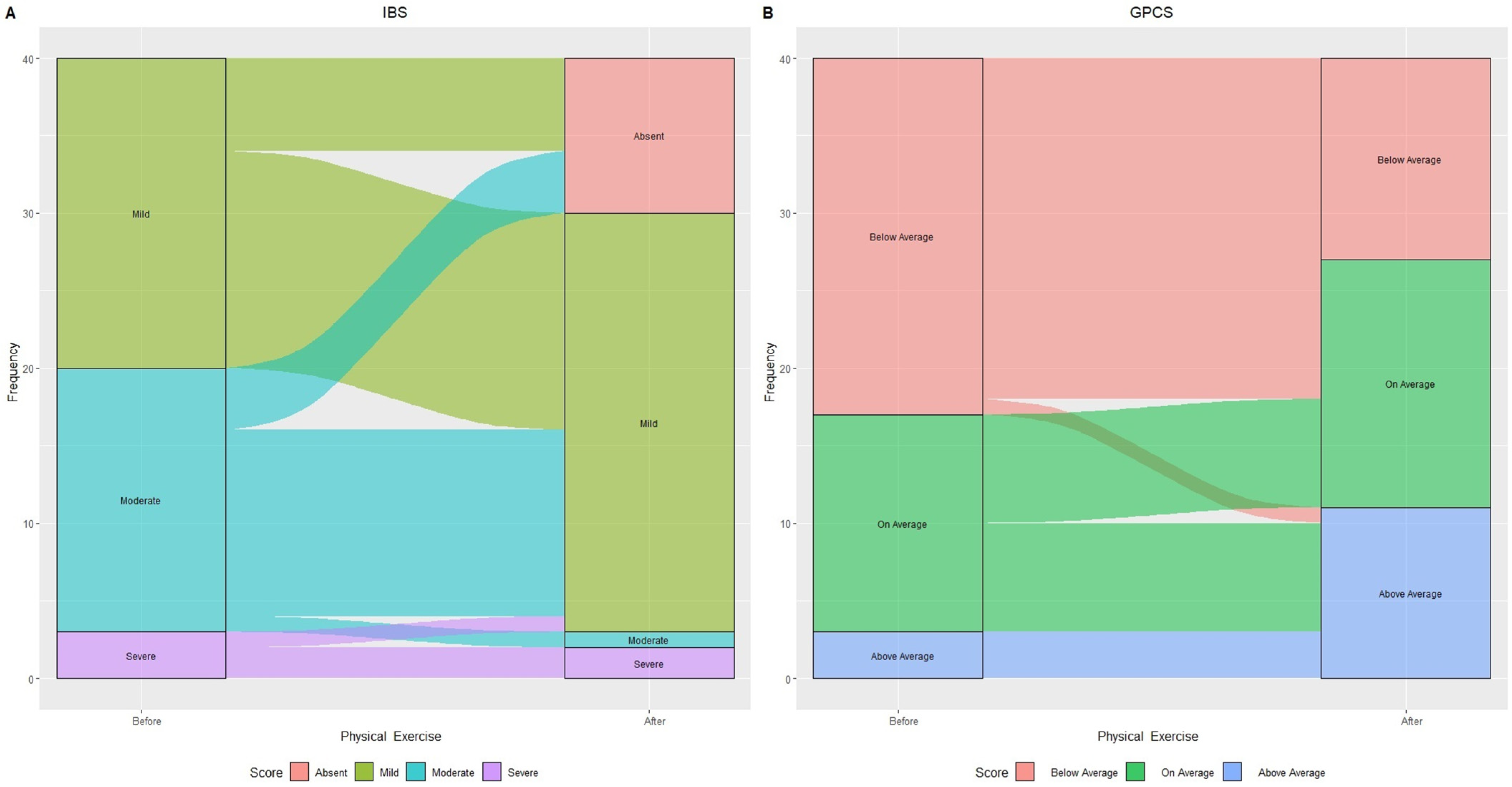
3.2. Modifications in IBS Categories Based on IBS-SSS and GPCS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No IBS | ||
|---|---|---|
| Exercise Intervention | ||
| PRE | POST | |
| N. | 21M/17F | 21M/17F |
| GPCS | 2.21 ± 1.76 | 3.13 ± 1.88 |
| IBS-SSS | 28.45 ± 26.02 | 22.26 ± 28.78 |
| BMI | 29.96 ± 5.84 | 29.47 ± 5.45 |
| WC | 98.69 ± 13.71 | 96.32 ± 11.19 |
| HC | 106.53 ± 11.26 | 106.27 ± 10.02 |
References
- Vandvik, P.O.; Lydersen, S.; Farup, P.G. Prevalence, comorbidity and impact of irritable bowel syndrome in Norway. Scand. J. Gastroenterol. 2006, 41, 650–656. [Google Scholar] [CrossRef]
- Wilson, S.; Roberts, L.; Roalfe, A.; Bridge, P.; Singh, S. Prevalence of irritable bowel syndrome: A community survey. Br. J. Gen. Pract. 2004, 54, 495–502. [Google Scholar]
- Thompson, W.G.; Longstreth, G.; Drossman, D.; Heaton, K.; Irvine, E.; Müller-Lissner, S. Functional bowel disorders and functional abdominal pain. Gut 1999, 45, II43–II47. [Google Scholar] [CrossRef] [PubMed]
- Kearney, D.J.; Brown-Chang, J. Complementary and alternative medicine for IBS in adults: Mind–body interventions. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 624–636. [Google Scholar] [CrossRef]
- Spanier, J.A.; Howden, C.W.; Jones, M.P. A systematic review of alternative therapies in the irritable bowel syndrome. Arch. Intern. Med. 2003, 163, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Rakel, D. Behavioral and complementary approaches for the treatment of irritable bowel syndrome. Nutr. Clin. Pract. 2008, 23, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C. Complementary and alternative medicine modalities for the treatment of irritable bowel syndrome: Facts or myths? Gastroenterol. Hepatol. 2010, 6, 705. [Google Scholar]
- Cozma-Petruţ, A.; Loghin, F.; Miere, D.; Dumitraşcu, D.L. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J. Gastroenterol. 2017, 23, 3771. [Google Scholar] [CrossRef]
- Ooi, S.L.; Correa, D.; Pak, S.C. Probiotics, prebiotics, and low FODMAP diet for irritable bowel syndrome–What is the current evidence? Complement. Ther. Med. 2019, 43, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Orlando, A.; Tutino, V.; Notarnicola, M.; Riezzo, G.; Linsalata, M.; Clemente, C.; Prospero, L.; Martulli, M.; D’Attoma, B.; De Nunzio, V. Improved symptom profiles and minimal inflammation in IBS-D patients undergoing a long-term low-FODMAP diet: A lipidomic perspective. Nutrients 2020, 12, 1652. [Google Scholar] [CrossRef]
- Riezzo, G.; Prospero, L.; Orlando, A.; Linsalata, M.; D’Attoma, B.; Ignazzi, A.; Giannelli, G.; Russo, F. A Tritordeum-Based Diet for Female Patients with Diarrhea-Predominant Irritable Bowel Syndrome: Effects on Abdominal Bloating and Psychological Symptoms. Nutrients 2023, 15, 1361. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Babyak, M.A.; Doraiswamy, P.M.; Watkins, L.; Hoffman, B.M.; Barbour, K.A.; Herman, S.; Craighead, W.E.; Brosse, A.L.; Waugh, R. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom. Med. 2007, 69, 587. [Google Scholar] [CrossRef]
- Mannerkorpi, K. Exercise in fibromyalgia. Curr. Opin. Rheumatol. 2005, 17, 190–194. [Google Scholar] [CrossRef]
- Steindorf, K.; Jedrychowski, W.; Schmidt, M.; Popiela, T.; Penar, A.; Galas, A.; Wahrendorf, J. Case-control study of lifetime occupational and recreational physical activity and risks of colon and rectal cancer. Eur. J. Cancer Prev. 2005, 14, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Wolin, K.Y.; Lee, I.M.; Colditz, G.A.; Glynn, R.J.; Fuchs, C.; Giovannucci, E. Leisure-time physical activity patterns and risk of colon cancer in women. Int. J. Cancer 2007, 121, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, E.; Li, Y.; Jia, Y.; Li, F. Exercise therapy of patients with irritable bowel syndrome: A systematic review of randomized controlled trials. Neurogastroenterol. Motil. 2019, 31, e13461. [Google Scholar] [CrossRef]
- Costantino, A.; Pessarelli, T.; Vecchiato, M.; Vecchi, M.; Basilisco, G.; Ermolao, A. A practical guide to the proper prescription of physical activity in patients with irritable bowel syndrome. Dig. Liver Dis. 2022, 54, 1600–1604. [Google Scholar] [CrossRef] [PubMed]
- Belvederi Murri, M.; Folesani, F.; Zerbinati, L.; Nanni, M.G.; Ounalli, H.; Caruso, R.; Grassi, L. Physical activity promotes health and reduces cardiovascular mortality in depressed populations: A literature overview. Int. J. Environ. Res. Public Health 2020, 17, 5545. [Google Scholar] [CrossRef]
- Villoria, A.; Serra, J.; Azpiroz, F.; Malagelada, J.-R. Physical activity and intestinal gas clearance in patients with bloating. Off. J. Am. Coll. Gastroenterol. ACG 2006, 101, 2552–2557. [Google Scholar] [CrossRef]
- Dainese, R.; Serra, J.; Azpiroz, F.; Malagelada, J.-R. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. Am. J. Med. 2004, 116, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, E.; Ringström, G.; Abrahamsson, H.; Sadik, R. Intervention to increase physical activity in irritable bowel syndrome shows long-term positive effects. World J. Gastroenterol. WJG 2015, 21, 600. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, E.; Simrén, M.; Strid, H.; Bajor, A.; Sadik, R. Physical activity improves symptoms in irritable bowel syndrome: A randomized controlled trial. Off. J. Am. Coll. Gastroenterol. ACG 2011, 106, 915–922. [Google Scholar] [CrossRef]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report; US Department of Health and Human Services: Washington, DC, USA, 2008; A1-H14. [Google Scholar]
- Lee, I.-M.; Skerrett, P.J. Physical activity and all-cause mortality: What is the dose-response relation? Med. Sci. Sports Exerc. 2001, 33, S459–S471. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.H.; Nevill, A.M.; Murtagh, E.M.; Holder, R.L. The effect of walking on fitness, fatness and resting blood pressure: A meta-analysis of randomised, controlled trials. Prev. Med. 2007, 44, 377–385. [Google Scholar] [CrossRef]
- Hu, F.B.; Sigal, R.J.; Rich-Edwards, J.W.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Speizer, F.E.; Manson, J.E. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA 1999, 282, 1433–1439. [Google Scholar] [CrossRef]
- Fox, K. At Least Five a Week: Evidence on the Impact of Physical Activity and Its Relationship to Health—A Report from the Chief Medical Officer; Senior Scientific Editor for Department of Health: London, UK, 2004. [Google Scholar]
- MIND. The MIND Guide to Physical Activity; MIND: London, UK, 2008. [Google Scholar]
- Kwak, L.; Kremers, S.; Walsh, A.; Brug, H. How is your walking group running? Health Educ. 2006, 106, 21–31. [Google Scholar] [CrossRef]
- Doughty, K. Walking together: The embodied and mobile production of a therapeutic landscape. Health Place 2013, 24, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Pate, R.R. A new definition of youth fitness. Physician Sportsmed. 1983, 11, 77–83. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126. [Google Scholar]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef]
- Khalil, S.F.; Mohktar, M.S.; Ibrahim, F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, R.; Oja, P.; Pasanen, M.; Vuori, I. Validity of a two kilometre walking test for estimating maximal aerobic power in overweight adults. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1992, 16, 263–268. [Google Scholar]
- Pearn, J.; Bullock, K. A portable hand-grip dynamometer. J. Paediatr. Child Health 1979, 15, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, W.W.; Hopkins, D.R. A comparison of the sit and reach and the modified sit and reach in the measurement of flexibility in women. Res. Q. Exerc. Sport 1992, 63, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- Foster, C.; Porcari, J.P.; Anderson, J.; Paulson, M.; Smaczny, D.; Webber, H.; Doberstein, S.T.; Udermann, B. The talk test as a marker of exercise training intensity. J. Cardiopulm. Rehabil. Prev. 2008, 28, 24–30. [Google Scholar] [CrossRef]
- Wilson, R.C.; Jones, P. A comparison of the visual analogue scale and modified Borg scale for the measurement of dyspnoea during exercise. Clin. Sci. 1989, 76, 277–282. [Google Scholar] [CrossRef]
- Bouchard, D.R.; Soucy, L.; Sénéchal, M.; Dionne, I.J.; Brochu, M. Impact of resistance training with or without caloric restriction on physical capacity in obese older women. Menopause 2009, 16, 66–72. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Warburton, D.E.; Bredin, S.S. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef]
- Warburton, D.E.; Charlesworth, S.; Ivey, A.; Nettlefold, L.; Bredin, S.S. A systematic review of the evidence for Canada’s Physical Activity Guidelines for Adults. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 39. [Google Scholar] [CrossRef]
- Costa, R.; Snipe, R.; Kitic, C.; Gibson, P. Systematic review: Exercise-induced gastrointestinal syndrome—Implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Peters, H.P.; De Vries, W.R.; Vanberge-Henegouwen, G.P.; Akkermans, L.M. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 2001, 48, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef]
- Madsbad, S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 2014, 16, 9–21. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Caes, L.; Orchard, A.; Christie, D. Connecting the mind–body split: Understanding the relationship between symptoms and emotional well-being in chronic pain and functional gastrointestinal disorders. Healthcare 2017, 5, 93. [Google Scholar] [CrossRef]
- Omagari, K.; Murayama, T.; Tanaka, Y.; Yoshikawa, C.; Inoue, S.-i.; Ichimura, M.; Hatanaka, M.; Saimei, M.; Muto, K.; Tobina, T. Mental, physical, dietary, and nutritional effects on irritable bowel syndrome in young Japanese women. Intern. Med. 2013, 52, 1295–1301. [Google Scholar] [CrossRef]
- De Schryver, A.M.; Keulemans, Y.C.; Peters, H.P.; Akkermans, L.M.; Smout, A.J.; De Vries, W.R.; Van Berge-Henegouwen, G.P. Effects of regular physical activity on defecation pattern in middle-aged patients complaining of chronic constipation. Scand. J. Gastroenterol. 2005, 40, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Colwell, L.; Prather, C.; Phillips, S.; Zinsmeister, A. Effects of an irritable bowel syndrome educational class on health-promoting behaviors and symptoms. Am. J. Gastroenterol. 1998, 93, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G.; Hughes, S. The exercise intensity–affect relationship: Evidence and implications for exercise behavior. J. Exerc. Sci. Fit. 2009, 7, S34–S41. [Google Scholar] [CrossRef]
- Hanson, S.; Jones, A. Is there evidence that walking groups have health benefits? A systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef] [PubMed]
| Exercise Intervention | ||||
|---|---|---|---|---|
| No IBS (n.38) | IBS Pre (n. 40) | IBS Post (40) | p | |
| Sex (male/female) | 21M/17F | 11M/29F | ||
| Age (years) | 53.71 ± 7.27 | 52.10 ± 7.72 | ||
| Body mass index | 29.96 ± 5.84 a | 29.04 ± 5.12 a | 28.80 ± 5.15 a | 0.0690 |
| Waist circumference | 98.69 ± 13.71 a | 93.04 ± 13.41a | 92.71 ± 13.62 a | 0.1149 |
| Hip circumference | 106.53 ± 11.26 a | 106.22 ± 10.07 a | 105.51 ± 10.07 a | 0.0465 |
| Waist/hip ratio | 0.93 ± 0.11 a | 0.87 ± 0.12 a | 0.88 ± 0.12 a | 0.0899 |
| PhA (degrees) | 6.76 ± 1.06 a | 6.46 ± 1.07 a | 6.44 ± 0.98 a | 0.3671 |
| BCM (kg) | 32.51 ± 7.22 a | 28.90 ± 7.04 b | 28.62 ± 6.6 b | 0.0380 |
| FM (kg) | 27.82 ± 12.10 a | 27.21 ± 10.84 a | 26.72 ± 10.91 a | 0.9734 |
| FFM (kg) | 56.70 ± 10.14 a | 51.60 ± 9.29 b | 51.22 ± 8.88 b | 0.0292 |
| TBW (liters) | 41.32 ± 7.68 a | 37.60 ± 6.84 b | 37.33 ± 6.44 b | 0.0340 |
| ECW (liters) | 17.57 ± 3.35 a | 16.44 ± 2.64 a | 16.36 ± 2.51 a | 0.2289 |
| Global Physical Capacity Score | 2.21 ± 1.76 a | 2.40 ± 1.53 a | 3.32 ± 1.68 b | <0.0001 |
| IBS scores | ||||
| Abdominal pain intensity | 1.84 ± 6.62 a | 24.50 ± 27.26 b | 12.58 ± 21.92 b | <0.0001 |
| Abdominal pain frequency | 1.58 ± 5.94 a | 21.75 ± 29.86 b | 8.62 ± 18.15 b | 0.0003 |
| Abdominal Distension | 9.21 ± 14.36 a | 45.75 ± 23.95 b | 27.80 ± 21.37 c | <0.0001 |
| Dissatisfaction with bowel habits | 10.39 ± 12.81a | 47.75 ± 32.20 b | 31.25 ± 23.88 b | 0.0002 |
| Interference on life in general | 5.39 ± 9.96 a | 43.38 ± 25.68 b | 31.50 ± 27.20 b | 0.0077 |
| Total score | 28.45 ± 26.02 a | 183.10 ± 79.43 b | 111.80 ± 76.84 c | <0.0001 |
| 2Km Walking Test | p * | |||
| Under the mean | 20 (52.6%) | 21 (52.5%) | 11 (27.5%) | |
| In mean | 16 (42.1%) | 15 (37.5%) | 20 (50%) | 0.0516 |
| Above the mean | 2 (5.3%) | 4 (10.0%) | 9 (22.5%) | |
| Sit and Reach Test | ||||
| Under the mean | 23 (60.5%) | 22 (55.0%) | 16 (40%) | |
| In mean | 2 (5.3%) | 4 (10.0%) | 4 (10%) | 0.4907 |
| Above the mean | 13 (34.2%) | 14 (35.0%) | 20 (50%) | |
| Hand-Grip Test | ||||
| Under the mean | 14 (36.8%) | 12 (30.0%) | 9 (22.5%) | |
| In mean | 12 (31.6%) | 15 (37.5%) | 11 (27.5%) | 0.3771 |
| Above the mean | 12 (31.6%) | 13 (32.5%) | 20 (50%) | |
| IPAQ Categories ° | ||||
| <700 | 13 (34.2%) | 11 (27.5%) | ||
| 700–2519 | 19 (50.0%) | 19 (47.5%) | 0.5723 | |
| ≥2520 | 6 (15.8%) | 10 (25.0%) | ||
| GPCS | Odds Ratio | p-Value | 95% CI |
|---|---|---|---|
| Below the mean | 1.00 | ||
| In mean | 0.32 | 0.10 | [0.08, 1.27] |
| Above the mean | 0.04 | 0.00 | [0.00, 0.31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, A.; Russo, F.; Franco, I.; Riezzo, G.; Donghia, R.; Curci, R.; Bonfiglio, C.; Prospero, L.; D’Attoma, B.; Ignazzi, A.; et al. Enhanced Physical Capacity and Gastrointestinal Symptom Improvement in Southern Italian IBS Patients following Three Months of Moderate Aerobic Exercise. J. Clin. Med. 2023, 12, 6786. https://doi.org/10.3390/jcm12216786
Bianco A, Russo F, Franco I, Riezzo G, Donghia R, Curci R, Bonfiglio C, Prospero L, D’Attoma B, Ignazzi A, et al. Enhanced Physical Capacity and Gastrointestinal Symptom Improvement in Southern Italian IBS Patients following Three Months of Moderate Aerobic Exercise. Journal of Clinical Medicine. 2023; 12(21):6786. https://doi.org/10.3390/jcm12216786
Chicago/Turabian StyleBianco, Antonella, Francesco Russo, Isabella Franco, Giuseppe Riezzo, Rossella Donghia, Ritanna Curci, Caterina Bonfiglio, Laura Prospero, Benedetta D’Attoma, Antonia Ignazzi, and et al. 2023. "Enhanced Physical Capacity and Gastrointestinal Symptom Improvement in Southern Italian IBS Patients following Three Months of Moderate Aerobic Exercise" Journal of Clinical Medicine 12, no. 21: 6786. https://doi.org/10.3390/jcm12216786
APA StyleBianco, A., Russo, F., Franco, I., Riezzo, G., Donghia, R., Curci, R., Bonfiglio, C., Prospero, L., D’Attoma, B., Ignazzi, A., Campanella, A., & Osella, A. R. (2023). Enhanced Physical Capacity and Gastrointestinal Symptom Improvement in Southern Italian IBS Patients following Three Months of Moderate Aerobic Exercise. Journal of Clinical Medicine, 12(21), 6786. https://doi.org/10.3390/jcm12216786








