Usefullness of Heparin Calibrated Anti-Xa Activity to Assess Anticoagulant Activity of Apixaban and Rivaroxaban in Emergency Patients Scheduled for Acute Interventions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Retrospective Part: The Derivation Cohort
Conversion factor CF = CF1 + CF2 + CF3..../n samples
2.2. The Prospective Part: The Validation Cohort
2.3. Statistical Analysis
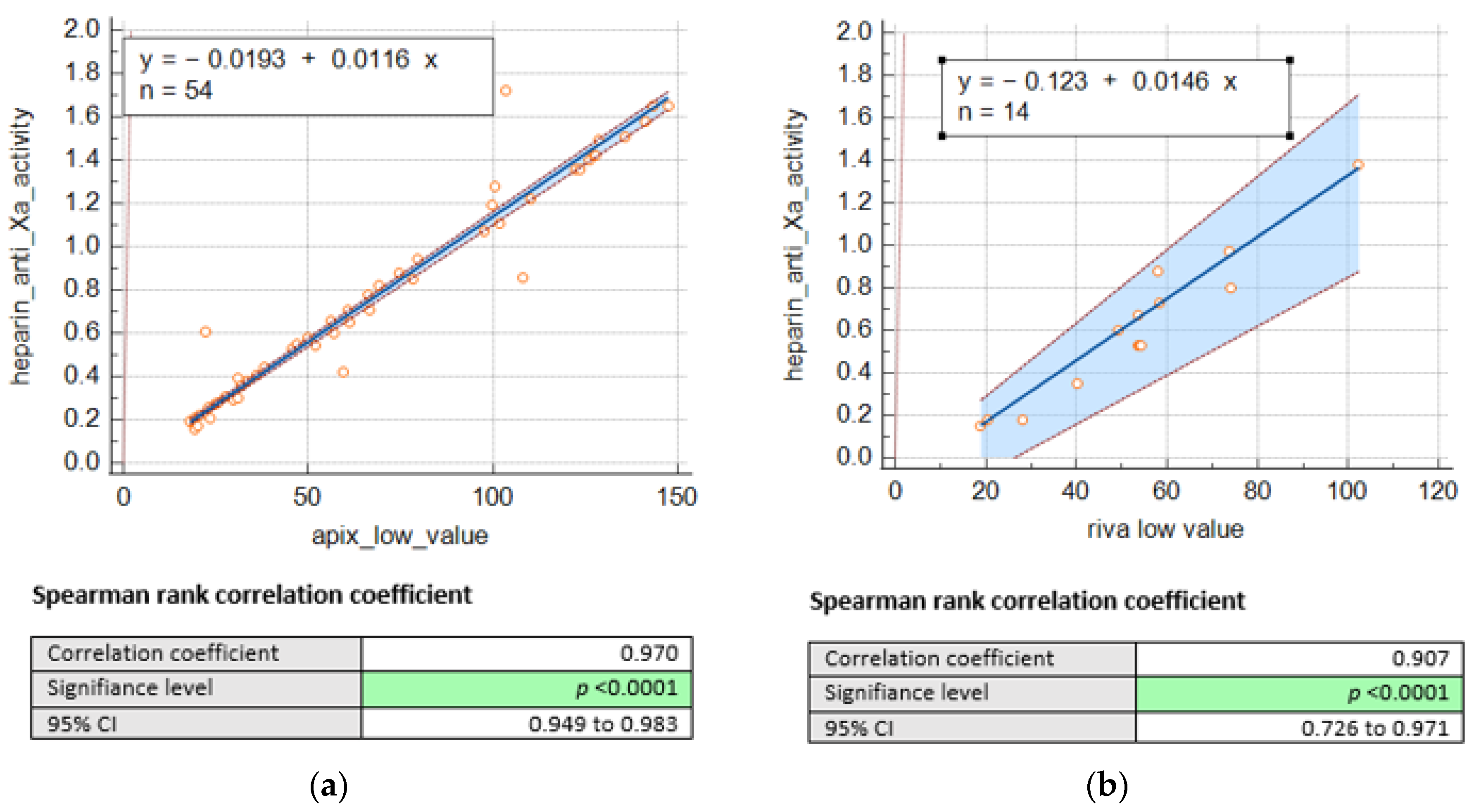
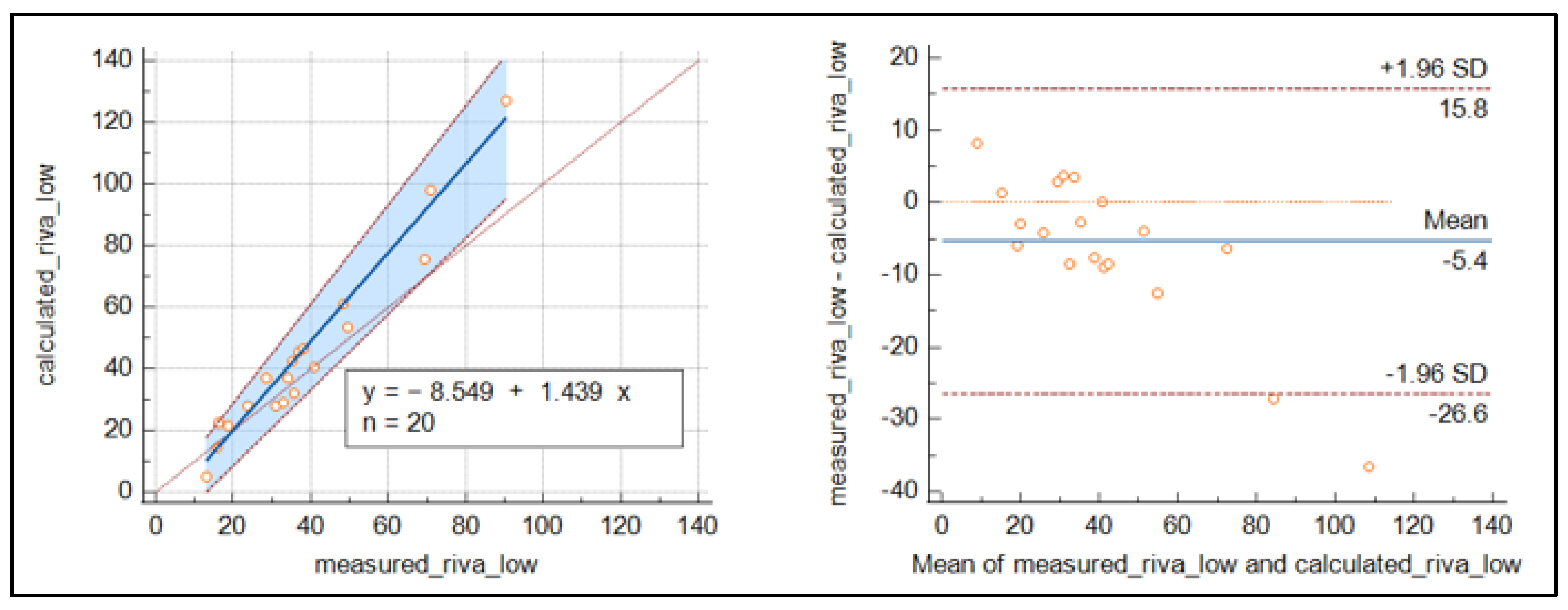
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, G.D.; Lucas, E.; Alexander, G.C.; Goldberger, Z.D. National Trends in Ambulatory Oral Anticoagulant Use. Am. J. Med. 2015, 128, 1300–1305.e2. [Google Scholar] [CrossRef] [PubMed]
- Heuvel, J.M.v.D.; Hövels, A.M.; Büller, H.R.; Mantel-Teeuwisse, A.K.; de Boer, A.; der Zee, A.H.M.-V. NOACs replace VKA as preferred oral anticoagulant among new patients: A drug utilization study in 560 pharmacies in The Netherlands. Thromb. J. 2018, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.T.; Hill, N.R.; Luo, X.; Masseria, C.; Abariga, S.A.; Ashaye, A.O. A systematic review of network meta-analyses among patients with nonvalvular atrial fibrillation: A comparison of efficacy and safety following treatment with direct oral anticoagulants. Int. J. Cardiol. 2018, 269, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Mekaj, A.Y.; Mekaj, Y.H.; Duci, S.B.; Miftari, E. New oral anticoagulants: Their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther. Clin. Risk Manag. 2015, 11, 967–977. [Google Scholar] [CrossRef]
- Schmitz, E.M.H.; Boonen, K.; Heuvel, D.v.D.; van Dongen, J.; Schellings, M.W.M.; Emmen, J.M.A.; van der Graaf, F.; Brunsveld, L.; van de Kerkhof, D. Determination of dabigatran, rivaroxaban and apixaban by ultra-performance liquid chromatography—Tandem mass spectrometry (UPLC-MS/MS) and coagulation assays for therapy monitoring of novel direct oral anticoagulants. J. Thromb. Haemost. 2014, 12, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, S.; Gray, E.; Mackie, I.; Baglin, T.; Makris, M. Measurement of non-Coumarin anticoagulants and their effects on tests of Haemostasis: Guidance from the British Committee for Standards in Haematology. Br. J. Haematol. 2014, 166, 830–841. [Google Scholar] [CrossRef]
- Jabet, A.; Stepanian, A.; Golmard, J.-L.; Flaujac, C.; Joly, B.S.; Gouin-Thibault, I.; Siguret, V. Are Screening Tests Reliable to Rule Out Direct Oral Anticoagulant Plasma Levels at Various Thresholds (30, 50, or 100 ng/mL) in Emergency Situations? Chest 2018, 153, 288–290. [Google Scholar] [CrossRef]
- Levy, J.H.; Ageno, W.; Chan, N.C.; Crowther, M.; Verhamme, P.; Weitz, J.I. When and how to use antidotes for the reversal of direct oral anticoagulants: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 623–627. [Google Scholar] [CrossRef]
- Pernod, G.; Albaladejo, P.; Godier, A.; Samama, C.M.; Susen, S.; Gruel, Y.; Blais, N.; Fontana, P.; Cohen, A.; Llau, J.V.; et al. Management of major bleeding complications and emergency surgery in patients on longterm treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: Proposals of the working group on perioperative haemostasis (GIHP). Arch. Cardiovasc. Dis. 2013, 106, 382–393. [Google Scholar] [CrossRef]
- Touzé, E.; Gruel, Y.; Gouin-Thibault, I.; De Maistre, E.; Susen, S.; Sie, P.; Derex, L. Intravenous thrombolysis for acute ischaemic stroke in patients on direct oral anticoagulants. Eur. J. Neurol. 2018, 25, 747-e52. [Google Scholar] [CrossRef]
- Faraoni, D.; Levy, J.H.; Albaladejo, P.; Samama, C.-M.; Groupe d’Intérêt en Hémostase Périopératoire. Updates in the perioperative and emergency management of non-vitamin K antagonist oral anticoagulants. Crit. Care 2015, 19, 203. [Google Scholar] [CrossRef] [PubMed]
- Erdoes, G.; De Arroyabe, B.M.L.; Bolliger, D.; Ahmed, A.B.; Koster, A.; Agarwal, S.; Boer, C.; von Heymann, C. International consensus statement on the peri-operative management of direct oral anticoagulants in cardiac surgery. Anaesthesia 2018, 73, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.C.; Adcock, D.M.; Bates, S.M.; Douxfils, J.; Favaloro, E.J.; Gouin-Thibault, I.; Guillermo, C.; Kawai, Y.; Lindhoff-Last, E.; Kitchen, S. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Arthritis Res. Ther. 2018, 118, 437–450. [Google Scholar] [CrossRef] [PubMed]
- ISO 15189:2022; Medical Laboratories. Requirements for Quality and Competence. ISO: Geneva, Switzerland, 2022.
- Billoir, P.; Barbay, V.; Joly, L.M.; Fresel, M.; Chrétien, M.H.; Duchez, V.L.C. Anti-Xa Oral Anticoagulant Plasma Concentration Assay in Real Life: Rivaroxaban and Apixaban Quantification in Emergency with LMWH Calibrator. Ann. Pharmacother. 2019, 53, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Boissier, E.; Senage, T.; Babuty, A.; Gouin-Thibault, I.; Rozec, B.; Roussel, J.-C.; Sigaud, M.; Ternisien, C.; Trossaert, M.; Fouassier, M.; et al. Heparin Anti-Xa Activity, a Readily Available Unique Test to Quantify Apixaban, Rivaroxaban, Fondaparinux, and Danaparoid Levels. Obstet. Anesth. Dig. 2021, 132, 707–716. [Google Scholar] [CrossRef]
- Maier, C.L.; Asbury, W.H.; Duncan, A.; Robbins, A.; Ingle, A.; Webb, A.; Stowell, S.R.; Roback, J.D. Using an old test for new tricks: Measuring direct oral anti-Xa drug levels by conventional heparin-calibrated anti-Xa assay. Am. J. Hematol. 2019, 94, E132–E134. [Google Scholar] [CrossRef]
- Willekens, G.; Studt, J.; Mendez, A.; Alberio, L.; Fontana, P.; Wuillemin, W.A.; Schmidt, A.; Graf, L.; Gerber, B.; Bovet, C.; et al. A universal anti-Xa assay for rivaroxaban, apixaban, and edoxaban measurements: Method validation, diagnostic accuracy and external validation. Br. J. Haematol. 2021, 193, 1203–1212. [Google Scholar] [CrossRef]
- Lim, M.S.; Hayes, R.; Sharma, A.; Kitiponchai, T.; Mohamed, M.; Mcrae, S. Prospective cohort study on the use of low molecular weight heparin calibrated anti-Xa assay for measurement of direct oral Xa inhibitors in ex vivo patient samples. Pathology 2022, 54, 599–605. [Google Scholar] [CrossRef]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef]
- Meihandoest, T.; Studt, J.-D.; Mendez, A.; Alberio, L.; Fontana, P.; Wuillemin, W.A.; Schmidt, A.; Graf, L.; Gerber, B.; Amstutz, U.; et al. Accuracy of a Single, Heparin-Calibrated Anti-Xa Assay for the Measurement of Rivaroxaban, Apixaban, and Edoxaban Drug Concentrations: A Prospective Cross-Sectional Study. Front. Cardiovasc. Med. 2022, 9, 817826. [Google Scholar] [CrossRef]
- Smahi, M.; De Pooter, N.; Hollestelle, M.J.; Toulon, P. Monitoring unfractionated heparin therapy: Lack of standardization of anti-Xa activity reagents. J. Thromb. Haemost. 2020, 18, 2613–2621. [Google Scholar] [CrossRef] [PubMed]
- Hollestelle, M.J.; van der Meer, F.J.; Meijer, P. Quality performance for indirect Xa inhibitor monitoring in patients using international external quality data. Clin. Chem. Lab. Med. 2020, 58, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
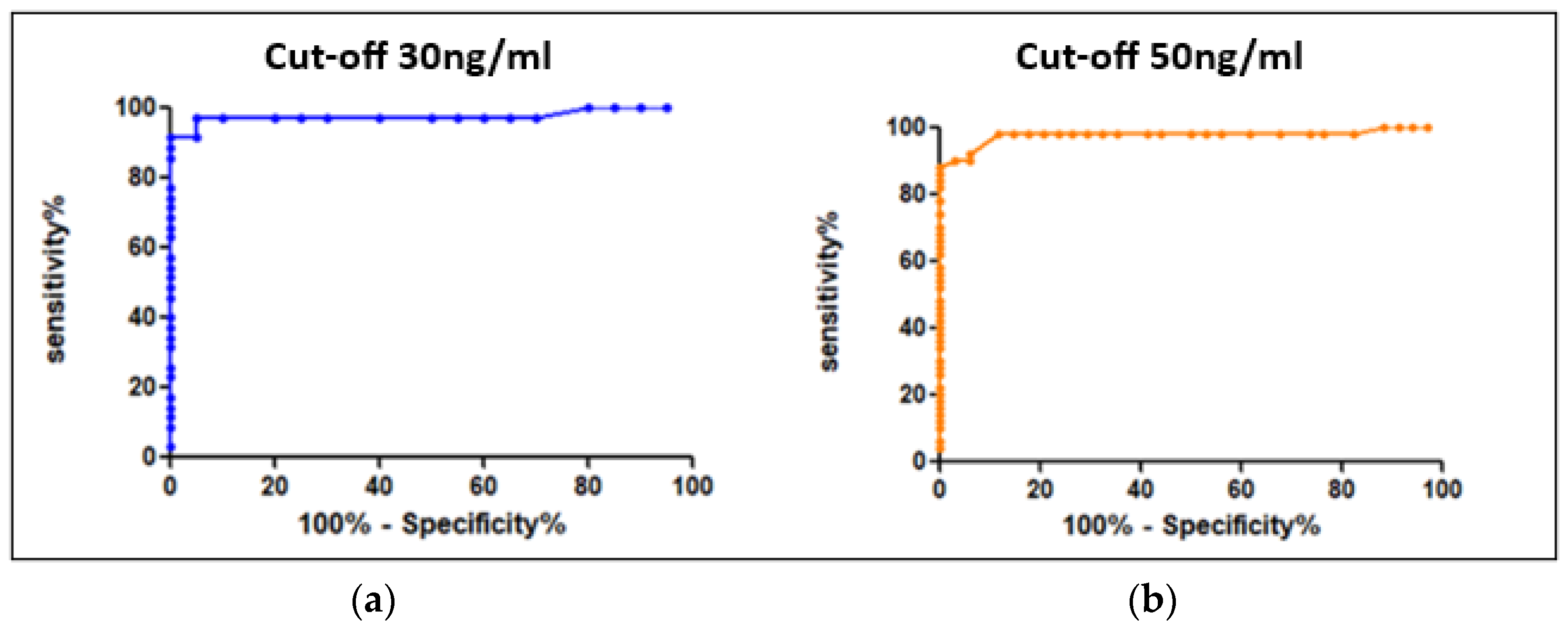

| APIX and RIVA Cut-Offs (ng/mL) | AUC | Sensitivity | Specificity | APIX and RIVA Cutoffs (UI/mL) |
|---|---|---|---|---|
| 30 ng/mL | 0.986 | 98% | 95% | 0.3 |
| 50 ng/mL | 0.983 | 98% | 88.2% | 0.51 |
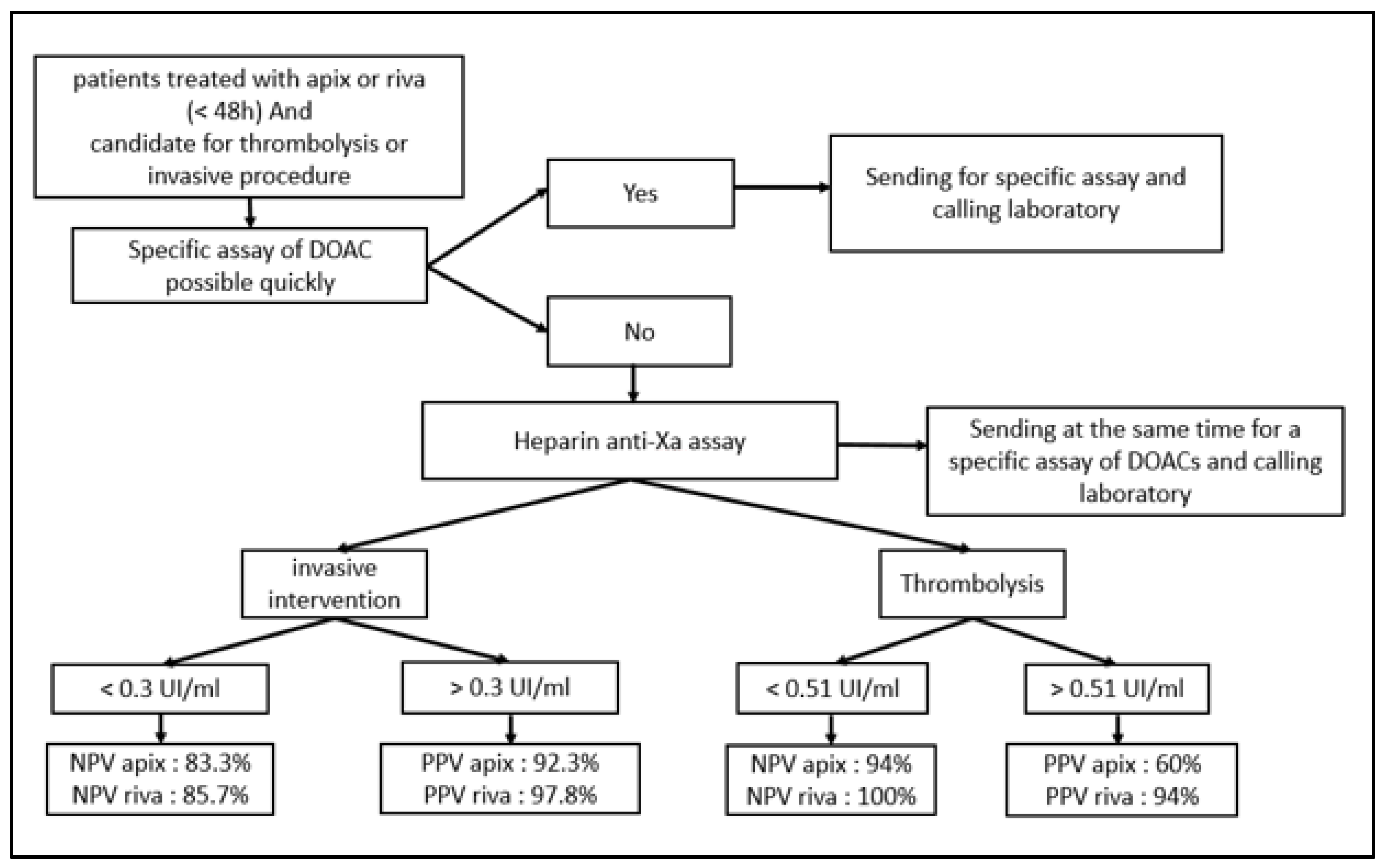
| DOAC | DOAC | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|
| Cutoff 0.3 UI/mL | APIX | 97.6% | 83.3% | 97.6% | 83.3% |
| RIVA | 92.3% | 85.7% | 92.3% | 85.7% | |
| Cutoff 0.51 UI/mL | APIX | 97% | 88.2% | 94% | 94% |
| RIVA | 100% | 88.2% | 60% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riahi, N.; Rozen, L.; Demulder, A. Usefullness of Heparin Calibrated Anti-Xa Activity to Assess Anticoagulant Activity of Apixaban and Rivaroxaban in Emergency Patients Scheduled for Acute Interventions. J. Clin. Med. 2023, 12, 6785. https://doi.org/10.3390/jcm12216785
Riahi N, Rozen L, Demulder A. Usefullness of Heparin Calibrated Anti-Xa Activity to Assess Anticoagulant Activity of Apixaban and Rivaroxaban in Emergency Patients Scheduled for Acute Interventions. Journal of Clinical Medicine. 2023; 12(21):6785. https://doi.org/10.3390/jcm12216785
Chicago/Turabian StyleRiahi, Nada, Laurence Rozen, and Anne Demulder. 2023. "Usefullness of Heparin Calibrated Anti-Xa Activity to Assess Anticoagulant Activity of Apixaban and Rivaroxaban in Emergency Patients Scheduled for Acute Interventions" Journal of Clinical Medicine 12, no. 21: 6785. https://doi.org/10.3390/jcm12216785
APA StyleRiahi, N., Rozen, L., & Demulder, A. (2023). Usefullness of Heparin Calibrated Anti-Xa Activity to Assess Anticoagulant Activity of Apixaban and Rivaroxaban in Emergency Patients Scheduled for Acute Interventions. Journal of Clinical Medicine, 12(21), 6785. https://doi.org/10.3390/jcm12216785






