Robot-Assisted Radical Prostatectomy in Renal Transplant Recipients: A Systematic Review
Abstract
1. Introduction
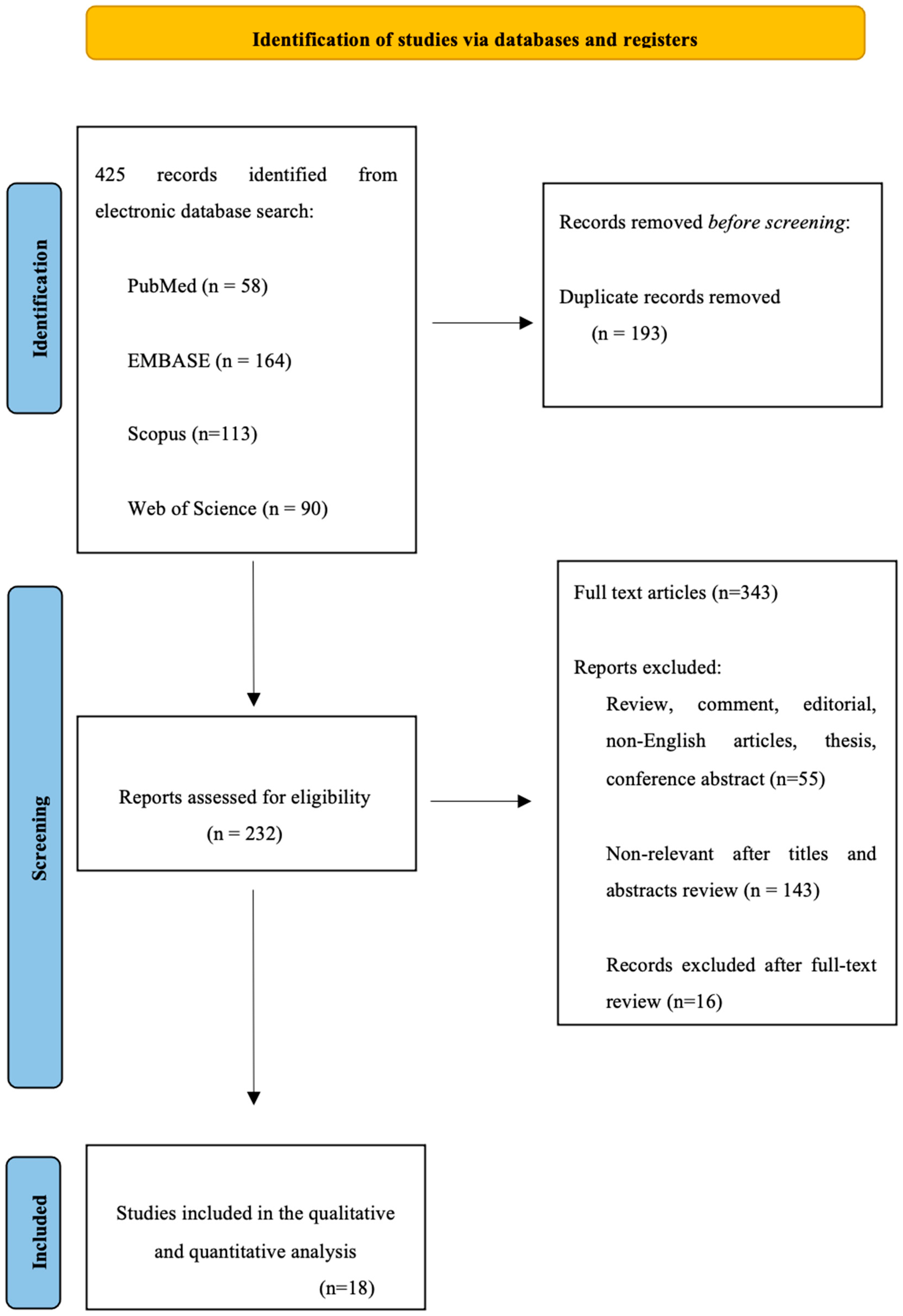
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
- (P): adults (age > 18 yrs) who underwent RARP or modified RARP for prostate cancer after kidney transplantation;
- (I): RARP or modified RARP;
- (C): either comparative or noncomparative studies;
- (O): incidence of positive surgical margins, intra- and postoperative complications and functional outcomes;
- (S): prospective or retrospective studies.
2.3. Data Extraction
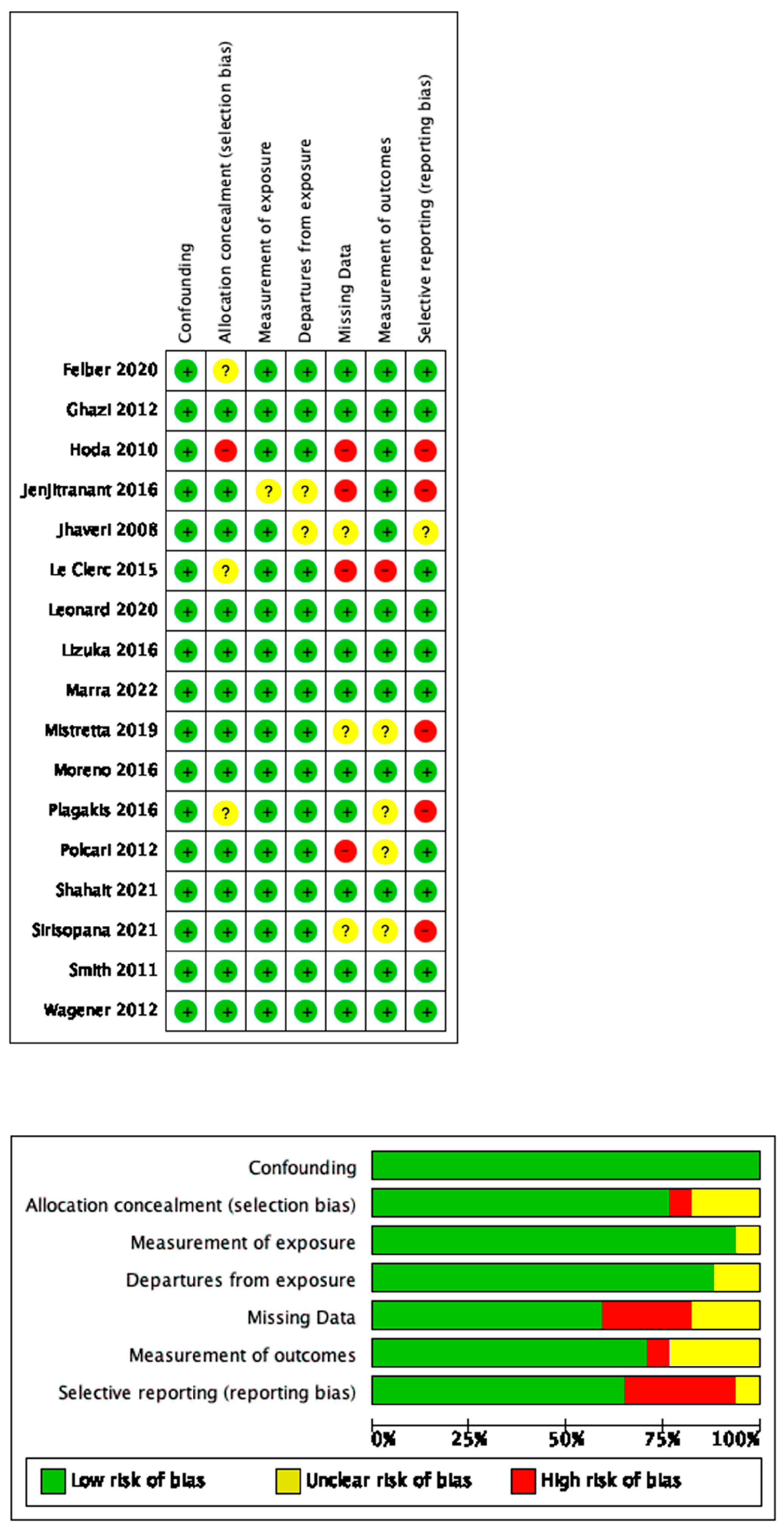
2.4. Risk-of-Bias Assessment
2.5. Data Extraction and Analysis
3. Evidence Synthesis
3.1. Study Characteristics
3.2. Baseline Characteristics
3.3. Surgical Technique
3.4. Perioperative Outcomes
3.5. Oncologic Outcomes
4. Discussion
4.1. Port Placement
4.2. Development of the Space of Retzius
4.3. Lymphadenectomy
4.4. Perioperative Outcomes
4.5. Oncologic Outcomes
4.6. Critical Reflections
4.7. Alternative Treatment Strategies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kasiske, B.L.; Snyder, J.J.; Gilbertson, D.T.; Wang, C. Cancer after Kidney Transplantation in the United States. Am. J. Transplant. 2004, 4, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Nunes, P.; Dinis, P.; Antunes, H.; Parada, B.; Marconi, L.; Moreira, P.; Roseiro, A.; Bastos, C.; Rolo, F.; et al. Prostate Cancer in Renal Transplant Recipients: Diagnosis and Treatment. Transplant. Proc. 2017, 49, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.C.; Pfeiffer, R.M.; Segev, D.L.; Engels, E.A. Cumulative incidence of cancer after solid organ transplantation. Cancer 2013, 119, 2300–2308. [Google Scholar] [CrossRef] [PubMed]
- Cormier, L.; Lechevallier, E.; Barrou, B.; Benoit, G.; Bensadoun, H.; Boudjema, K.; Descottes, J.-L.; Doré, B.; Guy, L.; Malavaud, B.; et al. Diagnosis and treatment of prostate cancers in renal-transplant recipients. Transplantation 2003, 75, 237–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Binsaleh, S. Diagnosis and treatment of prostate cancer in renal-transplant recipients. Int. Urol. Nephrol. 2011, 44, 149–155. [Google Scholar] [CrossRef]
- Marra, G.; Dalmasso, E.; Agnello, M.; Munegato, S.; Bosio, A.; Sedigh, O.; Biancone, L.; Gontero, P. Prostate cancer treatment in renal transplant recipients: A systematic review. BJU Int. 2017, 121, 327–344. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines on Prostate Cancer—Uroweb. Uroweb—European Association of Urology n.d. Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 9 August 2023).
- Zeng, J.; Christiansen, A.J.; Pooli, A.; Qiu, F.; LaGrange, C.A. Safety and Clinical Outcomes of Robot-Assisted Radical Prostatectomy in Kidney Transplant Patients: A Systematic Review. J. Endourol. 2018, 32, 935–943. [Google Scholar] [CrossRef]
- Marra, G.; Agnello, M.; Giordano, A.; Soria, F.; Oderda, M.; Dariane, C.; Timsit, M.-O.; Branchereau, J.; Hedli, O.; Mesnard, B.; et al. Robotic Radical Prostatectomy for Prostate Cancer in Renal Transplant Recipients: Results from a Multicenter Series. Eur. Urol. 2022, 82, 639–645. [Google Scholar] [CrossRef]
- Felber, M.; Drouin, S.J.; Grande, P.; Vaessen, C.; Parra, J.; Barrou, B.; Matillon, X.; Crouzet, S.; Leclerc, Q.; Rigaud, J.; et al. Morbidity, perioperative outcomes and complications of robot-assisted radical prostatectomy in kidney transplant patients: A French multicentre study. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 599.e15–599.e21. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Jhaveri, J.K.; Tan, G.Y.; Scherr, D.S.; Tewari, A.K. Robot-Assisted Laparoscopic Radical Prostatectomy in the Renal Allograft Transplant Recipient. J. Endourol. 2008, 22, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Hoda, M.R.; Hamza, A.; Greco, F.; Wagner, S.; Reichelt, O.; Heynemann, H.; Fischer, K.; Fornara, P. Management of localized prostate cancer by retropubic radical prostatectomy in patients after renal transplantation. Nephrol. Dial. Transplant. 2010, 25, 3416–3420. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Jellison, F.C.; Heldt, J.P.; Tenggardjaja, C.; Bowman, R.J.; Jin, D.H.; Chamberlin, J.; Lui, P.D.; Baldwin, D.D. Robot-Assisted Radical Prostatectomy in Patients with Previous Renal Transplantation. J. Endourol. 2011, 25, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, A.; Erturk, E.; Joseph, J.V. Modifications to Facilitate Extraperitoneal Robot-Assisted Radical Prostatectomy Post Kidney Transplant. JSLS J. Soc. Laparosc. Robot. Surg. 2012, 16, 314–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Polcari, A.J.; Allen, J.C.; Nunez-Nateras, R.; Mmeje, C.O.; Andrews, P.E.; Milner, J.E.; Castle, E.P.; Woods, M.E. Multicenter Experience With Robot-assisted Radical Prostatectomy in Renal Transplant Recipients. Urology 2012, 80, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Wagener, N.; Nyarangi-Dix, J.; Teber, D.; Zeier, M.; Hohenfellner, M. Applicability of Robot-Assisted Laparoscopic Radical Prostatectomy in Renal Allograft Recipients. Transplant. Proc. 2012, 44, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Le Clerc, Q.-C.; Lecornet, E.; Leon, G.; Rigaud, J.; Glemain, P.; Branchereau, J.; Karam, G. Technical feasibility of robot-assisted laparoscopic radical prostatectomy in renal transplant recipients: Results of a series of 12 consecutive cases. Can. Urol. Assoc. J. 2015, 9, E490–E493. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.M.; Paniagua, M.C.; Romo, M.G.; Pérez, J.S.; González, E.R.; Herrero, M.G.; Gómez, N.N.; Izquierdo, J.B. Robot Assisted Radical Prostatectomy in Kidney Transplant Recipients. Our Clinical Experience and a Systematic Review. Urol. Int. 2016, 97, 440–444. [Google Scholar] [CrossRef]
- Jenjitranant, P.; Sangkum, P.; Sirisreetreerux, P.; Viseshsindh, W.; Patcharatrakul, S.; Kongcharoensombat, W. Retzius Space Preservation Technique for Robotic-Assisted Laparoscopic Radical Prostatectomy in a Kidney Transplant Patient: First Case in Thailand and Our First Experience. Transplant. Proc. 2016, 48, 3130–3133. [Google Scholar] [CrossRef]
- Plagakis, S.; Foreman, D.; Sutherland, P.; Fuller, A.; Zeng, J.; Christiansen, A.; Pooli, A.; Qiu, F.; LaGrange, C.A. Transperitoneal Robot-Assisted Radical Prostatectomy Should Be Considered in Prostate Cancer Patients with Pelvic Kidneys. J. Endourol. Case Rep. 2016, 2, 38–40. [Google Scholar] [CrossRef]
- Iizuka, J.; Hashimoto, Y.; Kondo, T.; Takagi, T.; Inui, M.; Nozaki, T.; Omoto, K.; Shimizu, T.; Ishida, H.; Tanabe, K. Robot-Assisted Radical Prostatectomy for Localized Prostate Cancer in Asian Renal Transplant Recipients. Transplant. Proc. 2016, 48, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Mistretta, F.A.; Galfano, A.; Di Trapani, E.; Di Trapani, D.; Russo, A.; Secco, S.; Ferro, M.; Musi, G.; Bocciardi, A.M.; de Cobelli, O. Robot assisted radical prostatectomy in kidney transplant recipients: Surgical, oncological and functional outcomes of two different robotic approaches. Int. Braz. J. Urol. 2019, 45, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Léonard, G.; Pradère, B.; Monléon, L.; Boutin, J.-M.; Branchereau, J.; Karam, G.; Rigaud, J.; Bruyère, F. Oncological and Postoperative Outcomes of Robot-Assisted Laparoscopic Radical Prostatectomy in Renal Transplant Recipients: A Multicenter and Comparative Study. Transplant. Proc. 2020, 52, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Sirisopana, K.; Jenjitranant, P.; Sangkum, P.; Kijvikai, K.; Pacharatakul, S.; Leenanupunth, C.; Kochakarn, W.; Kongchareonsombat, W. Radical prostatectomy outcomes in renal transplant recipients: A retrospective case series of Thai patients. BMC Urol. 2021, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Shahait, M.; Al Majali, F.; Dobbs, R.W.; Sandberg, A.; El-Achkar, A.; El-Fahmawi, A.; Mucksavage, P.; Lee, D.I. Oncological and Functional Outcomes of Robot-Assisted Radical Prostatectomy in Kidney Transplant Recipients. JSLS J. Soc. Laparosc. Robot. Surg. 2021, 25, e2021.00045. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Falagario, U.G.; Villers, A.; Dell’oglio, P.; Mazzone, E.; Autorino, R.; Moschovas, M.C.; Buscarini, M.; Bravi, C.A.; Briganti, A.; et al. Contemporary Techniques of Prostate Dissection for Robot-assisted Prostatectomy. Eur. Urol. 2020, 78, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Territo, A.; Diana, P.; Gaya, J.M.; Gallioli, A.; Piana, A.; Breda, A. Robot-assisted kidney transplantation: State of art. Arch. Esp. Urol. 2021, 74, 970–978. [Google Scholar]
- Breda, A.; Diana, P.; Territo, A.; Gallioli, A.; Piana, A.; Gaya, J.M.; Gavrilov, P.; Desender, L.; Van Parys, B.; Van Praet, C.; et al. Intracorporeal Versus Extracorporeal Robot-assisted Kidney Autotransplantation: Experience of the ERUS RAKT Working Group. Eur. Urol. 2021, 81, 168–175. [Google Scholar] [CrossRef]
- Albisinni, S.; Dasnoy, C.; Diamand, R.; Mjaess, G.; Aoun, F.; Esperto, F.; Porpiglia, F.; Fiori, C.; Roumeguere, T.; DE Nunzio, C. Anterior vs. Retzius-sparing robotic assisted radical prostatectomy: Can the approach really make a difference? Eur. Urol. Suppl. 2022, 74, 137–145. [Google Scholar] [CrossRef]
- Kumar, A.; Patel, V.R.; Panaiyadiyan, S.; Bhat, K.R.S.; Moschovas, M.C.; Nayak, B. Nerve-sparing robot-assisted radical prostatectomy: Current perspectives. Asian J. Urol. 2020, 8, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Wagaskar, V.G.; Mittal, A.; Sobotka, S.; Ratnani, P.; Lantz, A.; Falagario, U.G.; Martini, A.; Dovey, Z.; Treacy, P.-J.; Pathak, P.; et al. Hood Technique for Robotic Radical Prostatectomy—Preserving Periurethral Anatomical Structures in the Space of Retzius and Sparing the Pouch of Douglas, Enabling Early Return of Continence Without Compromising Surgical Margin Rates. Eur. Urol. 2020, 80, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Checcucci, E.; Pecoraro, A.; DE Cillis, S.; Manfredi, M.; Amparore, D.; Aimar, R.; Piramide, F.; Granato, S.; Volpi, G.; Autorino, R.; et al. The importance of anatomical reconstruction for continence recovery after robot assisted radical prostatectomy: A systematic review and pooled analysis from referral centers. Eur. Urol. Suppl. 2021, 73, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Fiori, C.; Checcucci, E.; Stura, I.; Amparore, D.; De Cillis, S.; Piana, A.; Granato, S.; Volpi, G.; Sica, M.; Piramide, F.; et al. Development of a novel nomogram to identify the candidate to extended pelvic lymph node dissection in patients who underwent mpMRI and target biopsy only. Prostate Cancer Prostatic Dis. 2022, 26, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Fossati, N.; Zaffuto, E.; Bandini, M.; Dell’oglio, P.; Bravi, C.A.; Fallara, G.; Pellegrino, F.; Nocera, L.; Karakiewicz, P.I.; et al. Development and Internal Validation of a Novel Model to Identify the Candidates for Extended Pelvic Lymph Node Dissection in Prostate Cancer. Eur. Urol. 2017, 72, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Moretti, T.B.C.; Magna, L.A.; Reis, L.O. Surgical Results and Complications for Open, Laparoscopic, and Robot-assisted Radical Prostatectomy: A Reverse Systematic Review. Eur. Urol. Open Sci. 2022, 44, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.R.; Coelho, R.F.; Rocco, B.; Orvieto, M.; Sivaraman, A.; Palmer, K.J.; Kameh, D.; Santoro, L.; Coughlin, G.D.; Liss, M.; et al. Positive Surgical Margins After Robotic Assisted Radical Prostatectomy: A Multi-Institutional Study. J. Urol. 2011, 186, 511–517. [Google Scholar] [CrossRef]
- Coughlin, G.D.; Yaxley, J.W.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Teloken, P.; Dunglison, N.; Williams, S.; Lavin, M.F.; et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018, 19, 1051–1060. [Google Scholar] [CrossRef]
- Soeterik, T.F.W.; Bergh, R.C.N.v.D.; van Melick, H.H.E.; Kelder, H.; Peretti, F.; Dariane, C.; Timsit, M.-O.; Branchereau, J.; Mesnard, B.; Tilki, D.; et al. Active surveillance in renal transplant patients with prostate cancer: A multicentre analysis. World J. Urol. 2023, 41, 725–732. [Google Scholar] [CrossRef]
- Hevia, V.; Boissier, R.; Rodríguez-Faba, Ó.; Fraser-Taylor, C.; Hassan-Zakri, R.; Lledo, E.; Regele, H.; Buddde, K.; Figueiredo, A.; Olsburgh, J.; et al. Management of Localised Prostate Cancer in Kidney Transplant Patients: A Systematic Review from the EAU Guidelines on Renal Transplantation Panel. Eur. Urol. Focus 2018, 4, 153–162. [Google Scholar] [CrossRef]
- Spatafora, P.; Sessa, F.; Grisanti, S.C.; Bisegna, C.; Saieva, C.; Roviello, G.; Polverino, P.; Rivetti, A.; Verdelli, L.; Zanazzi, M.; et al. Prostate Cancer Characteristics in Renal Transplant Recipients: A 25-Year Experience from a Single Centre. Front. Surg. 2021, 8, 716861. [Google Scholar] [CrossRef]
| Study | Number of Patients | Age (Years) | OR Time (Console Time, mins) | Blood Loss (mL) | LOS (Days) | Complications (Clavien Classification) | Grade Group | Preoperative PSA | Pathologic Staging | PSM | BCR | F/U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [12] Jhaveri 2008 | 1 | 54 | 200 | 400 | 2 | 0/1 | 2 | 8.5 | pT2c | 0/1 | 0/1 | 6 weeks |
| [13] Hoda 2010 | 16 | 61.8 ± 8 (51–66) | 108.3 ± 3.9 (88–188) | 211.1 ± 87.1 (128–498) | 10.1 ± 3.4 (7–18) | 1/16 Prolonged hematuria | 1 in 87% 2 in 13% 4 in 0% | 4.7 ± 1.4 | pT2a 14% pT2b 24% pT2c 62% pT3a/b 0% | 1/16 | 0/16 | 28 months |
| [14] Smith 2011 | 3 | 48 54 61 | 244 400 322 | 50 100 75 | 2 2 3 | 0/3 | 1 | N/A | pT2c | 1/3 | 0/3 | 13 months |
| [15] Ghazi 2012 | 1 | 68 | 130 | 125 | 1 | 0/1 | 2 | 6.93 | pT2b | 0/1 | 0/1 | N/A |
| [16] Polcari 2012 | 7 | 63.3 | 186 | N/A | 1.8 | 3/7 Hematuria(II) Atrial fibrillation(II) Urosepsis(II) | 1 in 2 2 in 4 5 in 1 | 6.17 | pT2c in 4 pT3a in 3 | 2/7 | 1/7 | 16 months |
| [17] Wagener 2012 | 1 | 71 | 220 | 300 | N/A | 0/1 | 2 | 12.4 | pT2 | 0/1 | 0/1 | 9 months |
| [18] Le Clerc 2015 | 12 | 61.92 ± 2.98 | 241.3 ± 35.6 | 587.9 ± 261.3 | N/A | 1/12, acute renal failure (III) | 1 in 8 3 in 4 | 7.34 | pT2b in 1 pT2c in 8 pT3a in 2 | 4/12 | 2/12 | 31.2 months |
| [19] Moreno 2016 | 4 | 61.25 ± 7.76 | 196 ± 20.8 | N/A | 3.2 ± 0.9 | 0/4 | 1 in 2 2 in 1 5 in 1 | 7.1 ±2.8 | N/A | 2/4 | 1/4 | 33 ± 6.7 months |
| [20] Jenjitranant 2016 | 1 | 73 | 210 | 250 | 6 | 0/1 | 3 | 11.53 | pT2c | 1/1 | N/A | 1 month |
| [21] Plagakis 2016 | 1 | 60 | 139 | 190 | 2 | 0/1 | 2 | 13 | pT2c | 0/1 | 0/1 | 10 years |
| [22] Iizuka 2016 | 3 | 59 60 67 | 163(109) 195(153) 127(80) | 75 30 50 | 8 9 7 | 1/3, urinary retention (II) | 3 2 2 | 10.6 17 8.58 | pT2 pT2 pT2 | 0/3 | 1/3 | 24 months 23 months 8 months |
| [8] Zeng 2018 | 1 | 65 | 207 | 500 | 3 | 1/1, superfi- cial surgical site infection (II) | 5 | 6.65 | pT3b | 1/1 | 1/1 | 3 months |
| [23] Mistretta 2019 | 9 | 60 (56–63) | 160 (145–183) | 100 (100–200) | 4 (3–6) | 1/9 (Urosepsis) | 1 in 4 2 in 3 3 in 2 | 5.6 (5–15) | pT2a 1/9 pT2c 6/9 pT3a 1/9 pT3b 1/9 | 2/9 | 2/9 | 12 months |
| [10] Felber 2020 | 39 | 62 (58–67) | 180 (125−227) | 150 (150−400) | 4 (3;5) | 16/39 Pyelonephritis, hematoma and anaemia (I-II) 4/39 Lymphoceles (IVa) | 1 in 14 2 in 18 3 in 4 4 in 0 5 in 3 | 6.8 | pT2a 5/39 pT2b 2/39 pT2c 21/39 pT3a 9/39 pT3b 2/39 | 5/39 | 3/39 | 47.9 months (42.3–52.5) |
| [24] Léonard 2020 | 27 | 63.3 (43–73) | 244 (120–480) | 571.3 (100–1500) | 5.7 (3–16) | 2/27 hematoma of the Retzius space (IIIb) acute pulmonary edema (IVa) | 1 in 12 2 in 8 3 in 5 4 in 0 5 in 2 | 8.9 (4.4–19) | pT2a 15/27 pT2b 9/27 pT2c 2/27 pT3a 1/27 pT3b 0/27 | 12/27 | 2/27 | 34.9 months (0.5–85.5) |
| [25] Sirisopana 2021 | 5 | 67 64 74 66 79 | 365 210 210 190 210 | 630 300 250 150 100 | 13 6 8 5 7 | 5/5 Blood transfusion and postoperative fever | 4 1 3 4 2 | 25.66 10.84 11.53 130 9.63 | pT3b pT2a pT2c pT3b pT2a | 3/5 | 1/5 | 129 months 47 months 63 months 31 months 6 months |
| [26] Shahait 2021 | 14 | 60.2 | 129.7 ± 26.3 | 110 ± 44.6 | 1 | 0/14 | 1 in 4 2–3 in 9 4–5 in 1 | 6.9 (4–8.6) | 6/14 (pT3a pT3b) | 4/14 | 3/14 | 12 months |
| [9] Marra 2022 | 41 | 60 (57–64) | 210 (170– 250) | 300 (200–400) | 4 (2–6) | 4/41 postoperative hemorrhage(IIIa) pyelonephritis(II) urinary tract infection(II) renal insufficiency(II) | 1 in 11 2 in 20 3 in 4 4 in 3 5 in 1 | 6.5 (5.2–10.2) | pT2 29/41 pT3 11/41 | 7/41 | 2/41 | 42 months |
| Study | Number of Patients | Port Placement | Lymphadenectomy | Development of the Space of Retzius | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Changed | No Change | Not Described | Bilateral | Contralateral | Not Performed | Not Described | No Change | Extraperitoneal | Retzius Sparing | Contralateral | Not Described | ||
| [12] Jhaveri 2008 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |||
| [13] Hoda 2010 | 16 | 16 | 16 | 16 | |||||||||
| [14] Smith 2011 | 3 | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | |||
| [15] Ghazi 2012 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |||||
| [16] Polcari 2012 | 7 | 7 | 0 | 0 | 4 | 3 | 0 | 0 | 0 | 7 | |||
| [17] Wagener 2012 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |||
| [18] Le Clerc 2015 | 12 | 0 | 12 | 0 | 12 | 0 | 12 | ||||||
| [19] Moreno 2016 | 4 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 3 | ||
| [20] Jenjitranant 2016 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | |||
| [21] Plagakis 2016 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | |||
| [22] Iizuka 2016 | 3 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | |||
| [8] Zeng 2018 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | |||
| [23] Mistretta 2019 | 9 | 9 | 0 | 1 | 1 | 0 | 7 | 0 | 0 | 9 | 0 | ||
| [10] Felber 2020 | 39 | 0 | 39 | 1 | 12 | 26 | 39 | ||||||
| [24] Léonard 2020 | 27 | 27 | 0 | 12 | 13 | 2 | 27 | ||||||
| [25] Sirisopana 2021 | 5 | 5 | 0 | 5 | 5 | 0 | 0 | 0 | |||||
| [26] Shahait 2021 | 14 | 0 | 14 | 14 | 14 | 0 | 0 | 0 | |||||
| [9] Marra 2022 | 41 | 0 | 41 | 2 | 10 | 0 | 29 | 41 | 0 | 0 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piana, A.; Pecoraro, A.; Sidoti, F.; Checcucci, E.; Dönmez, M.İ.; Prudhomme, T.; Bañuelos Marco, B.; López Abad, A.; Campi, R.; Boissier, R.; et al. Robot-Assisted Radical Prostatectomy in Renal Transplant Recipients: A Systematic Review. J. Clin. Med. 2023, 12, 6754. https://doi.org/10.3390/jcm12216754
Piana A, Pecoraro A, Sidoti F, Checcucci E, Dönmez Mİ, Prudhomme T, Bañuelos Marco B, López Abad A, Campi R, Boissier R, et al. Robot-Assisted Radical Prostatectomy in Renal Transplant Recipients: A Systematic Review. Journal of Clinical Medicine. 2023; 12(21):6754. https://doi.org/10.3390/jcm12216754
Chicago/Turabian StylePiana, Alberto, Alessio Pecoraro, Flavio Sidoti, Enrico Checcucci, Muhammet İrfan Dönmez, Thomas Prudhomme, Beatriz Bañuelos Marco, Alicia López Abad, Riccardo Campi, Romain Boissier, and et al. 2023. "Robot-Assisted Radical Prostatectomy in Renal Transplant Recipients: A Systematic Review" Journal of Clinical Medicine 12, no. 21: 6754. https://doi.org/10.3390/jcm12216754
APA StylePiana, A., Pecoraro, A., Sidoti, F., Checcucci, E., Dönmez, M. İ., Prudhomme, T., Bañuelos Marco, B., López Abad, A., Campi, R., Boissier, R., Di Dio, M., Porpiglia, F., Breda, A., & Territo, A., on behalf of the EAU Young Academic Urologists (YAU) Working Group on Kidney Transplantation. (2023). Robot-Assisted Radical Prostatectomy in Renal Transplant Recipients: A Systematic Review. Journal of Clinical Medicine, 12(21), 6754. https://doi.org/10.3390/jcm12216754









