Celecoxib Suppresses NF-κB p65 (RelA) and TNFα Expression Signaling in Glioblastoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis
2.2. Ethics Statement
Tissue Samples
2.3. Quantitative qRT-PCR Analysis
2.4. ELISA
Statistics
2.5. Cell Line and Culture Conditions
2.6. MTT Cellular Proliferation Assay
2.7. Quantitative Expression Analysis by qRT-PCR
3. Results
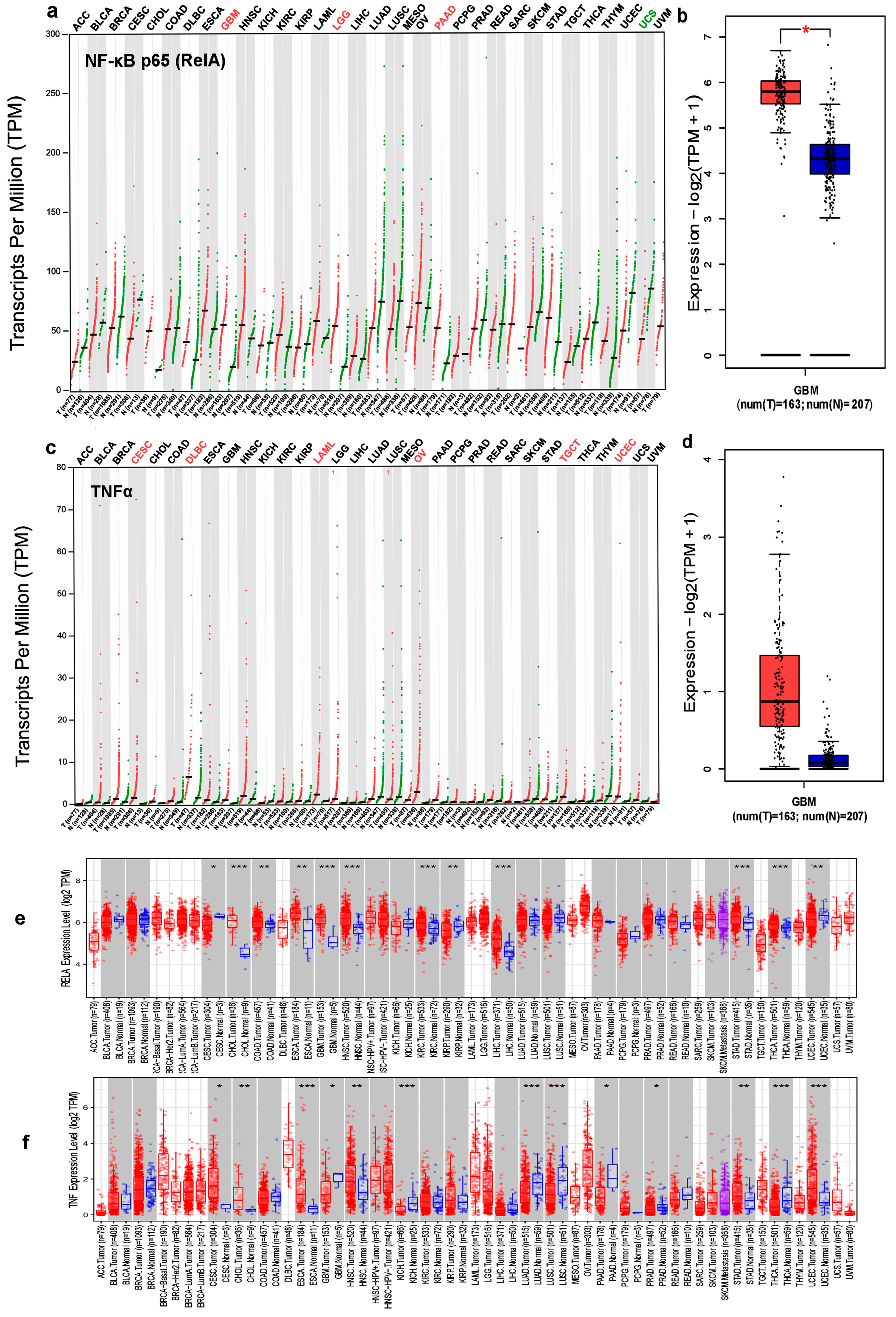
3.1. Expression Levels of NF-κB p65 (RelA) and TNFα in Different Cancer Types
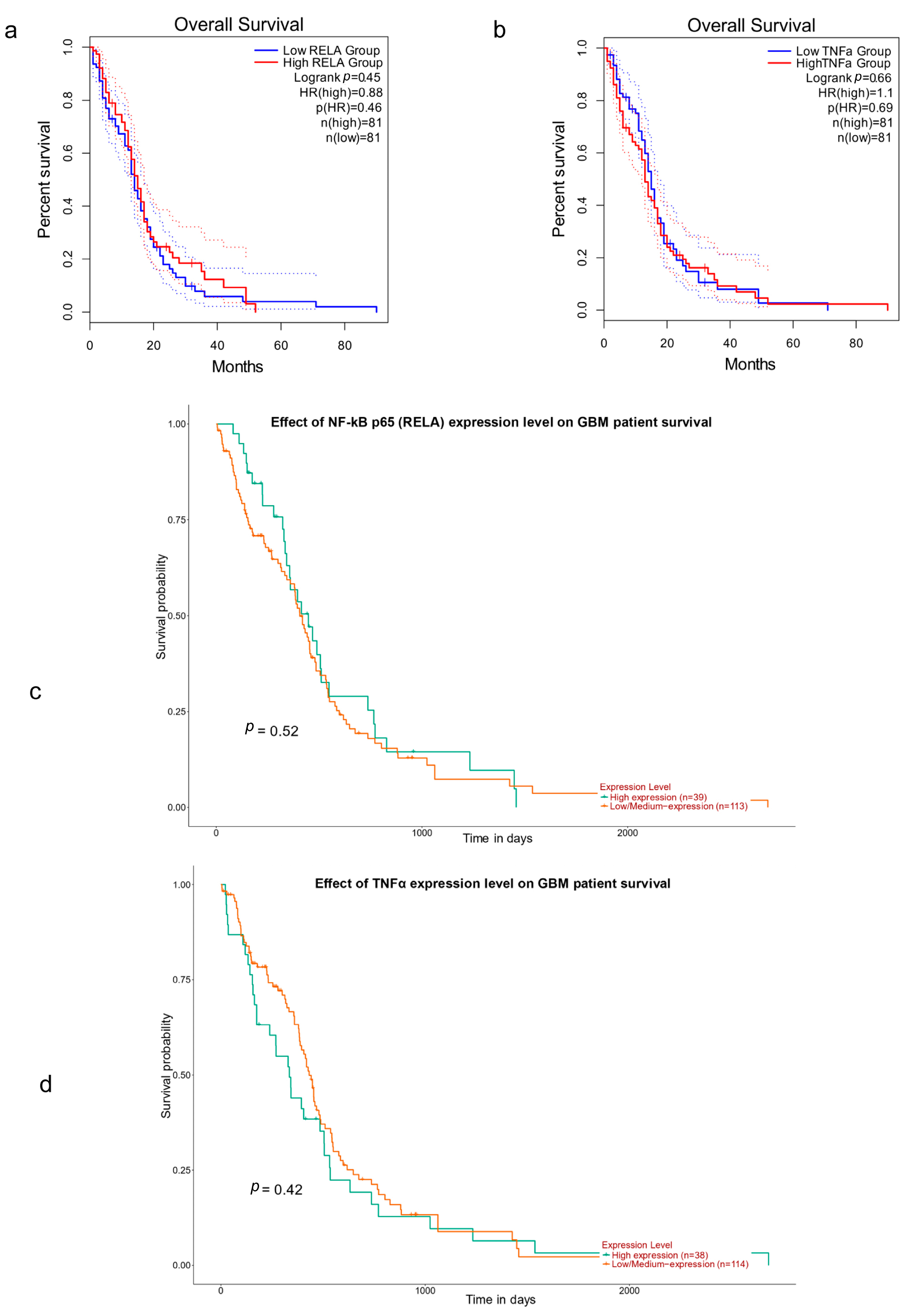
3.2. NF-κB p65 (RelA), TNFα and Survival in GBM
3.3. PPI Network and Functional Enrichment Analyses
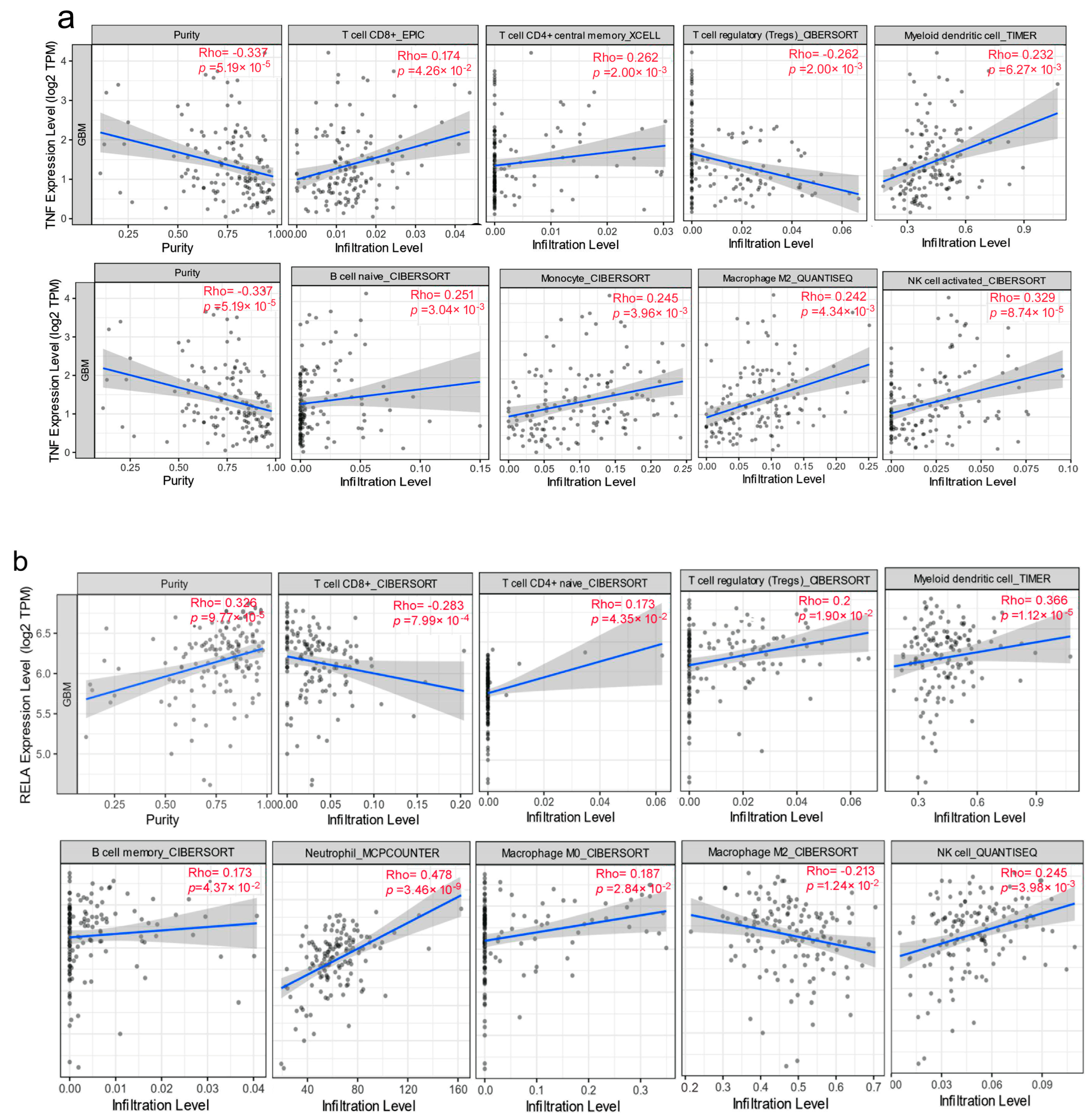
3.4. Correlation between Expression Levels and Immune Cell Infiltration Levels
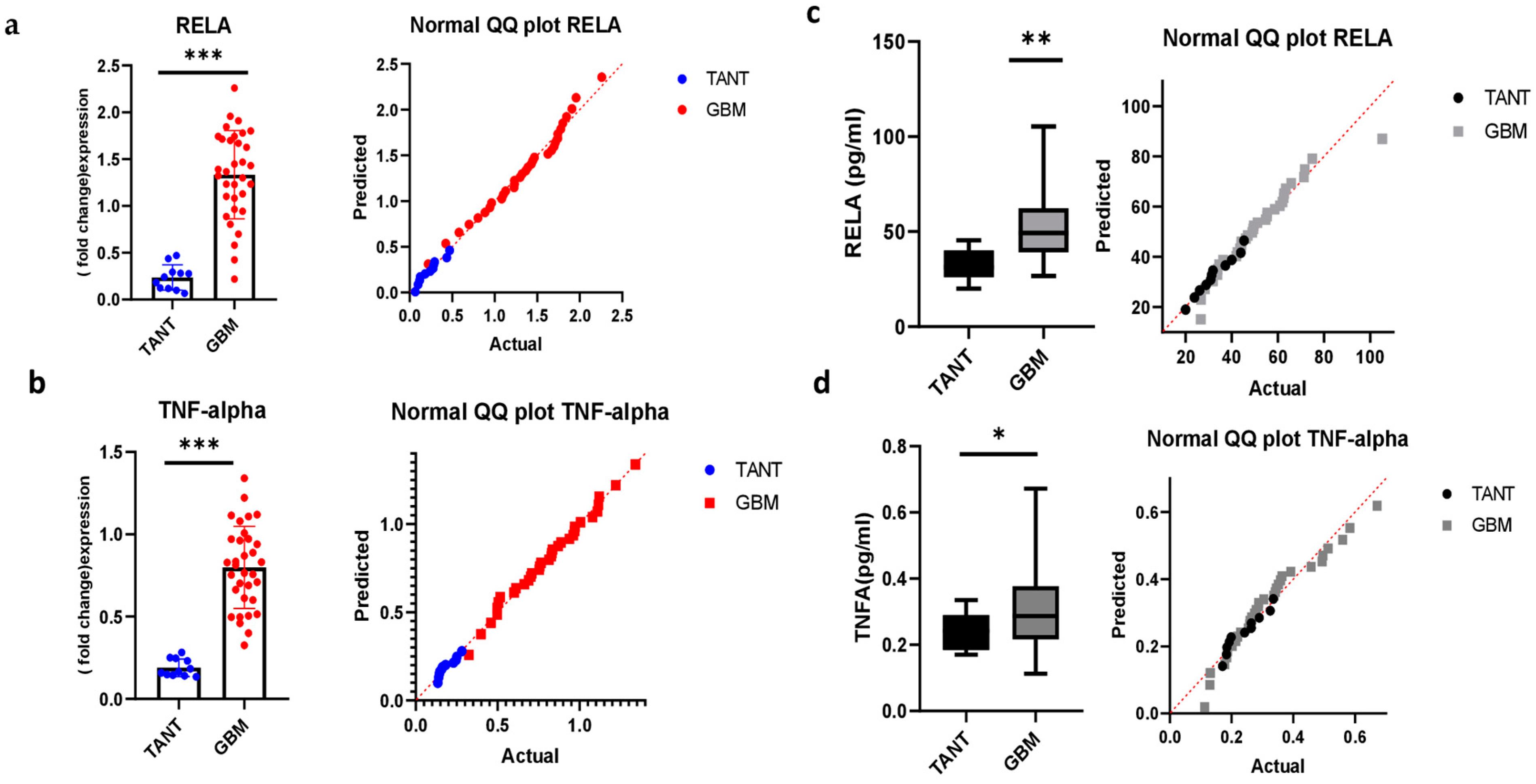
3.5. Consistency of mRNA Expression Profiling and Validation in a GBM Patient Sample
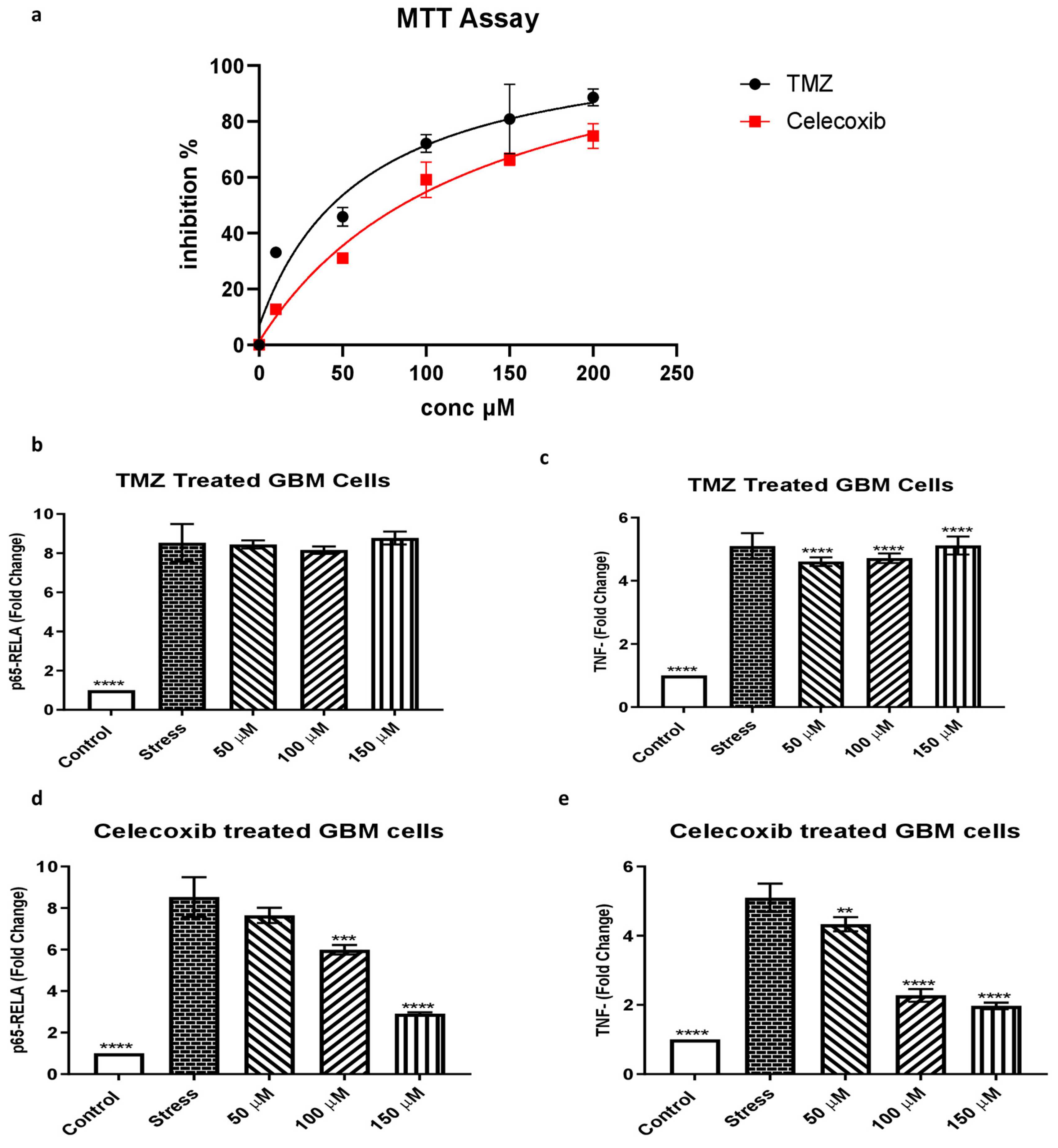
3.6. The Effect of Temozolomide and Celecoxib Treatment on Glioblastoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laug, D.; Glasgow, S.M.; Deneen, B. A glial blueprint for gliomagenesis. Nat. Rev. Neurosci. 2018, 19, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinov, D.; Stock, E.M.; Dandashi, J.A.; Vasquez, E.A.; Mao, Y.; Dayawansa, S.; Zhang, J.; Wu, E.; Fonkem, E.; Huang, J.H. Prognostication of survival outcomes in patients diagnosed with glioblastoma. World Neurosurg. 2018, 109, e67–e74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, W.; Cao, W.-D.; Cheng, G.; Zhang, Y.-Q. Glioblastoma multiforme: Molecular characterization and current treatment strategy. Exp. Ther. Med. 2012, 3, 9–14. [Google Scholar] [CrossRef]
- Armocida, D.; Pesce, A.; Di Giammarco, F.; Frati, A.; Santoro, A.; Salvati, M. Long term survival in patients suffering from glio-blastoma multiforme: A single-center observational cohort study. Diagnostics 2019, 9, 209. [Google Scholar] [CrossRef]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Perkins, N.D. The Rel/NF-κB family: Friend and foe. Trends Biochem. Sci. 2000, 25, 434–440. [Google Scholar] [CrossRef]
- Raychaudhuri, B.; Han, Y.; Lu, T.; Vogelbaum, M.A. Aberrant constitutive activation of nuclear factor κB in glioblastoma multiforme drives invasive phenotype. J. Neuro-Oncol. 2007, 85, 39–47. [Google Scholar] [CrossRef]
- Blahovcova, E.; Richterova, R.; Kolarovszki, B.; Dobrota, D.; Racay, P.; Hatok, J. Apoptosis-related gene expression in tumor tissue samples obtained from patients diagnosed with glioblastoma multiforme. Int. J. Mol. Med. 2015, 36, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Waetzig, G.H.; Rosenstiel, P.; Arlt, A.; Till, A.; Bräutigam, K.; Schäfer, H.; Rose-John, S.; Seegert, D.; Schreiber, S. Soluble tumor necrosis factor (TNF) receptor-1 induces apoptosis via reverse TNF signaling and autocrine transforming growth factor-β1. FASEB J. 2005, 19, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, M.; Liu, X.; Guo, S.-W. Constitutive and tumor necrosis factor-α-induced activation of nuclear factor-κB in adenomyosis and its inhibition by andrographolide. Fertil. Steril. 2013, 100, 568–577. [Google Scholar] [CrossRef]
- Burri, S.H.; Gondi, V.; Brown, P.D.; Mehta, M.P. The evolving role of tumor treating fields in managing glioblastoma: Guide for oncologists. Am. J. Clin. Oncol. 2018, 41, 191. [Google Scholar] [CrossRef] [PubMed]
- Jendrossek, V. Targeting apoptosis pathways by Celecoxib in cancer. Cancer Lett. 2013, 332, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Geeviman, K.; Ramulu, C.; Babu, P.P. The nonsteroidal anti-inflammatory drug celecoxib suppresses the growth and induces apoptosis of human glioblastoma cells via the NF-κB pathway. J. Neuro-Oncol. 2012, 106, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Chaki, R.; Mandal, V.; Mandal, S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010, 62, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Penas-Prado, M.; Hess, K.R.; Fisch, M.J.; Lagrone, L.W.; Groves, M.D.; Levin, V.A.; De Groot, J.F.; Puduvalli, V.K.; Colman, H.; Volas-Redd, G. Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro-Oncol. 2015, 17, 266–273. [Google Scholar] [CrossRef]
- Kesari, S.; Schiff, D.; Henson, J.W.; Muzikansky, A.; Gigas, D.C.; Doherty, L.; Batchelor, T.T.; Longtine, J.A.; Ligon, K.L.; Weaver, S. Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults. Neuro-Oncol. 2008, 10, 300–308. [Google Scholar] [CrossRef]
- Zhu, Y.; Qiu, P.; Ji, Y. TCGA-assembler: Open-source software for retrieving and processing TCGA data. Nat. Methods 2014, 11, 599–600. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, S.; Shi, J.; Zheng, M.; He, C.; Meng, H.; Han, Y.; Chen, J.; Han, J.; Yuan, Z. The association between several autophagy-related genes and their prognostic values in hepatocellular carcinoma: A study on the foundation of TCGA, GEPIA and HPA databases. Mol. Biol. Rep. 2022, 49, 10269–10277. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Yu, A.; Deng, K.; Chen, J.; Wang, Z.; Huang, L.; Ma, S.; Dai, X. FANCI is associated with poor prognosis and immune infiltration in liver hepatocellular carcinoma. Int. J. Med. Sci. 2023, 20, 918. [Google Scholar] [CrossRef]
- Espinoza, I.; Agarwal, S.; Reddy, A.; Shenoy, V.; Subramony, C.; Sakiyama, M.; Fair, L.; Poosarla, T.; Zhou, X.; Shannon Orr, W. Expression of trefoil factor 3 is decreased in colorectal cancer. Oncol. Rep. 2021, 45, 254–264. [Google Scholar] [CrossRef]
- Dillies, M.-A.; Rau, A.; Aubert, J.; Hennequet-Antier, C.; Jeanmougin, M.; Servant, N.; Keime, C.; Marot, G.; Castel, D.; Estelle, J. A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis. Brief. Bioinform. 2013, 14, 671–683. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Aditya, J.; Girija, A.S.; Paramasivam, A.; Priyadharsini, J.V. Genetic alterations in Wnt family of genes and their putative association with head and neck squamous cell carcinoma. Genom. Inform. 2021, 19, e5. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, W60–W64. [Google Scholar] [CrossRef]
- Yadav, R.; Srivastava, P. Clustering, pathway enrichment, and protein-protein interaction analysis of gene expression in neurodevelopmental disorders. Adv. Pharmacol. Pharm. Sci. 2018, 2018, 3632159. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Yang, Y.; Wang, L. Analysis of the correlation between the expression of WFDC2 in B cells of lung adenocarcinoma tissue and the survival rate of patients. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2019, 35, 425–433. [Google Scholar]
- Sturm, D.; Orr, B.A.; Toprak, U.H.; Hovestadt, V.; Jones, D.T.; Capper, D.; Sill, M.; Buchhalter, I.; Northcott, P.A.; Leis, I. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 2016, 164, 1060–1072. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Lee, M.-S.; Lee, H.; Kim, Y.J.; Lee, W.Y.; Yun, S.H.; Kim, H.C.; Hong, H.K.; Hannenhalli, S. Transcriptomes of the tumor-adjacent normal tissues are more informative than tumors in predicting recurrence in colorectal cancer patients. J. Transl. Med. 2023, 21, 209. [Google Scholar] [CrossRef]
- Wang, L.-B.; Karpova, A.; Gritsenko, M.A.; Kyle, J.E.; Cao, S.; Li, Y.; Rykunov, D.; Colaprico, A.; Rothstein, J.H.; Hong, R. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 2021, 39, 509–528.e20. [Google Scholar] [CrossRef]
- Liu, Z.; Rao, M.; Poiret, T.; Nava, S.; Meng, Q.; von Landenberg, A.; Bartek Jr, J.; Xie, S.; Sinclair, G.; Peredo, I. Mesothelin as a novel biomarker and immunotherapeutic target in human glioblastoma. Oncotarget 2017, 8, 80208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhong, X.; Wu, T.; Yang, T.; Chen, G.; Xie, X.; Wei, Y.; Ye, M.; Zhou, Y.; Du, Z. Identification of a NFKBIA polymorphism associated with lower NFKBIA protein levels and poor survival outcomes in patients with glioblastoma multiforme. Int. J. Mol. Med. 2014, 34, 1233–1240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McFarland, B.C.; Hong, S.W.; Rajbhandari, R.; Twitty Jr, G.B.; Gray, G.K.; Yu, H.; Benveniste, E.N.; Nozell, S.E. NF-κB-induced IL-6 ensures STAT3 activation and tumor aggressiveness in glioblastoma. PLoS ONE 2013, 8, e78728. [Google Scholar] [CrossRef]
- Weller, M.; Kaulich, K.; Hentschel, B.; Felsberg, J.; Gramatzki, D.; Pietsch, T.; Simon, M.; Westphal, M.; Schackert, G.; Tonn, J.C. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int. J. Cancer 2014, 134, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Batool, S.M.; Muralidharan, K.; Hsia, T.; Falotico, S.; Gamblin, A.S.; Rosenfeld, Y.B.; Khanna, S.K.; Balaj, L.; Carter, B.S.J.C.C.R. Highly sensitive EGFRvIII detection in circulating extracellular vesicle RNA of glioma patients. Clin. Cancer Res. 2022, 28, 4070–4082. [Google Scholar] [CrossRef]
- Hübner, M.; Effinger, D.; Wu, T.; Strauß, G.; Pogoda, K.; Kreth, F.-W.; Kreth, S.J.C. The IL-1 antagonist anakinra attenuates glioblastoma aggressiveness by dampening tumor-associated inflammation. Cances 2020, 12, 433. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Shamsdin, S.A.; Mehrafshan, A.; Rakei, S.M.; Mehrabani, D. Evaluation of VEGF, FGF and PDGF and serum levels of inflammatory cytokines in patients with glioma and meningioma in Southern Iran. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 2883. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011, 70, i104–i108. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Mamun, A.A.; Sarwar, M.S.; Nasrin, F.; Emran, T.B.; Alanazi, I.S.; Rauf, A.; Albadrani, G.M.; Sayed, A.A. Natural small molecules targeting NF-κB signaling in glioblastoma. Front. Pharmacol. 2021, 12, 703761. [Google Scholar] [CrossRef]
- Cahill, K.E.; Morshed, R.A.; Yamini, B. Nuclear factor-κB in glioblastoma: Insights into regulators and targeted therapy. Neuro-Oncol. 2015, 18, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, L.; Ruiz-Ontañon, P.; Vazquez-Barquero, A.; Moris, F.; Fernandez-Luna, J.L. The NFκB pathway: A therapeutic target in glioblastoma. Oncotarget 2011, 2, 646. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, C.; Irshad, K.; Gupta, Y.; Sarkar, C.; Suri, A.; Chattopadhyay, P.; Sinha, S.; Chosdol, K. NFkB is a critical transcriptional regulator of atypical cadherin FAT1 in glioma. BMC Cancer 2020, 20, 62. [Google Scholar] [CrossRef] [PubMed]
- Salam, R.; Saliou, A.; Bielle, F.; Bertrand, M.; Antoniewski, C.; Carpentier, C.; Alentorn, A.; Capelle, L.; Sanson, M.; Huillard, E. Cellular senescence in malignant cells promotes tumor progression in mouse and patient Glioblastoma. Nat. Commun. 2023, 14, 441. [Google Scholar] [CrossRef]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Shrivastava, R.; Gandhi, P.; Gothalwal, R. The road-map for establishment of a prognostic molecular marker panel in glioma using liquid biopsy: Current status and future directions. Clin. Transl. Oncol. 2022, 24, 1702–1714. [Google Scholar] [CrossRef]
- Hou, D.; Wang, Z.; Li, H.; Liu, J.; Liu, Y.; Jiang, Y.; Lou, M. Circular RNA circASPM promotes the progression of glioblastoma by acting as a competing endogenous RNA to regulate miR-130b-3p/E2F1 axis. J. Cancer 2022, 13, 1664. [Google Scholar] [CrossRef]
- Shuklaa, S.; Patrica, I.R.P.; SivaarumugamThinagararjana, S.S.; Mondala, B.; Hegdeb, A.S.; Chandramoulic, B.A.; Santoshd, V.; Arivazhaganc, A.; Somasundarama, K. The NPTX2-PTEN-NFkB nexus is an essential component of a prognostic DNA methylation signature of glioblastoma. Cancer Res. 2013, 73, 6563–6573. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. J. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef]
- Basheer, A.S.; Abas, F.; Othman, I.; Naidu, R. Role of inflammatory mediators, macrophages, and neutrophils in glioma maintenance and progression: Mechanistic understanding and potential therapeutic applications. Cancers 2021, 13, 4226. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, H.; Roncali, L.; Rousseau, A.; Chérel, M.; Delneste, Y.; Jeannin, P.; Hindré, F.; Garcion, E. Targeting tumor associated macrophages to overcome conventional treatment resistance in glioblastoma. Front. Pharmacol. 2020, 11, 368. [Google Scholar] [CrossRef] [PubMed]
- Colombo, F.; Zambrano, S.; Agresti, A. NF-κB, the importance of being dynamic: Role and insights in cancer. Biomedicines 2018, 6, 45. [Google Scholar] [CrossRef]
- Laver, T. Mechanism of Interferon-Beta-Mediated Inhibition of IL-8 Gene Expression; The University of Alabama at Birmingham: Birmingham, AL, USA, 2008. [Google Scholar]
- Stark, L.A.; Dunlop, M.G. Nucleolar sequestration of RelA (p65) regulates NF-κB-driven transcription and apoptosis. Mol. Cell. Biol. 2005, 25, 5985–6004. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Tan, Y.; Deng, G.; Sun, Z.; Liu, B.; Wang, Y.; Yuan, F.; Sun, Q.; Hu, P.; Gao, L. TGF-β induces GBM mesenchymal transition through upregulation of CLDN4 and nuclear translocation to activate TNF-α/NF-κB signal pathway. Cell Death Dis. 2022, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Maletínská, L.; Blakely, E.A.; Bjornstad, K.A.; Deen, D.F.; Knoff, L.J.; Forte, T.M. Human glioblastoma cell lines: Levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000, 60, 2300–2303. [Google Scholar]
- Horescu, C.; Elena Cioc, C.; Tuta, C.; Sevastre, A.-S.; Tache, D.E.; Alexandru, O.; Artene, S.-A.; Danoiu, S.; Dricu, A.; Stefana Oana, P. The effect of temozolomide in combination with doxorubicin in glioblastoma cells in vitro. J. Immunoass. Immunochem. 2020, 41, 1033–1043. [Google Scholar] [CrossRef]
- Jiapaer, S.; Furuta, T.; Tanaka, S.; Kitabayashi, T.; Nakada, M. Potential strategies overcoming the temozolomide resistance for glioblastoma. Neurol. Med.-Chir. 2018, 58, 405–421. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Rabé, M.; Dumont, S.; Álvarez-Arenas, A.; Janati, H.; Belmonte-Beitia, J.; Calvo, G.F.; Thibault-Carpentier, C.; Séry, Q.; Chauvin, C.; Joalland, N.; et al. Identification of a transient state during the acquisition of temozolomide resistance in glioblastoma. Cell Death Dis. 2020, 11, 19. [Google Scholar] [CrossRef]
- Pang, L.Y.; Hurst, E.A.; Argyle, D.J. Cyclooxygenase-2: A role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells Int. 2016, 2016, 2048731. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, C.; Toi, M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer 2005, 5, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, C.; Legrand, N. Celecoxib analogues for cancer treatment: An update on OSU-03012 and 2, 5-dimethyl-celecoxib. Biomolecules 2021, 11, 1049. [Google Scholar] [CrossRef]
- Uram, Ł.; Markowicz, J.; Misiorek, M.; Filipowicz-Rachwał, A.; Wołowiec, S.; Wałajtys-Rode, E. Celecoxib substituted biotinylated poly (amidoamine) G3 dendrimer as potential treatment for temozolomide resistant glioma therapy and anti-nematode agent. Eur. J. Pharm. Sci. 2020, 152, 105439. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Koul, D.; Aggarwal, B.B. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-κB activation through inhibition of activation of IκBα kinase and Akt in human non-small cell lung carcinoma: Correlation with suppression of COX-2 synthesis. J. Immunol. 2004, 173, 2011–2022. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Wang, L.; Zhang, X. NF-κB signaling pathway, inflammation and colorectal cancer. Cell. Mol. Immunol. 2009, 6, 327–334. [Google Scholar] [CrossRef]
- Pramanik, K.C.; Makena, M.R.; Bhowmick, K.; Pandey, M.K. Advancement of NF-κB signaling pathway: A novel target in pancreatic cancer. Int. J. Mol. Sci. 2018, 19, 3890. [Google Scholar] [CrossRef]
- Li, F.; Sethi, G. Targeting transcription factor NF-κB to overcome chemoresistance and radioresistance in cancer therapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2010, 1805, 167–180. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sethi, G.; Baladandayuthapani, V.; Krishnan, S.; Shishodia, S. Targeting cell signaling pathways for drug discovery: An old lock needs a new key. J. Cell. Biochem. 2007, 102, 580–592. [Google Scholar] [CrossRef]
- Yin, D.; Jin, G.; He, H.; Zhou, W.; Fan, Z.; Gong, C.; Zhao, J.; Xiong, H. Celecoxib reverses the glioblastoma chemo-resistance to temozolomide through mitochondrial metabolism. Aging 2021, 13, 21268. [Google Scholar] [CrossRef]
- Tai, H.-H.; Chi, X.; Tong, M. Regulation of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) by non-steroidal anti-inflammatory drugs (NSAIDs). Prostaglandins Other Lipid Mediat. 2011, 96, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Kardosh, A.; Blumenthal, M.; Wang, W.J.; Chen, T.C.; Schonthal, A.H. Differential effects of selective COX-2 inhibitors on cell cycle regulation and proliferation of glioblastoma cell lines. Cancer Biol. Ther. 2004, 3, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Kesanakurti, D.; Kirti, P.B.; Babu, P.P. Nonsteroidal anti-inflammatory drugs diclofenac and celecoxib attenuates Wnt/β-catenin/Tcf signaling pathway in human glioblastoma cells. Neurochem. Res. 2013, 38, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Batlouni, M. Nonsteroidal anti-inflammatory drugs: Cardiovascular, cerebrovascular and renal effects. Arq. Bras. Cardiol. 2010, 94, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Yeomans, N.D.; Solomon, D.H.; Lüscher, T.F.; Libby, P.; Husni, M.E.; Graham, D.Y.; Borer, J.S.; Wisniewski, L.M.; Wolski, K.E. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N. Engl. J. Med. 2016, 375, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Vardeny, O.; Solomon, S.D. Cyclooxygenase-2 inhibitors, nonsteroidal anti-inflammatory drugs, and cardiovascular risk. Cardiol. Clin. 2008, 26, 589–601. [Google Scholar] [CrossRef]
- Gajraj, N.M. Cyclooxygenase-2 inhibitors. Anesth. Analg. 2003, 96, 1720–1738. [Google Scholar] [CrossRef]
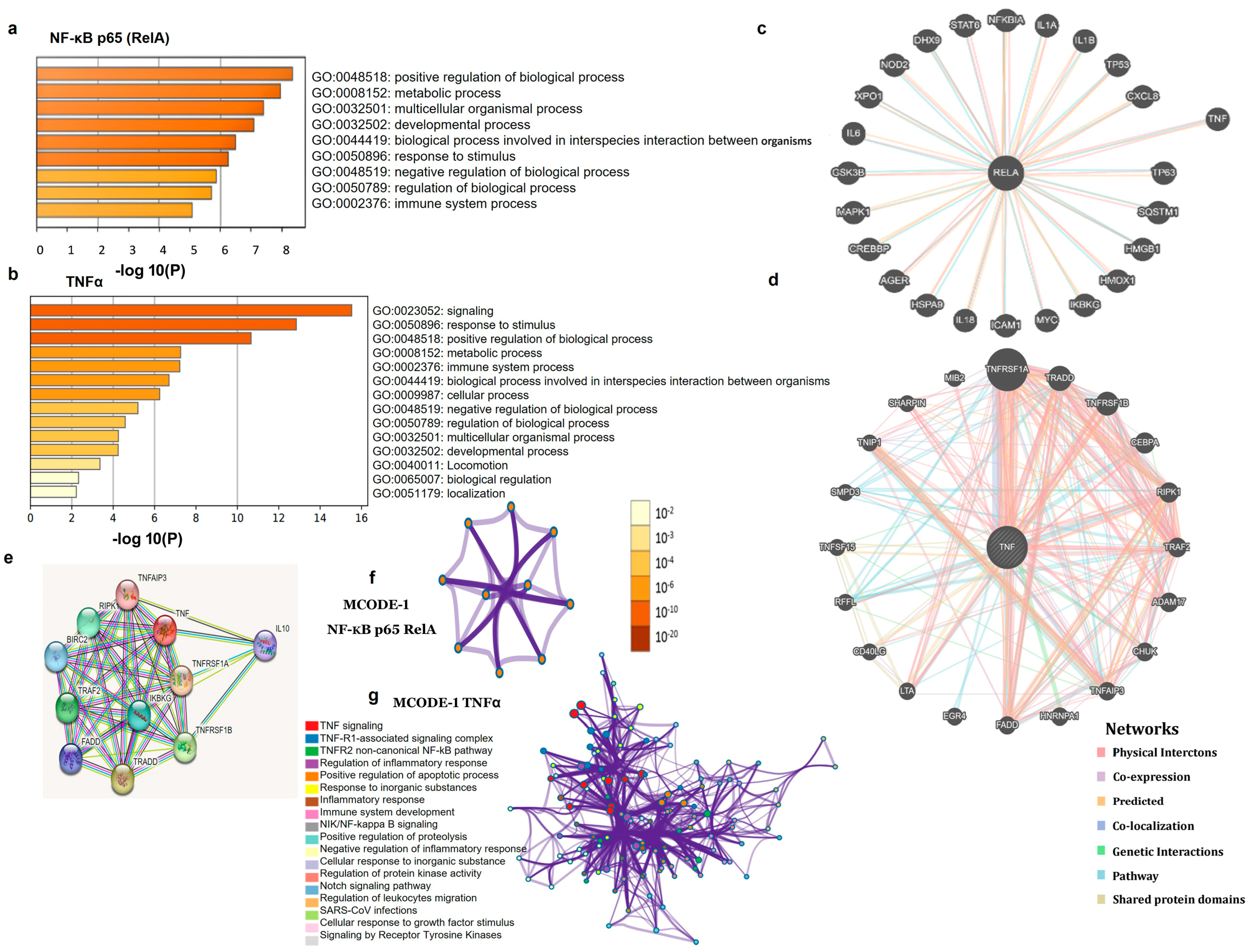
| MCODE | GO | Description | Log10(P) |
|---|---|---|---|
| MCODE_1 p-65 RELA | GO:1902895 | Positive regulation of miRNA transcription. | −8.3 |
| MCODE_1 p-65 RELA | GO:2000630 | Positive regulation of miRNA metabolic process. | −8.1 |
| MCODE_1 p-65 RELA | WP4329 | miRNAs involvement in the immune response in sepsis. | −8 |
| MCODE_1 TNFα | M128 | PID TNF Pathway. | −21.9 |
| MCODE_1 TNFα | R-HSA-5357905 | Regulation of TNFR1 signaling. | −21.7 |
| MCODE_1 TNFα | R-HSA-75893 | TNF signaling. | −21.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahsan, H.; Malik, S.I.; Shah, F.A.; El-Serehy, H.A.; Ullah, A.; Shah, Z.A. Celecoxib Suppresses NF-κB p65 (RelA) and TNFα Expression Signaling in Glioblastoma. J. Clin. Med. 2023, 12, 6683. https://doi.org/10.3390/jcm12206683
Ahsan H, Malik SI, Shah FA, El-Serehy HA, Ullah A, Shah ZA. Celecoxib Suppresses NF-κB p65 (RelA) and TNFα Expression Signaling in Glioblastoma. Journal of Clinical Medicine. 2023; 12(20):6683. https://doi.org/10.3390/jcm12206683
Chicago/Turabian StyleAhsan, Hina, Shaukat Iqbal Malik, Fawad Ali Shah, Hamed A. El-Serehy, Amin Ullah, and Zafar Abbas Shah. 2023. "Celecoxib Suppresses NF-κB p65 (RelA) and TNFα Expression Signaling in Glioblastoma" Journal of Clinical Medicine 12, no. 20: 6683. https://doi.org/10.3390/jcm12206683
APA StyleAhsan, H., Malik, S. I., Shah, F. A., El-Serehy, H. A., Ullah, A., & Shah, Z. A. (2023). Celecoxib Suppresses NF-κB p65 (RelA) and TNFα Expression Signaling in Glioblastoma. Journal of Clinical Medicine, 12(20), 6683. https://doi.org/10.3390/jcm12206683







