Direct Stenting versus Conventional Stenting in Patients with ST-Segment Elevation Myocardial Infarction—A COMPARE CRUSH Sub-Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definitions and Endpoints
2.3. Statistical Analysis
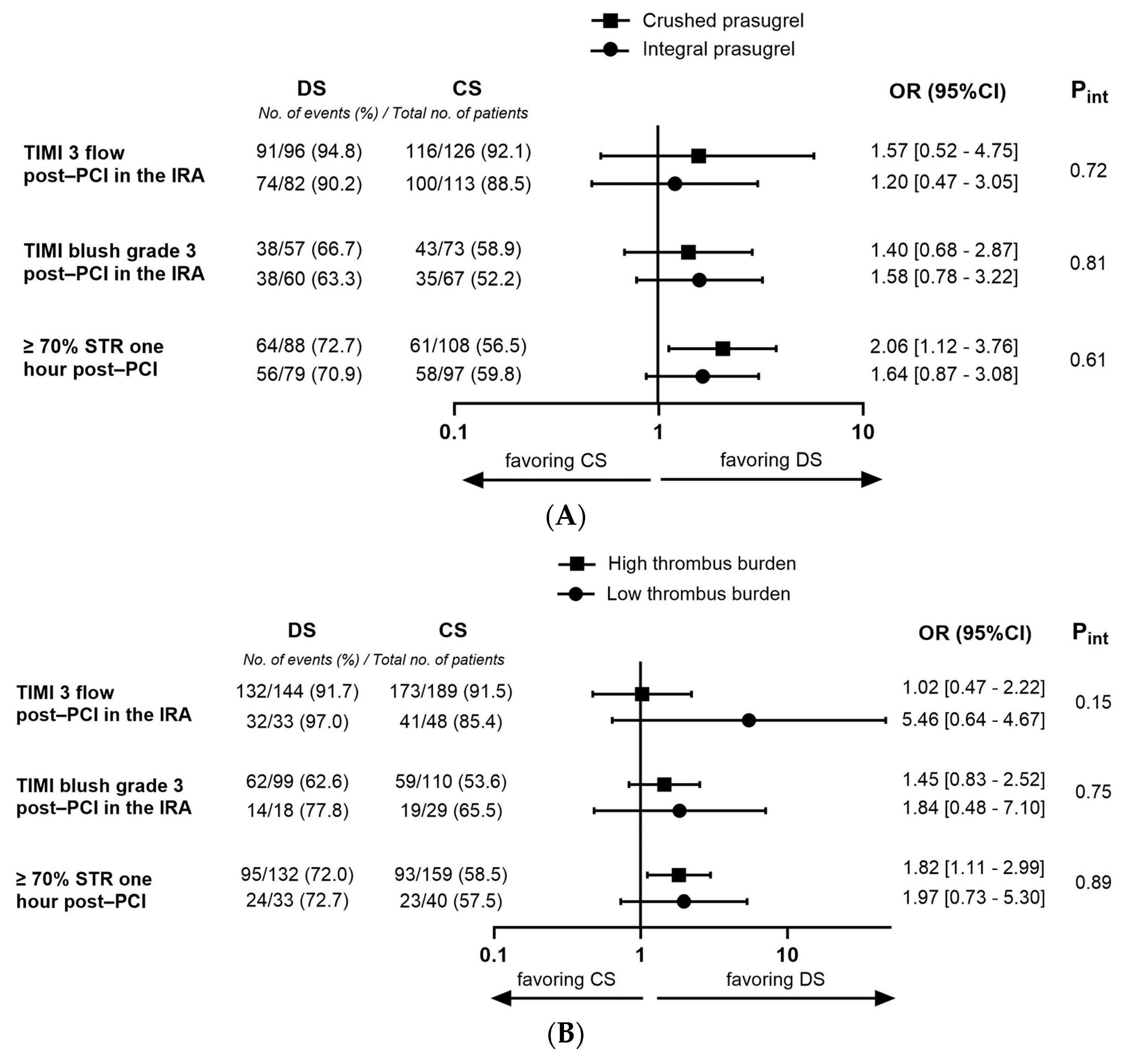
3. Results
3.1. Baseline Characteristics
3.2. Stent Dimensions
3.3. Early Myocardial Reperfusion Markers
3.4. Clinical Outcomes
3.5. Subgroup with High Thrombus Burden at the Beginning of Angiography
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angiolillo, D.J.; Galli, M.; Collet, J.P.; Kastrati, A.; O’Donoghue, M.L. Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention 2022, 17, e1371–e1396. [Google Scholar] [CrossRef]
- Pinelli, S.; Agrinier, N.; Bouchahda, N.; Metzdorf, P.A.; Camenzind, E.; Popovic, B. Myocardial reperfusion for acute myocardial infarction under an optimized antithrombotic medication: What can you expect in daily practice? Cardiovasc. Revasc. Med. 2018, 19 Pt B, 820–825. [Google Scholar] [CrossRef]
- Lønborg, J.; Kelbæk, H.; Holmvang, L.; Helqvist, S.; Vejlstrup, N.; Jørgensen, E.; Saunamäki, K.; Dridi, N.P.; Kløvgaard, L.; Kaltoft, A.; et al. Comparison of Outcome of Patients With ST-Segment Elevation Myocardial Infarction and Complete Versus Incomplete ST-Resolution Before Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2016, 117, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Peterson, M.A.; Lansky, A.J.; Dangas, G.; Mehran, R.; Leon, M.B. Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J. Am. Coll. Cardiol. 2002, 39, 591–597. [Google Scholar] [CrossRef]
- Saad, M.; Stiermaier, T.; Fuernau, G.; Pöss, J.; de Waha-Thiele, S.; Desch, S.; Thiele, H.; Eitel, I. Impact of direct stenting on myocardial injury assessed by cardiac magnetic resonance imaging and prognosis in ST-elevation myocardial infarction. Int. J. Cardiol. 2019, 283, 88–92. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Mechanical strategies to enhance myocardial salvage during primary percutaneous coronary intervention in patients with STEMI. EuroIntervention 2016, 12, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Loubeyre, C.; Morice, M.C.; Lefèvre, T.; Piéchaud, J.F.; Louvard, Y.; Dumas, P. A randomized comparison of direct stenting with conventional stent implantation in selected patients with acute myocardial infarction. J. Am. Coll. Cardiol. 2002, 39, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, R.; Sezgin, A.T.; Barutcu, I.; Topal, E.; Gullu, H.; Acikgoz, N. Comparison of direct stenting versus conventional stent implantation on blood flow in patients with ST-segment elevation myocardial infarction. Angiology 2006, 57, 453–458. [Google Scholar] [CrossRef]
- Gasior, M.; Gierlotka, M.; Lekston, A.; Wilczek, K.; Zebik, T.; Hawranek, M.; Wojnar, R.; Szkodzinski, J.; Piegza, J.; Dyrbus, K.; et al. Comparison of outcomes of direct stenting versus stenting after balloon predilation in patients with acute myocardial infarction (DIRAMI). Am. J. Cardiol. 2007, 100, 798–805. [Google Scholar] [CrossRef]
- Vlachojannis, G.J.; Vogel, R.F.; Wilschut, J.M.; Lemmert, M.E.; Delewi, R.; Diletti, R.; van Vliet, R.; van Der Waarden, N.; Nuis, R.J.; Paradies, V.; et al. COMPARison of pre-hospital CRUSHed vs. uncrushed Prasugrel tablets in patients with STEMI undergoing primary percutaneous coronary interventions: Rationale and design of the COMPARE CRUSH trial. Am. Heart J. 2020, 224, 10–16. [Google Scholar] [CrossRef]
- Vlachojannis, G.J.; Wilschut, J.M.; Vogel, R.F.; Lemmert, M.E.; Delewi, R.; Diletti, R.; van Der Waarden, N.W.; Nuis, R.J.; Paradies, V.; Alexopoulos, D.; et al. Effect of Prehospital Crushed Prasugrel Tablets in Patients With ST-Segment-Elevation Myocardial Infarction Planned for Primary Percutaneous Coronary Intervention: The Randomized COMPARE CRUSH Trial. Circulation 2020, 142, 2316–2328. [Google Scholar] [CrossRef]
- Vogel, R.F.; Delewi, R.; Angiolillo, D.J.; Wilschut, J.M.; Lemmert, M.E.; Diletti, R.; van Vliet, R.; van der Waarden, N.W.; Nuis, R.J.; Paradies, V.; et al. Pharmacodynamic Effects of Pre-Hospital Administered Crushed Prasugrel in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2021, 14, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.F.; Delewi, R.; Wilschut, J.M.; Lemmert, M.E.; Diletti, R.; van Vliet, R.; van der Waarden, N.W.P.L.; Nuis, R.-J.; Paradies, V.; Alexopoulos, D.; et al. Pre-hospital treatment with crushed versus integral tablets of prasugrel in patients presenting with ST-Segment Elevation Myocardial Infarction-1-year follow-up results of the COMPARE CRUSH trial. Am. Heart J. 2022, 252, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Meeks, B.; Pogue, J.; Rokoss, M.J.; Kedev, S.; Thabane, L.; Stankovic, G.; Moreno, R.; et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N. Engl. J. Med. 2015, 372, 1389–1398. [Google Scholar] [CrossRef]
- Scarparo, P.; Improta, R.; Wilschut, J.; Kardys, I.; Den Dekker, W.K.; Daemen, J.; Zijlstra, F.; Van Mieghem, N.M.; Diletti, R. Very Long-Term Clinical Outcomes After Direct Stenting in Patients Presenting With ST-Segment Elevation Myocardial Infarction. Cardiovasc. Revasc. Med. 2022, 41, 144–150. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Azzalini, L.; Millán, X.; Ly, H.Q.; L’Allier, P.L.; Jolicoeur, E.M. Direct stenting versus pre-dilation in ST-elevation myocardial infarction: A systematic review and meta-analysis. J. Interv. Cardiol. 2015, 28, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Seto, A.; Kern, M. Direct stenting for STEMI: Does it really make a difference? Catheter Cardiovasc. Interv. 2014, 84, 932–933. [Google Scholar] [CrossRef]
- Wijns, W.; Verheye, S.; Manoharan, G.; Werner, G.S.; Grube, E.; De Bruyne, B.; Koolen, J.; Hamm, C.W.; Medina, A.; Bech, J.W.; et al. Angiographic, intravascular ultrasound, and fractional flow reserve evaluation of direct stenting vs. conventional stenting using BeStent2 in a multicentre randomized trial. Eur. Heart J. 2005, 26, 1852–1859. [Google Scholar] [CrossRef]
- Möckel, M.; Vollert, J.; Lansky, A.J.; Witzenbichler, B.; Guagliumi, G.; Peruga, J.Z.; Brodie, B.R.; Kornowski, R.; Dudek, D.; Farkouh, M.E.; et al. Comparison of direct stenting with conventional stent implantation in acute myocardial infarction. Am. J. Cardiol. 2011, 108, 1697–1703. [Google Scholar] [CrossRef]
- Dziewierz, A.; Siudak, Z.; Rakowski, T.; Kleczyński, P.; Zasada, W.; Dubiel, J.S.; Dudek, D. Impact of direct stenting on outcome of patients with ST-elevation myocardial infarction transferred for primary percutaneous coronary intervention (from the EUROTRANSFER registry). Catheter Cardiovasc. Interv. 2014, 84, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, A.; Oduncu, V.; Karabay, C.Y.; Erkol, A.; Tanalp, A.C.; Tanboga, I.H.; Candan, O.; Gecmen, C.; Izgi, I.A.; Kirma, C. Outcomes of direct stenting in patients with ST-elevated myocardial infarction. Herz 2018, 43, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Fröbert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angerås, O.; Calais, F.; Danielewicz, M.; et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Antoniucci, D.; Valenti, R.; Migliorini, A.; Parodi, G.; Memisha, G.; Santoro, G.M.; Sciagrà, R. Comparison of rheolytic thrombectomy before direct infarct artery stenting versus direct stenting alone in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Am. J. Cardiol. 2004, 93, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Dudek, D.; Mielecki, W.; Burzotta, F.; Gasior, M.; Witkowski, A.; Horvath, I.G.; Legutko, J.; Ochala, A.; Rubartelli, P.; Wojdyla, R.M.; et al. Thrombus aspiration followed by direct stenting: A novel strategy of primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Results of the Polish-Italian-Hungarian RAndomized ThrombEctomy Trial (PIHRATE Trial). Am. Heart J. 2010, 160, 966–972. [Google Scholar] [CrossRef]
- Capodanno, D.; Dangas, G. Facilitated/pharmaco-invasive approaches in STEMI. Curr. Cardiol. Rev. 2012, 8, 177–180. [Google Scholar] [CrossRef][Green Version]
| DS (n = 189) | CS (n = 257) | p-Value | |
|---|---|---|---|
| Patient characteristics | |||
| Demographics | |||
| Age—years | 60 + 11 | 64 + 12 | 0.001 |
| Female sex—no. (%) | 49 (25.9) | 58 (22.6) | 0.41 |
| Caucasian—no. (%) | 170 (90.9)/187 | 234 (92.1)/254 | 0.65 |
| BMI—kg/m2 | 28 + 5/116 | 27 + 4/187 | 0.09 |
| Cardiovascular risk factors—no. (%) | |||
| Hypertension | 61 (32.6)/187 | 106 (41.9)/253 | 0.047 |
| Diabetes mellitus | 28 (15.1)/185 | 41 (16.1)/254 | 0.78 |
| Dyslipidemia | 37 (20.9)/177 | 70 (28.6)/245 | 0.07 |
| Smoking | 85 (47.2)/180 | 107 (43.0)/249 | 0.38 |
| Family history of cardiovascular disease | 70 (38.9)/180 | 102 (41.8)/244 | 0.55 |
| Medical history | |||
| Previous PCI | 16 (8.5)/189 | 32 (12.5)/256 | 0.18 |
| Previous myocardial infarction | 8 (4.2)/189 | 26 (10.2)/256 | 0.020 |
| Presentation | |||
| Onset symptoms to first medical contact—min | 50 [29–122] | 65 [34–129] | 0.08 |
| Crushed prasugrel—no. (%) | 87 (46.0) | 120 (46.7) | 0.89 |
| Procedural characteristics | |||
| Culprit vessel—no. (%) | |||
| LAD | 63 (33.9)/186 | 112 (44.8)/250 | 0.021 |
| Cx | 28 (15.1)/186 | 43 (17.2)/250 | 0.55 |
| RCA | 93 (50.0)/186 | 91 (36.4)/250 | 0.004 |
| Multivessel disease | 70 (37.6)/186 | 117 (46.8)/250 | 0.07 |
| Procedure | |||
| Onset symptoms to wire crossing—min | 132 [103–218] | 150 [112–225] | 0.11 |
| Angiographic parameters pre-PCI | |||
| TIMI 3 flow IRA pre-PCI—no. (%) | 82 (44.6)/184 | 73 (29.2)/250 | 0.001 |
| TIMI thrombusgrade 3–5—no. (%) | 150 (81.1)/185 | 198 (79.9)/249 | 0.76 |
| Postdilatation | 98 (51.9) | 168 (65.4) | 0.004 |
| Total procedural time—min | 28 [22–39]/187 | 36 [27–51]/256 | <0.001 |
| Total fluoroscopy time—min | 6 [4–10]/157 | 9 [6–15]/229 | <0.001 |
| Odds Ratio (95%CI) | p-Value | |
|---|---|---|
| Stent dimensions in culprit lesion | ||
| One DES | 2.49 [1.55–4.01] | <0.001 |
| Early reperfusion parameters post-PCI | ||
| TIMI 3 flow in the IRA | 1.16 [0.56–2.39] | 0.69 |
| TIMI blush grade 3 | 1.31 [0.75–2.31] | 0.35 |
| cTFC ≤ 23 frames/s | 1,42 [0.87–2.31] | 0.16 |
| Complete ST-segment resolution at 1 h | 1.29 [0.77–2.16] | 0.34 |
| Clinical outcomes (1 year) | ||
| All-cause mortality | - | - |
| Cardiac death | - | - |
| Myocardial re-infarction | 1.86 [0.66–5.26] | 0.24 |
| Target lesion failure | 2.93 [0.52–16.49] | 0.22 |
| Stent thrombosis | 0.59 [0.05–6.88] | 0.67 |
| Stroke | - | - |
| Urgent revascularization | 2.92 [0.83–10.26] | 0.10 |
| Target lesion revascularization | 3.49 [0.31–38.93] | 0.31 |
| Composite of death and stent thrombosis | 0.18 [0.02–1.44] | 0.11 |
| Composite of death, myocardial re-infarction, stroke, stent thrombosis, and urgent revascularization | 0.93 [0.41–2.10] | 0.86 |
| Odds Ratio (95%CI) | p-Value | |
|---|---|---|
| Stent dimensions in culprit lesion | ||
| One DES | 2.56 [1.49–4.39] | 0.001 |
| Early reperfusion parameters post-PCI | ||
| TIMI 3 flow in the IRA | 0.78 [0.36–1.73] | 0.55 |
| TIMI blush grade 3 | 1.17 [0.64–2.14] | 0.60 |
| cTFC ≤ 23 frames/sec | 1.51 [0.88–2.59] | 0.13 |
| Complete ST-segment resolution | 1.34 [0.78–2.30] | 0.29 |
| Clinical outcomes (1 year) | ||
| All-cause mortality | - | - |
| Cardiac death | - | - |
| Myocardial re-infarction | 1.92 [0.61–6.03] | 0.27 |
| Target lesion failure | 3.10 [0.56–17.48] | 0.20 |
| Stent thrombosis | 1.96 [0.12–31.65] | 0.64 |
| Stroke | - | - |
| Urgent revascularization | 3.98 [0.71–22.19] | 0.12 |
| Target lesion revascularization | 3.68 [0.33–41.16] | 0.29 |
| Composite of death and stent thrombosis | 0.43 [0.05–3.92] | 0.45 |
| Composite of death, myocardial re-infarction, stroke, stent thrombosis, and urgent revascularization | 1.23 [0.46–3.32] | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogel, R.F.; Delewi, R.; Wilschut, J.M.; Lemmert, M.E.; Diletti, R.; van Vliet, R.; van der Waarden, N.W.P.L.; Nuis, R.-J.; Paradies, V.; Alexopoulos, D.; et al. Direct Stenting versus Conventional Stenting in Patients with ST-Segment Elevation Myocardial Infarction—A COMPARE CRUSH Sub-Study. J. Clin. Med. 2023, 12, 6645. https://doi.org/10.3390/jcm12206645
Vogel RF, Delewi R, Wilschut JM, Lemmert ME, Diletti R, van Vliet R, van der Waarden NWPL, Nuis R-J, Paradies V, Alexopoulos D, et al. Direct Stenting versus Conventional Stenting in Patients with ST-Segment Elevation Myocardial Infarction—A COMPARE CRUSH Sub-Study. Journal of Clinical Medicine. 2023; 12(20):6645. https://doi.org/10.3390/jcm12206645
Chicago/Turabian StyleVogel, Rosanne F., Ronak Delewi, Jeroen M. Wilschut, Miguel E. Lemmert, Roberto Diletti, Ria van Vliet, Nancy W. P. L. van der Waarden, Rutger-Jan Nuis, Valeria Paradies, Dimitrios Alexopoulos, and et al. 2023. "Direct Stenting versus Conventional Stenting in Patients with ST-Segment Elevation Myocardial Infarction—A COMPARE CRUSH Sub-Study" Journal of Clinical Medicine 12, no. 20: 6645. https://doi.org/10.3390/jcm12206645
APA StyleVogel, R. F., Delewi, R., Wilschut, J. M., Lemmert, M. E., Diletti, R., van Vliet, R., van der Waarden, N. W. P. L., Nuis, R.-J., Paradies, V., Alexopoulos, D., Zijlstra, F., Montalescot, G., Angiolillo, D. J., Krucoff, M. W., Van Mieghem, N. M., Smits, P. C., & Vlachojannis, G. J. (2023). Direct Stenting versus Conventional Stenting in Patients with ST-Segment Elevation Myocardial Infarction—A COMPARE CRUSH Sub-Study. Journal of Clinical Medicine, 12(20), 6645. https://doi.org/10.3390/jcm12206645







