Thromboembolic and Bleeding Events in Transthyretin Amyloidosis and Coagulation System Abnormalities: A Review
Abstract
1. Introduction
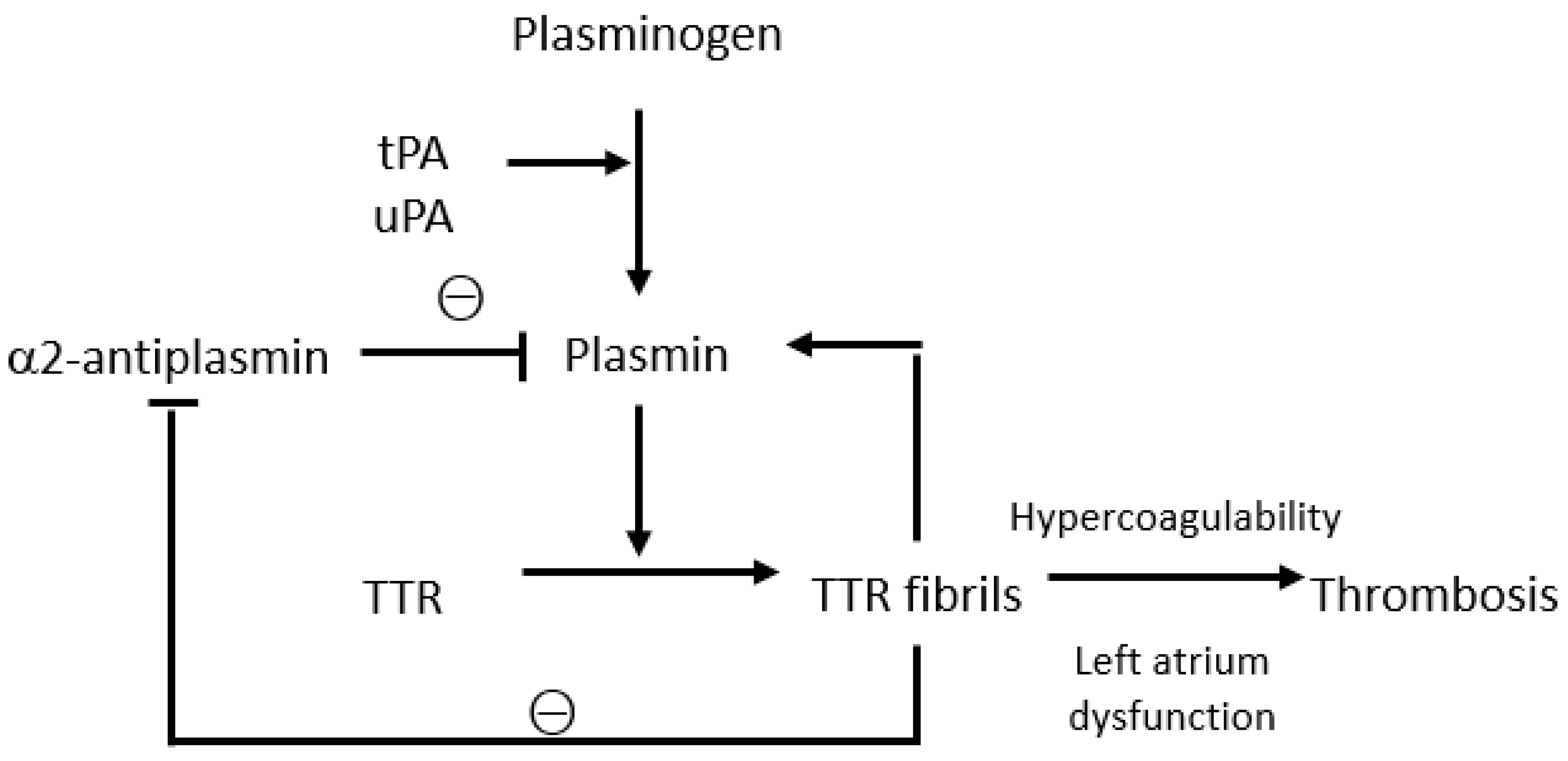
2. Interactions between Transthyretin and the Coagulation System
3. Thrombotic Events in Transthyretin Amyloidosis
3.1. The Role of Atrial Dysfunction in Transthyretin Cardiac Amyloidosis
| Thrombotic Events | Study, Date | Population | Prevalence | Predisposing Factors |
|---|---|---|---|---|
| Intracardiac thrombi | Feng, 2007 [29] | 55 ATTRwt 55 AL 4 AA | 33% | -AL subtype (51% vs. 16%, p < 0.001) -AF -Left ventricular diastolic dysfunction |
| Feng, 2009 [30] | 56 ATTRwt 17 ATTRv 3 AA 80 AL | 27% | -AL subtype (35% vs. 18%, p < 0.002) -AF -Lower left atrial appendage-emptying velocity -Diastolic dysfunction | |
| Martinez-Naharro, 2019 [31] | 166 TTR 155 AL | 6.2% | -AL subtype -AF -Atrial dilation -Higher ECV -Biventricular systolic dysfunction | |
| El-Am, 2019 [32] | 25 ATTRwt 4 ATTRv | 28% | -AF | |
| Cerebrovascular events | Mitrani, 2020 [33] | 290 TTR | 6% | |
| Donnellan, 2020 [34] | 111 ATTRwt 271 ATTRv | 16% | -Increased CHA2DS2VASC score -Non-anticoagulation | |
| Cerebrovascular events, peripheral embolism | Cappelli, 2020 [35] | 199 ATTRwt 73 ATTRv 134 AL | 7.6% | -AF -Left ventricular ejection fraction < 50% -CHA2DS2VASC score > 2 -CKD |
| Bukhari, 2021 [36] | 77 ATTRwt 68 ATTRwt-AF | 36.8% (ATTRwt-AF) | -AF | |
| Intracardiac thrombi, cerebrovascular events, peripheral embolism | Vilches, 2022 [22] | 1191 ATTR-CM | 16.2% | -AF -Non-anticoagulation -Age -African-American race -Peripheral vascular disease |
3.2. Intracardiac Thrombi
3.3. Cerebrovascular Events
3.4. Cerebrovascular Events and Peripheral Embolism
3.5. Intracardiac Thrombi, Cerebrovascular Events, and Peripheral Embolism
4. Bleeding Events in Transthyretin Amyloidosis
4.1. Spontaneous Bleeding Manifestations: Case Reports
4.2. Bleeding While Undergoing DOACs vs. VKAs
4.3. Type of Haemorrhagic Events According to Anticoagulant Therapy
4.4. Amyloid Angiopathy Associated with an Increased Fragility of Blood Vessels
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; et al. Systemic amyloidosis from A (AA) to T (ATTR): A review. J. Intern. Med. 2021, 289, 268–292. [Google Scholar] [CrossRef]
- Rapezzi, C.; Merlini, G.; Quarta, C.C.; Riva, L.; Longhi, S.; Leone, O.; Salvi, F.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009, 120, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Merlini, G.; Dispenzieri, A.; Sanchorawala, V.; Schönland, S.O.; Palladini, G.; Hawkins, P.N.; Gertz, M.A. Systemic immunoglobulin light chain amyloidosis. Nat. Rev. Dis. Primers 2018, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Stelmach-Gołdyś, A.; Zaborek-Łyczba, M.; Łyczba, J.; Garus, B.; Pasiarski, M.; Mertowska, P.; Małkowska, P.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P.; Grywalska, E. Physiology, Diagnosis and Treatment of Cardiac Light Chain Amyloidosis. J. Clin. Med. 2022, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Porcari, A.; Fontana, M.; Gillmore, J.D. Transthyretin cardiac amyloidosis. Cardiovasc. Res. 2023, 118, 3517–3535. [Google Scholar] [CrossRef] [PubMed]
- Falk, R.H.; Comenzo, R.L.; Skinner, M. The systemic amyloidoses. N. Engl. J. Med. 1997, 337, 898–909. [Google Scholar] [CrossRef]
- Quarta, C.C.; Kruger, J.L.; Falk, R.H. Cardiac Amyloidosis. Circulation 2012, 126, e178–e182. [Google Scholar] [CrossRef]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; de Haro-Del Moral, F.J.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef]
- Laptseva, N.; Rossi, V.A.; Sudano, I.; Schwotzer, R.; Ruschitzka, F.; Flammer, A.J.; Duru, F. Arrhythmic Manifestations of Cardiac Amyloidosis: Challenges in Risk Stratification and Clinical Management. J. Clin. Med. 2023, 12, 2581. [Google Scholar] [CrossRef]
- Frustaci, A.; Verardo, R.; Russo, M.A.; Caldarulo, M.; Alfarano, M.; Galea, N.; Miraldi, F.; Chimenti, C. Infiltration of Conduction Tissue Is a Major Cause of Electrical Instability in Cardiac Amyloidosis. J. Clin. Med. 2023, 12, 1798. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.S.; Schneiderman, H.; Kayani, N.; Radford, M.J.; Hager, W.D. Amyloid heart disease manifested by systemic arterial thromboemboli. Chest 1992, 102, 304–307. [Google Scholar] [CrossRef]
- Mangione, P.P.; Verona, G.; Corazza, A.; Marcoux, J.; Canetti, D.; Giorgetti, S.; Raimondi, S.; Stoppini, M.; Esposito, M.; Relini, A.; et al. Plasminogen activation triggers transthyretin amyloidogenesis in vitro. J. Biol. Chem. 2018, 293, 14192–14199. [Google Scholar] [CrossRef]
- Bouma, B.; Maas, C.; Hazenberg, B.P.C.; Lokhorst, H.M.; Gebbink, M.F.B.G. Increased plasmin-α2-antiplasmin levels indicate activation of the fibrinolytic system in systemic amyloidoses. J. Thromb. Haemost. 2007, 5, 1139–1142. [Google Scholar] [CrossRef]
- Wieczorek, E.; Ożyhar, A. Transthyretin: From Structural Stability to Osteoarticular and Cardiovascular Diseases. Cells 2021, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Mutimer, C.A.; Keragala, C.B.; Markus, H.S.; Werring, D.J.; Cloud, G.C.; Medcalf, R.L. Cerebral Amyloid Angiopathy and the Fibrinolytic System: Is Plasmin a Therapeutic Target? Stroke 2021, 52, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Zamolodchikov, D.; Berk-Rauch, H.E.; Oren, D.A.; Stor, D.S.; Singh, P.K.; Kawasaki, M.; Aso, K.; Strickland, S.; Ahn, H.J. Biochemical and structural analysis of the interaction between β-amyloid and fibrinogen. Blood 2016, 128, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Yaprak, E.; Kasap, M.; Akpinar, G.; Islek, E.E.; Sinanoglu, A. Abundant proteins in platelet-rich fibrin and their potential contribution to wound healing: An explorative proteomics study and review of the literature. J. Dent. Sci. 2018, 13, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Hindberg, K.; Solomon, T.; Smith, E.N.; Lapek, J.D., Jr.; Gonzalez, D.J.; Latysheva, N.; Frazer, K.A.; Braekkan, S.K.; Hansen, J.B. Discovery of novel plasma biomarkers for future incident venous thromboembolism by untargeted synchronous precursor selection mass spectrometry proteomics. J. Thromb. Haemost. 2018, 16, 1763–1774. [Google Scholar] [CrossRef]
- Zinellu, A.; Mangoni, A.A. Serum Prealbumin Concentrations, COVID-19 Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 638529. [Google Scholar] [CrossRef]
- Takahashi, R.; Ono, K.; Ikeda, T.; Akagi, A.; Noto, D.; Nozaki, I.; Sakai, K.; Asakura, H.; Iwasa, K.; Yamada, M. Coagulation and fibrinolysis abnormalities in familial amyloid polyneuropathy. Amyloid 2012, 19, 129–132. [Google Scholar] [CrossRef][Green Version]
- Nicol, M.; Siguret, V.; Vergaro, G.; Aimo, A.; Emdin, M.; Dillinger, J.G.; Baudet, M.; Cohen-Solal, A.; Villesuzanne, C.; Harel, S.; et al. Thromboembolism and bleeding in systemic amyloidosis: A review. ESC Heart Fail. 2022, 9, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Vilches, S.; Fontana, M.; Gonzalez-Lopez, E.; Mitrani, L.; Saturi, G.; Renju, M.; Griffin, J.M.; Caponetti, A.; Gnanasampanthan, S.; De Los Santos, J.; et al. Systemic embolism in amyloid transthyretin cardiomyopathy. Eur. J. Heart Fail. 2022, 24, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Martini, N.; Sinigiani, G.; De Michieli, L.; Mussinelli, R.; Perazzolo Marra, M.; Iliceto, S.; Zorzi, A.; Perlini, S.; Corrado, D.; Cipriani, A. Electrocardiographic features and rhythm disorders in cardiac amyloidosis. Trends Cardiovasc. Med. 2023, S1050-1738(23)00024-5. [Google Scholar] [CrossRef]
- Bandera, F.; Martone, R.; Chacko, L.; Ganesananthan, S.; Gilbertson, J.A.; Ponticos, M.; Lane, T.; Martinez-Naharro, A.; Whelan, C.; Quarta, C.; et al. Clinical Importance of Left Atrial Infiltration in Cardiac Transthyretin Amyloidosis. Cardiovasc. Imaging 2022, 15, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Dubrey, S.; Pollak, A.; Skinner, M.; Falk, R.H. Atrial thrombi occurring during sinus rhythm in cardiac amyloidosis: Evidence for atrial electromechanical dissociation. Heart 1995, 74, 541–544. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Versteylen, M.O.; Brons, M.; Teske, A.J.; Oerlemans, M.I.F.J. Restrictive Atrial Dysfunction in Cardiac Amyloidosis: Differences between Immunoglobulin Light Chain and Transthyretin Cardiac Amyloidosis Patients. Biomedicines 2022, 10, 1768. [Google Scholar] [CrossRef]
- Nochioka, K.; Quarta, C.C.; Claggett, B.; Roca, G.Q.; Rapezzi, C.; Falk, R.H.; Solomon, S.D. Left atrial structure and function in cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1128–1137. [Google Scholar] [CrossRef]
- Feng, D.; Edwards, W.D.; Oh, J.K.; Chandrasekaran, K.; Grogan, M.; Martinez, M.W.; Syed, I.I.; Hughes, D.A.; Lust, J.A.; Jaffe, A.S.; et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007, 116, 2420–2426. [Google Scholar] [CrossRef]
- Feng, D.; Syed, I.S.; Martinez, M.; Oh, J.K.; Jaffe, A.S.; Grogan, M.; Edwards, W.D.; Gertz, M.A.; Klarich, K.W. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009, 119, 2490–2497. [Google Scholar] [CrossRef]
- Martinez-Naharro, A.; Gonzalez-Lopez, E.; Corovic, A.; Mirelis, J.G.; Baksi, A.J.; Moon, J.C.; Garcia-Pavia, P.; Gillmore, J.D.; Hawkins, P.N.; Fontana, M. High prevalence of intracardiac thrombi in cardiac amyloidosis. J. Am. Coll. Cardiol. 2019, 73, 1733–1734. [Google Scholar] [CrossRef] [PubMed]
- El-Am, E.A.; Dispenzieri, A.; Melduni, R.M.; Ammash, N.M.; White, R.D.; Hodge, D.O.; Noseworthy, P.A.; Lin, G.; Pislaru, S.V.; Egbe, A.C.; et al. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J. Am. Coll. Cardiol. 2019, 73, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Mitrani, L.R.; De Los Santos, J.; Driggin, E.; Kogan, R.; Helmke, S.; Goldsmith, J.; Biviano, A.B.; Maurer, M.S. Anticoagulation with warfarin compared to novel oral anticoagulants for atrial fibrillation in adults with transthyretin cardiac amyloidosis: Comparison of thromboembolic events and major bleeding. Amyloid 2020, 28, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Elshazly, M.B.; Puri, R.; Saliba, W.; Kanj, M.; Vakamudi, S.; Patel, D.R.; Baranowski, B.; et al. Atrial fibrillation in transthyretin cardiac amyloidosis: Predictors, prevalence, and efficacy of rhythm control strategies. Clin. Electrophysiol. 2020, 6, 1118–1127. [Google Scholar] [CrossRef]
- Cappelli, F.; Tini, G.; Russo, D.; Emdin, M.; Del Franco, A.; Vergaro, G.; Di Bella, G.; Mazzeo, A.; Canepa, M.; Volpe, M.; et al. Arterial thrombo-embolic events in cardiac amyloidosis: A look beyond atrial fibrillation. Amyloid 2020, 28, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Barakat, A.F.; Eisele, Y.S.; Nieves, R.; Jain, S.; Saba, S.; Follansbee, W.P.; Brownell, A.; Soman, P. Prevalence of Atrial Fibrillation and Thromboembolic Risk in Wild-Type Transthyretin Amyloid Cardiomyopathy. Circulation 2021, 143, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Mumford, A.D.; O’Donnell, J.; Gillmore, J.D.; Manning, R.A.; Hawkins, P.N.; Laffan, M. Bleeding symptoms and coagulation abnormalities in 337 patients with AL-amyloidosis. Br. J. Haematol. 2000, 110, 454–460. [Google Scholar] [CrossRef]
- Schrutka, L.; Avanzini, N.; Seirer, B.; Rettl, R.; Dachs, T.; Duca, F.; Binder, C.; Dalos, D.; Eslam, R.B.; Bonderman, D. Bleeding events in patients with cardiac amyloidosis. Eur. Heart J. 2020, 41, 2122. [Google Scholar] [CrossRef]
- Ono, R.; Kajiyama, T.; Miyauchi, H.; Kobayashi, Y. Periorbital ecchymosis and shoulder pad sign in transthyretin amyloidosis. BMJ Case Rep. 2021, 14, e242614. [Google Scholar] [CrossRef]
- Jhawar, N.; Reynolds, J.; Nakhleh, R.; Lyle, M. Hereditary transthyretin amyloidosis presenting with spontaneous periorbital purpura: A case report. Eur. Heart J. Case Rep. 2023, 7, ytad108. [Google Scholar] [CrossRef]
- Yumoto, S.; Doi, K.; Higashi, T.; Shimao, Y.; Ueda, M.; Ishihara, A.; Adachi, Y.; Ishiodori, H.; Honda, S.; Baba, H. Intra-abdominal bleeding caused by amyloid transthyretin amyloidosis in the gastrointestinal tract: A case report. Clin. J. Gastroenterol. 2022, 15, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, T.; Kamran, A.; Shah, D.; Guha, A.; Salman Faisal, M.; Mewawalla, P. Senile Systemic Amyloidosis Presenting as Hematuria: A Rare Presentation and Review of Literature. Case Rep. Med. 2020, 2020, 5892707. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Chen, X.W.; Pan, J.L.; Li, H.; Xie, B.; Cai, S.J. Clinical features of retinal amyloid angiopathy with transthyretin Gly83Arg variant. Int. J. Ophthalmol. 2023, 16, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.L.; Schwartz, S.G.; Davis, J.L. An 82-year-old woman with retinal vascular sheathing and vitreous hemorrhage. Retin. Cases Brief Rep. 2023, 17, S36–S40. [Google Scholar] [CrossRef]
- Cariou, E.; Sanchis, K.; Rguez, K.; Blanchard, V.; Cazalbou, S.; Fournier, P.; Huart, A.; Roussel, M.; Cintas, P.; Galinier, M.; et al. New Oral Anticoagulants vs. Vitamin K Antagonists Among Patients With Cardiac Amyloidosis: Prognostic Impact. Front. Cardiovasc. Med. 2021, 8, 742428. [Google Scholar] [CrossRef]
- Thelander, U.; Westermark, G.T.; Antoni, G.; Estrada, S.; Zancanaro, A.; Ihse, E.; Westermark, P. Cardiac microcalcifications in transthyretin (ATTR) amyloidosis. Int. J. Cardiol. 2022, 352, 84–91. [Google Scholar] [CrossRef]
- Bulk, M.; Moursel, L.G.; van der Graaf, L.M.; van Veluw, S.J.; Greenberg, S.M.; van Duinen, S.G.; van Buchem, M.A.; van Rooden, S.; van der Weerd, L. Cerebral Amyloid Angiopathy With Vascular Iron Accumulation and Calcification. Stroke 2018, 49, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Grand Moursel, L.; van der Graaf, L.M.; Bulk, M.; van Roon-Mom, W.M.C.; van der Weerd, L. Osteopontin and phospho-SMAD2/3 are associated with calcification of vessels in D-CAA, an hereditary cerebral amyloid angiopathy. Brain Pathol. 2019, 29, 793–802. [Google Scholar] [CrossRef]
- Sud, K.; Narula, N.; Aikawa, E.; Arbustini, E.; Pibarot, P.; Merlini, G.; Rosenson, R.S.; Seshan, S.V.; Argulian, E.; Ahmadi, A.; et al. The contribution of amyloid deposition in the aortic valve to calcification and aortic stenosis. Nat. Rev. Cardiol. 2023, 20, 418–428. [Google Scholar] [CrossRef]
- Writing Committee; Kittleson, M.M.; Ruberg, F.L.; Ambardekar, A.V.; Brannagan, T.H.; Cheng, R.K.; Clarke, J.O.; Dember, L.M.; Frantz, J.G.; Hershberger, R.E.; et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis. J. Am. Coll Cardiol. 2023, 81, 1076–1126. [Google Scholar] [CrossRef]
- Tschöpe, C.; Elsanhoury, A. Treatment of Transthyretin Amyloid Cardiomyopathy: The Current Options, the Future, and the Challenges. J. Clin. Med. 2022, 11, 2148. [Google Scholar] [CrossRef] [PubMed]
| Studies, Date | Population | Type of Bleeding | Prevalence | Comments |
|---|---|---|---|---|
| Feng, 2009 [30] | 56 ATTRwt 17 ATTRv 3 AA 80 AL | Massive GI bleedings | 1.9% | |
| Mitrani, 2020 [33] | 290 ATTR | Miscellany | 7% | Labile INR |
| Cariou, 2021 [45] | 179 ATTRwt 25 ATTRv 69 AL | Miscellany | 15% Minor bleedings 8%; Major bleedings 7% | VKAs vs. NOACs (14% vs. 2%) |
| Bukhari, 2021 [36] | 77 ATTRwt | Miscellany | 11.7% Haemorrhagic stroke 4.4% Major extracranial bleedings 7.3% | No difference between ATTRwt-AF and AF-control |
| Vilches, 2022 [22] | 1191 ATTR-CM | Miscellany | 6.5% GI bleedings 46.9% Intracranial bleedings 12.5% | No difference between ATTRwt-AF and AF-control |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napolitano, A.; De Michieli, L.; Sinigiani, G.; Berno, T.; Cipriani, A.; Spiezia, L. Thromboembolic and Bleeding Events in Transthyretin Amyloidosis and Coagulation System Abnormalities: A Review. J. Clin. Med. 2023, 12, 6640. https://doi.org/10.3390/jcm12206640
Napolitano A, De Michieli L, Sinigiani G, Berno T, Cipriani A, Spiezia L. Thromboembolic and Bleeding Events in Transthyretin Amyloidosis and Coagulation System Abnormalities: A Review. Journal of Clinical Medicine. 2023; 12(20):6640. https://doi.org/10.3390/jcm12206640
Chicago/Turabian StyleNapolitano, Angela, Laura De Michieli, Giulio Sinigiani, Tamara Berno, Alberto Cipriani, and Luca Spiezia. 2023. "Thromboembolic and Bleeding Events in Transthyretin Amyloidosis and Coagulation System Abnormalities: A Review" Journal of Clinical Medicine 12, no. 20: 6640. https://doi.org/10.3390/jcm12206640
APA StyleNapolitano, A., De Michieli, L., Sinigiani, G., Berno, T., Cipriani, A., & Spiezia, L. (2023). Thromboembolic and Bleeding Events in Transthyretin Amyloidosis and Coagulation System Abnormalities: A Review. Journal of Clinical Medicine, 12(20), 6640. https://doi.org/10.3390/jcm12206640





