The Impact of Transfer-Related Ischemia on Free Flap Metabolism and Electrolyte Homeostasis—A New In Vivo Experimental Approach in Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia and Instrumentation
2.3. Surgical Preparation
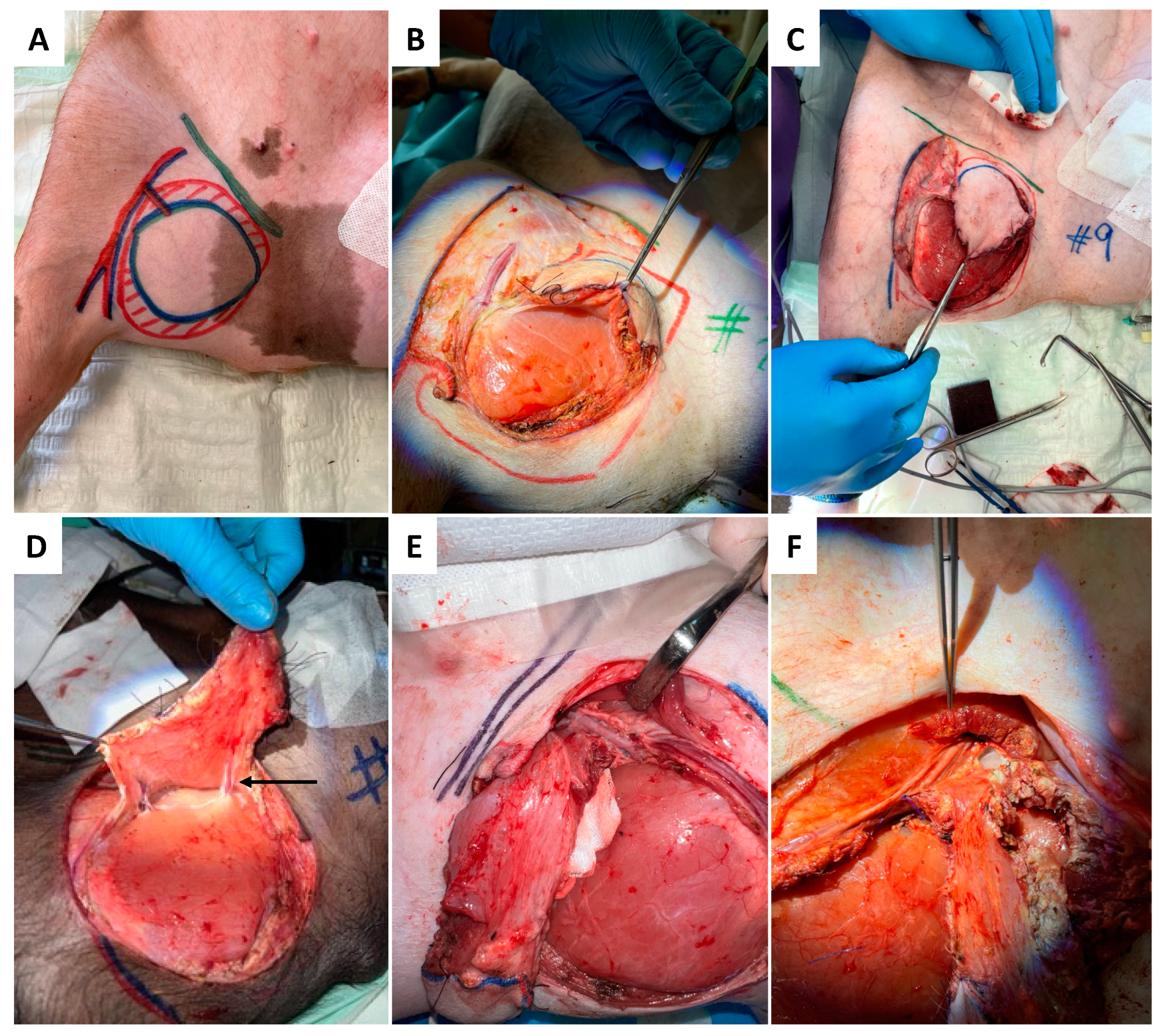
2.4. Surgery
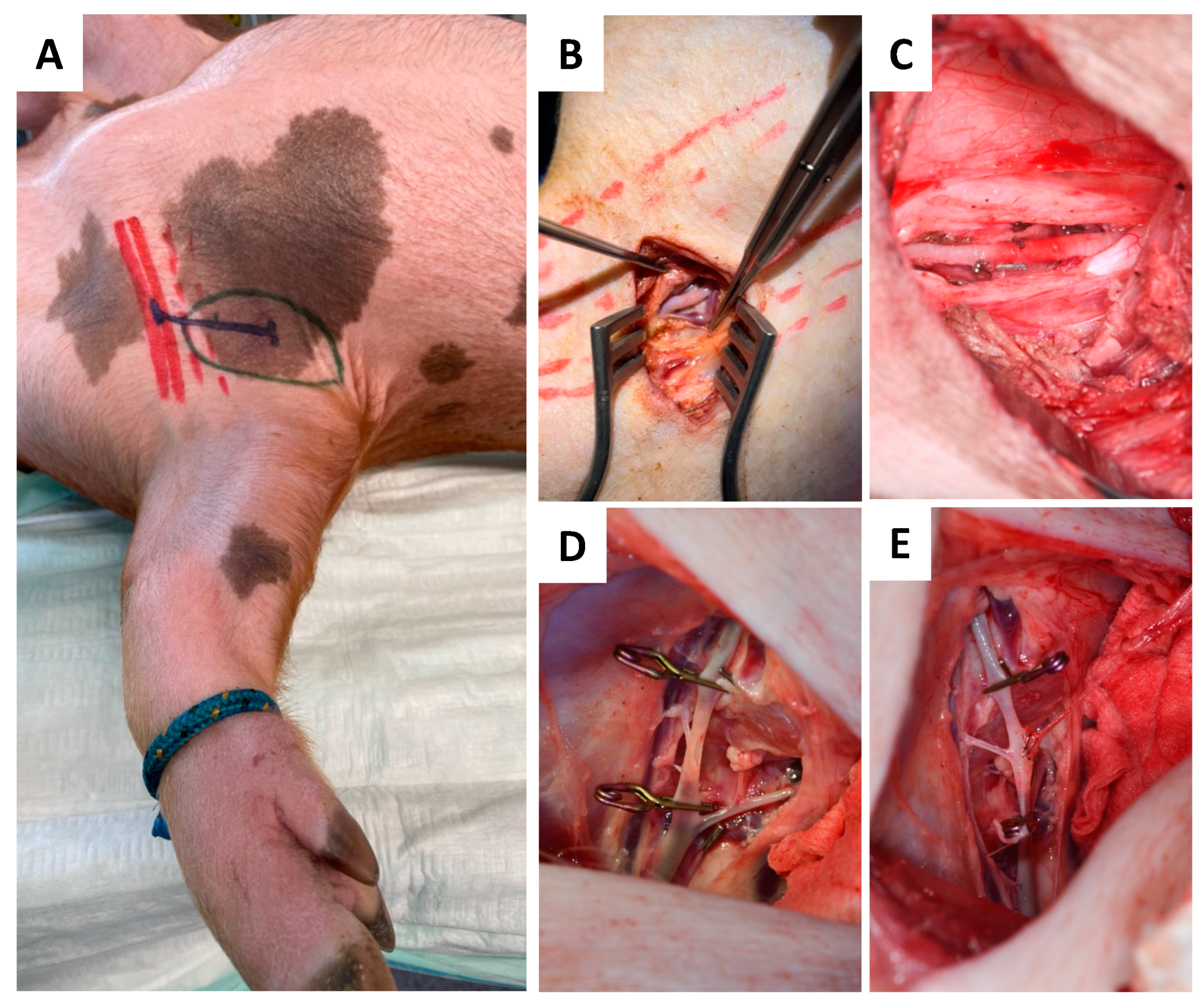
2.4.1. Donor Site
2.4.2. Recipient Site
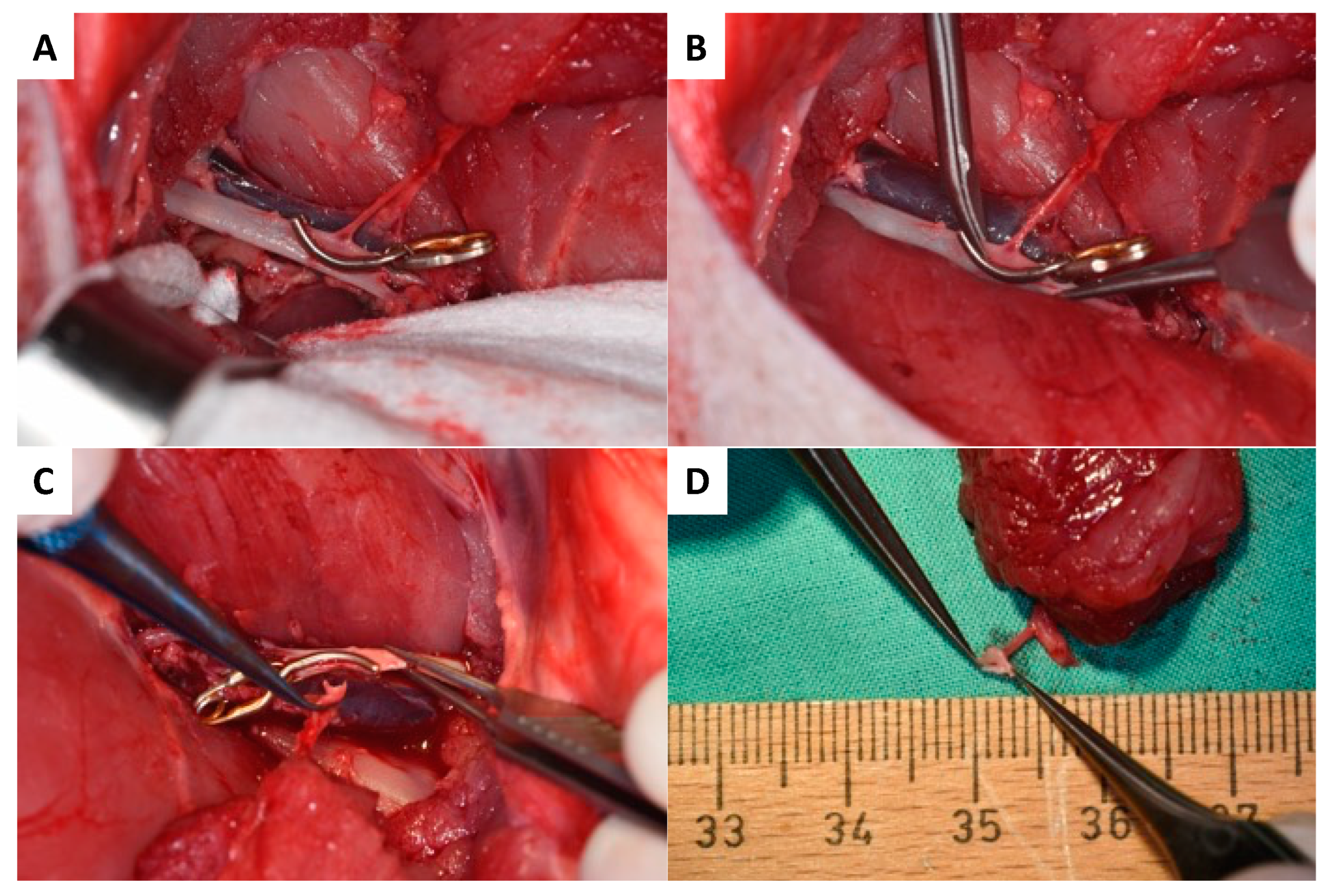
2.4.3. Anastomosis
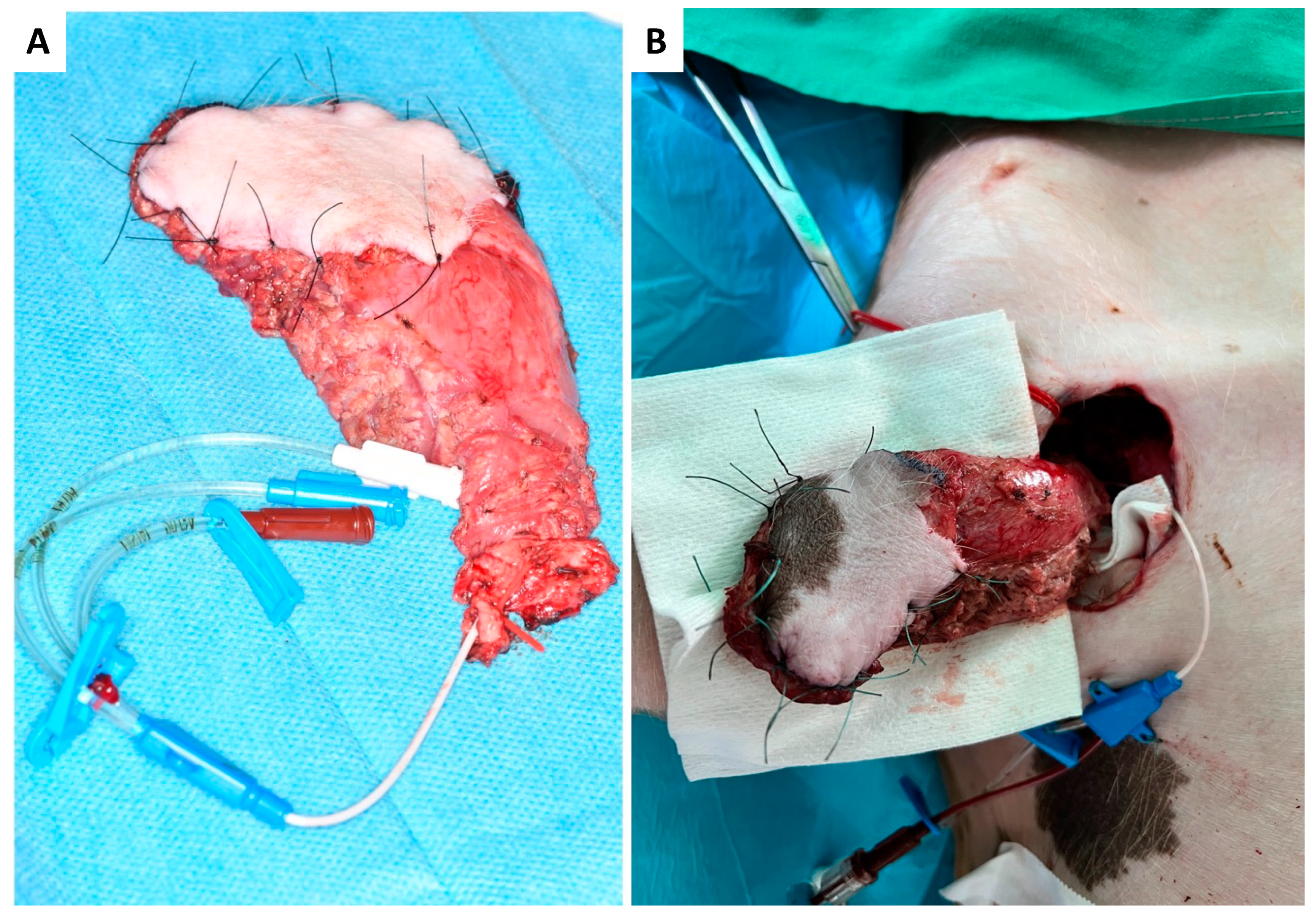
2.5. Flap Monitoring and Tissue Sampling
2.6. Statistical Analysis
3. Results
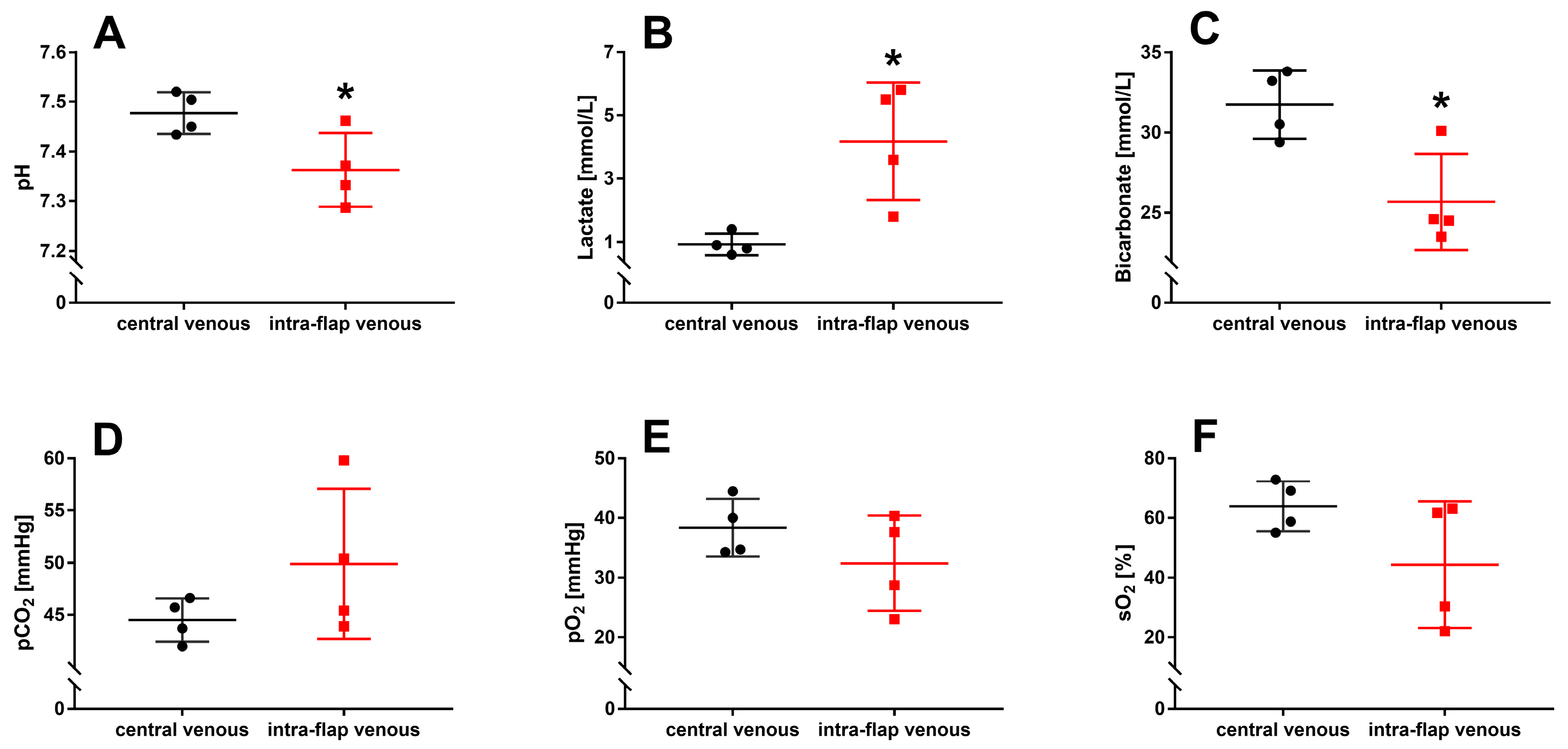
3.1. Ischemia during Flap Transfer Changes the Intra-Flap Acid–Base Balance
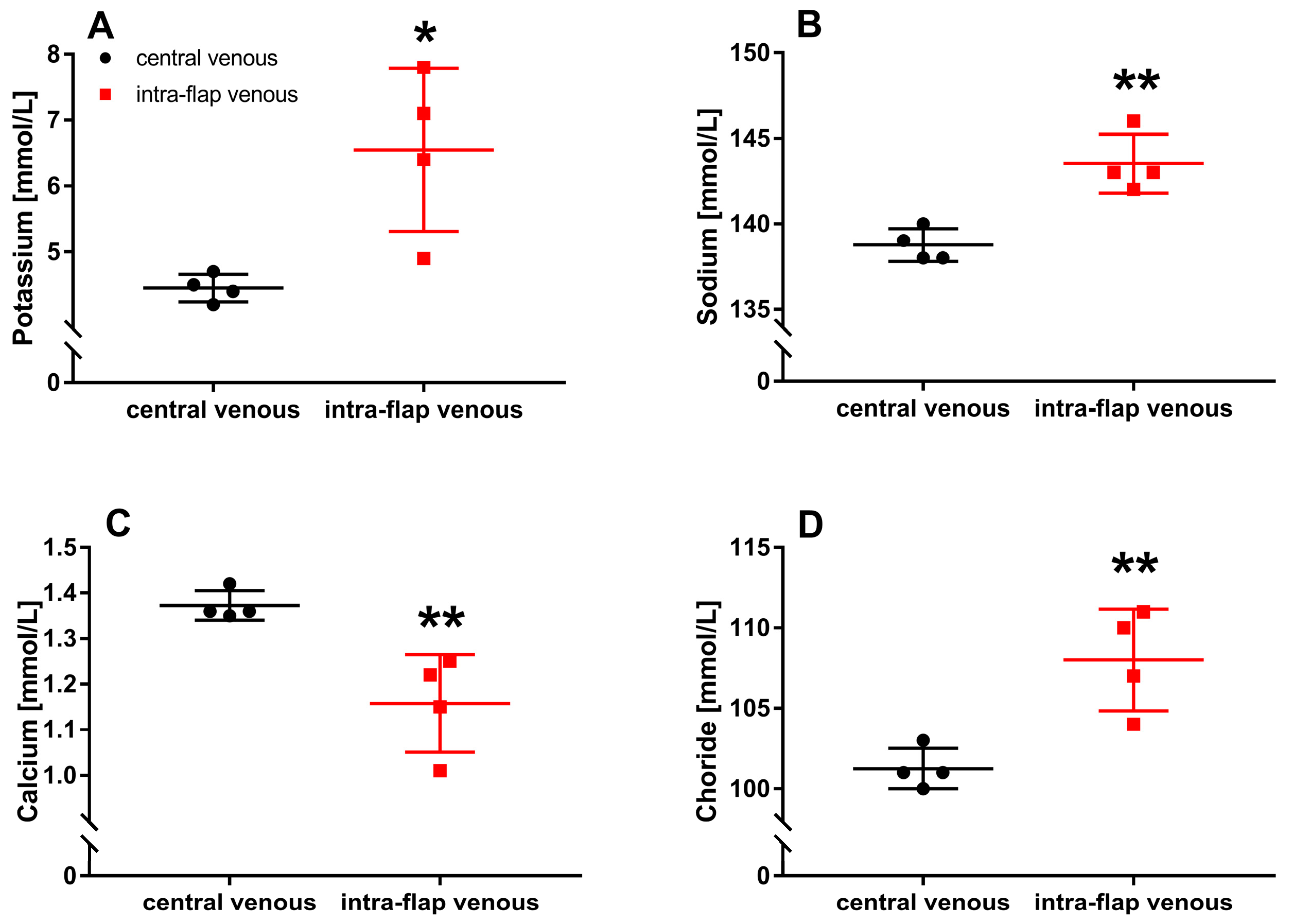
3.2. Ischemia during Flap Transfer Alters Intra-Flap Electrolyte Concentrations
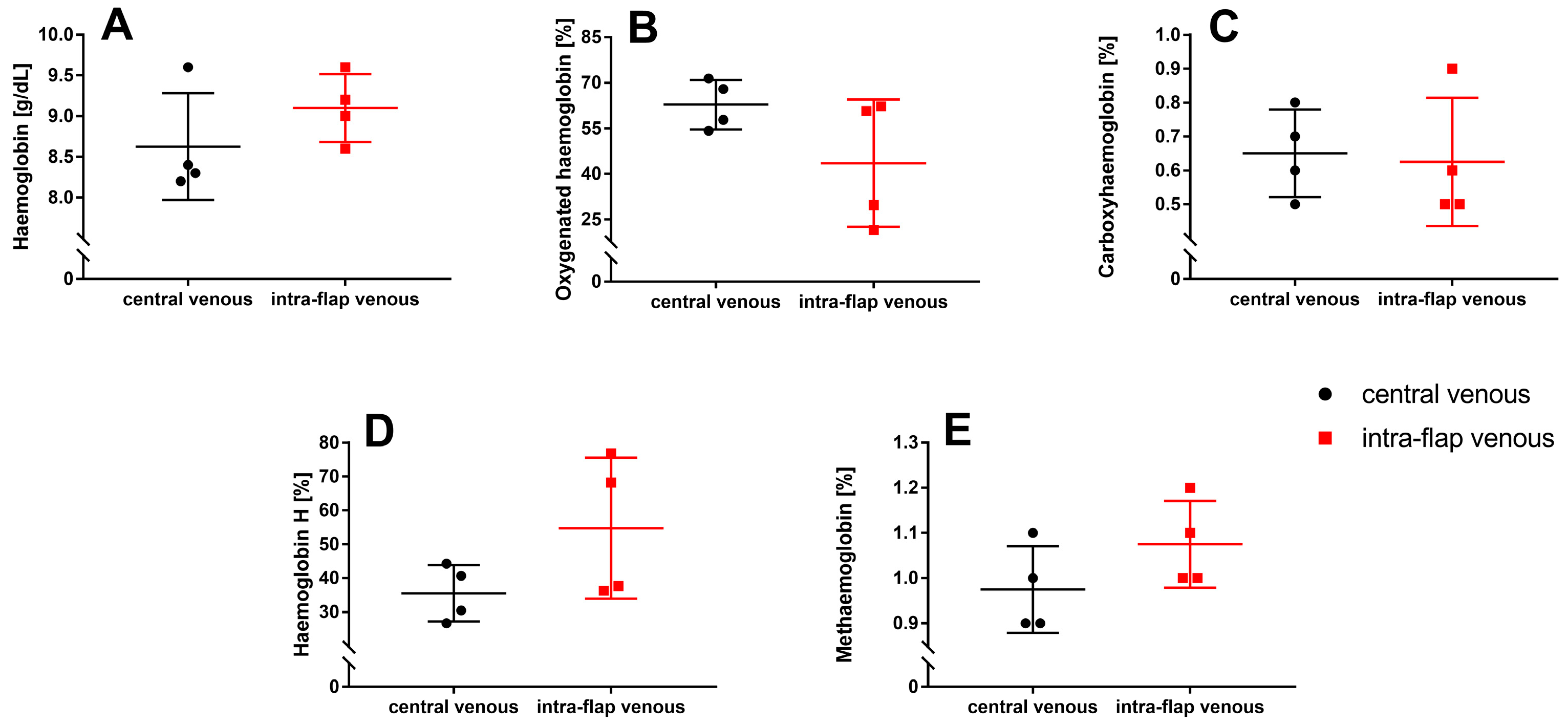
3.3. Ischemia during Flap Transfer Has No Impact on Hemoglobin Types and Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frederick, J.W.; Sweeny, L.; Carroll, W.R.; Peters, G.E.; Rosenthal, E.L. Outcomes in head and neck reconstruction by surgical site and donor site. Laryngoscope 2013, 123, 1612–1617. [Google Scholar] [CrossRef]
- Ferrari, S.; Copelli, C.; Bianchi, B.; Ferri, A.; Poli, T.; Ferri, T.; Sesenna, E. Free flaps in elderly patients: Outcomes and complications in head and neck reconstruction after oncological resection. J. Craniomaxillofac. Surg. 2013, 41, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Kohlert, S.; Quimby, A.E.; Saman, M.; Ducic, Y. Postoperative Free-Flap Monitoring Techniques. Semin. Plast. Surg. 2019, 33, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Thiem, D.G.E.; Frick, R.W.; Goetze, E.; Gielisch, M.; Al-Nawas, B.; Kämmerer, P.W. Hyperspectral analysis for perioperative perfusion monitoring-a clinical feasibility study on free and pedicled flaps. Clin. Oral Investig. 2021, 25, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Thiem, D.G.E.; Römer, P.; Blatt, S.; Al-Nawas, B.; Kämmerer, P.W. New Approach to the Old Challenge of Free Flap Monitoring-Hyperspectral Imaging Outperforms Clinical Assessment by Earlier Detection of Perfusion Failure. J. Per. Med. 2021, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.P.; Rozen, W.M.; Whitaker, I.S.; Chubb, D.; Grinsell, D.; Ashton, M.W.; Hunter-Smith, D.J.; Lineaweaver, W.C. Current evidence for postoperative monitoring of microvascular free flaps: A systematic review. Ann. Plast. Surg. 2015, 74, 621–632. [Google Scholar] [CrossRef]
- Dolan, R.; Butler, J.; Murphy, S.; Cronin, K. Health-related quality of life, surgical and aesthetic outcomes following microvascular free flap reconstructions: An 8-year institutional review. Ann. R Coll. Surg. Engl. 2012, 94, 43–51. [Google Scholar] [CrossRef]
- Kansy, K.; Mueller, A.A.; Mücke, T.; Koersgen, F.; Wolff, K.D.; Zeilhofer, H.-F.; Hölzle, F.; Pradel, W.; Schneider, M.; Kolk, A.; et al. Microsurgical reconstruction of the head and neck region: Current concepts of maxillofacial surgery units worldwide. J. Craniomaxillofac. Surg. 2015, 43, 1364–1368. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar]
- Sanada, S.; Komuro, I.; Kitakaze, M. Pathophysiology of myocardial reperfusion injury: Preconditioning, postconditioning, and translational aspects of protective measures. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1723–H1741. [Google Scholar] [CrossRef]
- Jennings, R.B.; Reimer, K.A. The cell biology of acute myocardial ischemia. Annu. Rev. Med. 1991, 42, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P. How and when do myocytes die during ischemia and reperfusion: The late phase. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C.; Mattiazzi, A.; Bassani, R.A.; Escobar, A.L.; Palomeque, J.; Valverde, C.A.; Petroff, M.V.; Bers, D.M.; Belliard, A.; et al. Ion transport and energetics during cell death and protection. Physiology 2008, 23, 115–123. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Ordy, J.; Wengenack, T.; Bialobok, P.; Coleman, P.; Rodier, P.; Baggs, R.; Dunlap, W.; Kates, B. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp. Neurol. 1993, 119, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Sapega, A.A.; Heppenstall, R.B.; Chance, B.; Park, Y.S.; Sokolow, D. Optimizing tourniquet application and release times in extremity surgery. A biochemical and ultrastructural study. J. Bone Joint Surg. Am. 1985, 67, 303–314. [Google Scholar] [CrossRef]
- Belkin, M.; Brown, R.D.; Wright, J.G.; LaMorte, W.W.; Hobson, R.W. A new quantitative spectrophotometric assay of ischemia-reperfusion injury in skeletal muscle. Am. J. Surg. 1988, 156, 83–86. [Google Scholar] [CrossRef]
- Jennische, E.; Hagberg, H.; Haljamäe, H. Extracellular potassium concentration and membrane potential in rabbit gastrocnemius muscle during tourniquet ischemia. Pflugers Arch. 1982, 392, 335–339. [Google Scholar] [CrossRef]
- Birke-Sørensen, H.; Toft, G.; Bengaard, J. Pure muscle transfers can be monitored by use of microdialysis. J. Reconstr. Microsurg. 2010, 26, 623–630. [Google Scholar] [CrossRef]
- Brix, M.; Muret, P.; Ricbourg, B.; Humbert, P. Monitoring free flaps with cutaneous microdialysis: Preliminary study in oral cavity reconstruction. Rev. Stomatol. Chir. Maxillofac. 2006, 107, 460–464. [Google Scholar] [CrossRef]
- Jyränki, J.; Suominen, S.; Vuola, J.; Bäck, L. Microdialysis in clinical practice: Monitoring intraoral free flaps. Ann. Plast. Surg. 2006, 56, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.T.; Gutberg, N.; Birke-Sorensen, H. Monitoring of intraoral free flaps with microdialysis. Br. J. Oral Maxillofac. Surg. 2011, 49, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Dakpé, S.; Colin, E.; Bettoni, J.; Davrou, J.; Diouf, M.; Devauchelle, B.; Testelin, S. Intraosseous microdialysis for bone free flap monitoring in head and neck reconstructive surgery: A prospective pilot study. Microsurgery 2020, 40, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Ziebart, A.; Möllmann, C.; Garcia-Bardon, A.; Kamuf, J.; Schäfer, M.; Thomas, R.; Hartmann, E.K. Effect of gelatin-polysuccinat on cerebral oxygenation and microcirculation in a porcine haemorrhagic shock model. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 15. [Google Scholar] [CrossRef]
- Finseth, F.; Zimmermann, J. Prevention of necrosis in island myocutaneous flaps in the pig by treatment with isoxsuprine. Plast. Reconstr. Surg. 1979, 64, 536–539. [Google Scholar] [CrossRef]
- Barone, A.A.L.; Leonard, D.A.; Torabi, R.; Mallard, C.; Glor, T.; Scalea, J.R.; Randolph, M.A.; Sachs, D.H.; Cetrulo, C.L. The gracilis myocutaneous free flap in swine: An advantageous preclinical model for vascularized composite allograft transplantation research. Microsurgery 2013, 33, 51–55. [Google Scholar] [CrossRef]
- Villamaria, C.Y.; Rasmussen, T.E.; Spencer, J.R.; Patel, S.; Davis, M.R. Microvascular porcine model for the optimization of vascularized composite tissue transplantation. J. Surg. Res. 2012, 178, 452–459. [Google Scholar] [CrossRef]
- Bodin, F.; Diana, M.; Koutsomanis, A.; Robert, E.; Marescaux, J.; Bruant-Rodier, C. Porcine model for free-flap breast reconstruction training. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 1402–1409. [Google Scholar] [CrossRef]
- González-García, J.A.; Chiesa-Estomba, C.M.; Larruscain, E.; Álvarez, L.; Sistiaga, J.A. Porcine experimental model for gracilis free flap transfer to the head and neck area with novel donor site description. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 111–117. [Google Scholar] [CrossRef]
- Gao, Y.; Aravind, S.; Patel, N.S.; Fuglestad, M.A.; Ungar, J.S.; Mietus, C.J.; Li, S.; Casale, G.P.; Pipinos, I.I.; Carlson, M.A. Collateral Development and Arteriogenesis in Hindlimbs of Swine After Ligation of Arterial Inflow. J. Surg. Res. 2020, 249, 168–179. [Google Scholar] [CrossRef]
- Thiem, D.G.E.; Siegberg, F.; Römer, P.; Blatt, S.; Pabst, A.; Heimes, D.; Al-Nawas, B.; Kämmerer, P.W. Long-Term Donor Site Morbidity and Flap Perfusion Following Radial versus Ulnar Forearm Free Flap-A Randomized Controlled Prospective Clinical Trial. J. Clin. Med. 2022, 11, 3601. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.K.; Nguyen, T.J.; Peric, M.; Shahabi, A.; Vidar, E.N.; Hwang, B.H.; Leilabadi, S.N.; Chan, L.S.; Urata, M.M. Analysis of risk factors associated with microvascular free flap failure using a multi-institutional database. Microsurgery 2015, 35, 6–12. [Google Scholar] [CrossRef]
- Irawati, N.; Every, J.; Dawson, R.; Leinkram, D.; Elliott, M.; Ch’ng, S.; Low, H.; Palme, C.E.; Clark, J.; Wykes, J. Effect of operative time on complications associated with free flap reconstruction of the head and neck. Clin. Otolaryngol. 2023, 48, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Serletti, J.M.; Higgins, J.P.; Moran, S.; Orlando, G.S. Factors affecting outcome in free-tissue transfer in the elderly. Plast. Reconstr. Surg. 2000, 106, 66–70. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephan, D.; Blatt, S.; Riedel, J.; Mohnke, K.; Ruemmler, R.; Ziebart, A.; Al-Nawas, B.; Kämmerer, P.W.; Thiem, D.G.E. The Impact of Transfer-Related Ischemia on Free Flap Metabolism and Electrolyte Homeostasis—A New In Vivo Experimental Approach in Pigs. J. Clin. Med. 2023, 12, 6625. https://doi.org/10.3390/jcm12206625
Stephan D, Blatt S, Riedel J, Mohnke K, Ruemmler R, Ziebart A, Al-Nawas B, Kämmerer PW, Thiem DGE. The Impact of Transfer-Related Ischemia on Free Flap Metabolism and Electrolyte Homeostasis—A New In Vivo Experimental Approach in Pigs. Journal of Clinical Medicine. 2023; 12(20):6625. https://doi.org/10.3390/jcm12206625
Chicago/Turabian StyleStephan, Daniel, Sebastian Blatt, Julian Riedel, Katja Mohnke, Robert Ruemmler, Alexander Ziebart, Bilal Al-Nawas, Peer W. Kämmerer, and Daniel G. E. Thiem. 2023. "The Impact of Transfer-Related Ischemia on Free Flap Metabolism and Electrolyte Homeostasis—A New In Vivo Experimental Approach in Pigs" Journal of Clinical Medicine 12, no. 20: 6625. https://doi.org/10.3390/jcm12206625
APA StyleStephan, D., Blatt, S., Riedel, J., Mohnke, K., Ruemmler, R., Ziebart, A., Al-Nawas, B., Kämmerer, P. W., & Thiem, D. G. E. (2023). The Impact of Transfer-Related Ischemia on Free Flap Metabolism and Electrolyte Homeostasis—A New In Vivo Experimental Approach in Pigs. Journal of Clinical Medicine, 12(20), 6625. https://doi.org/10.3390/jcm12206625





