1. Introduction
The results of the studies confirm that childhood obesity remains one of the most significant challenges in 21st-century medicine. The troubling increase in the number of children with excessive body weight is concerning. In 2020, excess body weight affected 39 million children under the age of 4. Despite the ongoing serious and unresolved issues of hunger and underweight, the majority of the global population resides in countries where deaths due to excessive body weight are more prevalent than due to underweight (with exceptions in parts of Sub-Saharan Africa and Asia) [
1]. Childhood obesity substantially elevates the risk of complications that impact both the quality and length of life. One of the more serious complications secondary to excessive body weight is metabolic disturbances, which can manifest as insulin resistance, impaired glucose tolerance, or type 2 diabetes. Their occurrence depends on the severity of obesity, the duration of the condition, visceral fat mass, and family history of diabetes. In obese children, the most common primary cause of carbohydrate metabolism disorders is peripheral tissue resistance to insulin action [
2,
3]. Currently, the greatest influence on the development of insulin resistance in adolescents is exerted by obesity.
There is a general consensus that insulin resistance predisposes the development of glucose metabolism disorders, dyslipidemia, and high blood pressure, which are components of metabolic syndrome. This, in turn, is a later factor in the development of type 2 diabetes, cardiovascular diseases, and many others, and can also serve as an independent predictor of cardiovascular risk [
2]. The prevalence of type 2 diabetes among children and adolescents has recently rapidly increased worldwide, which is correlated with the global rise in obesity rates among the youngest patients [
4,
5]. Children with obesity are 4-fold more likely to develop type 2 diabetes compared to children with a normal BMI [
6]. Thirty years ago, type 2 diabetes was considered a rare disease in the pediatric population. Up until the mid-1990s, only 1–2% of children with diabetes were classified as having type 2 diabetes. However, with the increase in obesity, the frequency of type 2 diabetes has risen depending on the studied population, ranging from 8 to 45% of all new diabetes cases reported in children and adolescents [
7]. A study conducted by Starzyk et al. revealed that DM2 accounts for 10% of all diagnosed cases of diabetes in Caucasian youth. Additionally, prediabetic conditions such as impaired glucose tolerance were diagnosed in 10–27% of obese children. The average age of DM2 diagnosis in youth was 13 years [
8,
9]. On the other hand, a comprehensive meta-analysis conducted by Cioana revealed that 75.27% of children with type 2 diabetes had obesity, and 77.24% were obese at diagnosis [
10].
In recent years, the causes of lipid disorders in children have also changed due to the steadily growing percentage of children with excessive body weight. Genetic disorders used to be the most common cause of dyslipidemia. In recent decades, an increase in dyslipidemia secondary to obesity has been observed [
11]. Most constitutional lipid disorders occur independently of excessive body weight, yet obesity can facilitate the expression of certain lipid disorders. In a Polish study examining lipid disorders published in 2020 performed on a group of 1948 children and adolescents aged 6–15 with excess body weight, at least one lipid disorder was described in 38.23% of girls and 40.51% of boys with overweight and obesity. The most prevalent lipid disorders were decreased high-density lipoprotein cholesterol (HDL-C) levels, found in 20.55% of girls and 23.79% of boys, and elevated low-density lipoprotein cholesterol (LDL-C), observed in 15.31% of girls and 14.25% of boys. There was no strong association between lipid disorders and age, sex, birth weight, gestational age at birth, or body composition [
12]. Moreover, cardiovascular risk indicators such as reduced HDL cholesterol fraction, high systolic and diastolic blood pressure, and elevated triglyceride levels increased with the severity of obesity [
13]. The rise in the frequency of excessive body weight and the large scale of this phenomenon have led to more attention being paid to coexisting diseases and complications resulting from obesity. The degree of obesity in children and adolescents has significant clinical implications. Some of them once considered long term and characteristic of adult obesity are now being increasingly diagnosed in children and adolescents. Therefore, the role of preventive programs funded by public resources is crucial.
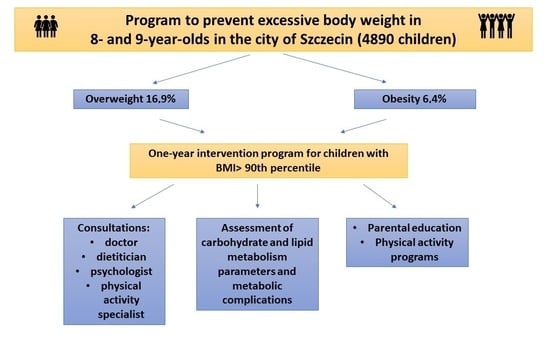
The objective of this study was not only to assess the biochemical parameters before and after the completion of a year-long intervention activities in 8- and 9-year-old children with excessive body weight but also to collect epidemiological data on the prevalence of overweight and obesity among this study population and to determine the effectiveness of medical intervention in the form of a year-long interdisciplinary collaboration. Studies assessing the prevalence of excess body weight in children living in Szczecin and evaluating the risk of obesity-related complications have not been conducted so far. The choice of the research topic stemmed from the need for epidemiological studies in this region providing information about the scale of the excessive bodyweight prevalence in children. The other aim of this study was to promote a healthy lifestyle and counteract the development of lifestyle diseases.
Given the insufficient level of knowledge in society regarding the causes and complications of excess body weight, the program had a significant societal character by introducing health-promoting measures and improving the awareness of the entire patient environment, especially their families and schools. Cooperation with educational institutions was crucial to ensure that preventive and intervention measures were as effective as possible. Importantly, this study had a societal nature and was directed at a large percentage of recipients to provide real assistance for as many children as possible. Furthermore, this research aimed to demonstrate how to formulate preventive and intervention programs to ensure their long-term effectiveness at the highest level.
4. Discussion
The prevalence of excessive body weight in studied population exceeded 23%, with 16.9% patients classified as overweight and 6.4% as obese. The most pronounced effects of decreasing anthropometric parameters were evident up to the 3rd visit (within the initial 6 months of the program), diminishing thereafter due to the extended interval between the 3rd and 4th visits (6 months). Notably, a gradual decline in both BMI percentile and BMI z-score was observed during all visits [
19]. The intervention program successfully achieved the intended effects inhibition of the increase in individual’s anthropometric parameters.
The primary objective of this study was an precise analysis of the biochemical parameters in the examined children, encompassing parameters related to carbohydrate and lipid metabolism, as well as the concentration of liver enzymes, serving as early indicators of liver steatosis. Initially, 4.7% of children exhibited abnormal fasting glycemia (>99 mg/dL), and 3.1% showed elevated insulin levels (>24.9 μIU/mL). Data were assessed based on two cut-off points for the HOMA-IR index: 2.5 and 3.16. A HOMA-IR index > 3.16 was identified in 21.8%, and >2.5 in 35.0% of the examined children. The relatively low percentage of children with abnormal glucose and insulin fasting levels may be attributed to the young age of the patients and the moderate degree of obesity. It is worth noting, that still significant proportion of children exhibited an elevated HOMA-IR index, indicating early disturbances in glucose homeostasis associated with excessive body weight. This underscores that impaired glucose levels or pre-diabetes manifest later than insulin resistance, which remains the dominant abnormality in glucose metabolism in children with obesity. It is plausible that fasting glucose levels within the norm range are maintained through a compensatory mechanism based on hyperinsulinemia, as reflected by HOMA-IR values. A simple criterion for assessing insulin resistance is the presence of fasting hyperinsulinemia. According to the 2012 OSCA recommendations (Obesity Services for Children), fasting insulin levels should be interpreted based on the degree of sexual maturation. For children in Tanner stages 1 and 2, fasting insulin levels should not exceed 15 mIU/L [
20]. In our study, a more permissive cut-off point for insulin (24.9 mIU/L) was adopted in alignment with the laboratory standards used for evaluating biochemical parameters. The HOMA-IR (homeostasis model assessment index) is a relatively straightforward method for assessing insulin resistance [
20,
21]. Thus far, no specific threshold value has been established above which the HOMA IR index in the pediatric patient group indicates pathology. Some authors suggest that insulin resistance can be identified if the HOMA-IR is greater than 2 or 3 [
2]. In adults, an HOMA-IR index > 2.5 indicates insulin resistance. However, Keskina et al., in their study, proposed a value greater than 3.16 [
22]. Shashaj stated that values exceeding 1.68 in individuals with normal body weight signify a “non-physiological state” (3.42 in the group of children and adolescents) and may pose an elevated risk of cardiovascular diseases [
23]. The 2012 OSCA guidelines recommended an HOMA-IR value > 4.5 as a cut-off point defining insulin resistance in children with excess body weight [
20]. Due to considerable disparities in the interpretation of the HOMA-IR index, the decision was made to conduct the analysis based on two cut-off points. Physiological insulin resistance occurs during adolescence when insulin sensitivity decreases by 25–50%. This trend improves after maturation is completed. Unfortunately, insulin resistance in adolescence is often exacerbated by excess body weight [
24]. Genetic, familial, and ethnic factors likely play a more substantial role in the development of insulin resistance than its severity or duration. This might elucidate why insulin resistance is not consistently diagnosed in children with advanced obesity. Nevertheless, the risk of developing insulin resistance escalates proportionally with visceral adipose tissue mass [
2].
Some studies suggest that physical activity may exert a greater impact on improving insulin sensitivity than reducing the body mass index (BMI). Modifying dietary habits and increasing physical activity seem to be the most important interventions even if they are not followed by BMI decrease [
25]. Regrettably, the current level of physical activity in children, especially those with excess body weight, is very low. A study conducted in 2018 in Szczecin, Poland, in which the main author participated, presented data evaluating physical fitness in a cohort of 3321 eight- and nine-year-old children. An analysis based on the Kasch Pulse Recovery Test Step revealed very poor physical fitness (test termination before completion due to excessively high heart rate HR > 180/min) in 151 children (4.5%), very poor physical fitness in 234 children (7%), poor physical fitness in 827 children (24.9%), satisfactory physical fitness in 961 children (29.2%), good in 650 (19.5%), very good in 428 children (12.8%), and excellent in 70 children (2.1%). Unfortunately, more than 1/3 of the tested population achieved an unsatisfactory physical fitness result [
26]. The presented results of the study on carbohydrate metabolism disorders may further indicate the need for the application of different, higher norms for both insulin concentrations and insulin resistance indices during the adolescent period. These topics remain controversial and debatable [
20,
27,
28]. It may also highlight the significant impact of excess body weight on metabolic disorders that appear relatively early, despite young age and despite improvements in anthropometric parameters.
Both parameters evaluating carbohydrate metabolism and those evaluating lipid metabolism could be specific indicators of increased risk of early disorders and complications related to excess body weight. Abnormalities in triglycerides compared to other lipids were distinctly noticeable, serving as the primary marker of lipid disorders associated with an improper diet and high consumption of simple sugars. Apart from elevated triglyceride levels, the most frequently observed disorder was elevated LDL cholesterol level. It is worth noting the significantly high percentage of borderline lipid levels in the studied population. Given the age of the studied children and the fact that they were not only obese but also overweight, such significant deviations in laboratory tests were not expected. Abnormalities in the studied biochemical parameters were found in over half of the examined population. In the analyzed group, lipid metabolism disorders were more prevalent than deviations in carbohydrate metabolism. It has also been demonstrated that the risk of developing lipid disorders is 2.8-fold higher in children with a BMI > 90th percentile compared to children with normal body weight. The prevalence of dyslipidemia in the population of obese children ranges from 27% to 43% [
29]. Both HDL cholesterol and triglyceride concentrations are proportional to visceral adipose tissue mass, unlike LDL cholesterol levels. It is important to note that the unfavorable lipid profile and metabolic disturbances observed in children with excess body weight can persist into adulthood. Excessive dietary fat intake, as well as other poor dietary habits, can lead to dyslipidemia regardless of whether they affect weight gain. The most common dyslipidemia among obese children is hypo-HDL-cholesterolemia. The triglyceride-to-HDL cholesterol ratio (TG/HDL-C) is associated with increased BMI and cardiometabolic risk factors [
2,
30], and it also serves as a good marker for insulin resistance and diabetes [
31]. A similar study focusing on the evaluation of biochemical parameters was conducted on a population of children with obesity in Portugal. The authors found decreased HDL cholesterol levels in 11% and elevated triglyceride levels in 13.4% of the studied population. No child showed elevated fasting blood glucose, but hyperinsulinemia was detected in 7.3% (>15 µUI/mL) and insulin resistance in 8.5% of the study group [
32]. In our study, the percentage of children with decreased HDL cholesterol fraction was equal to 9.7%, while the percentage of children with elevated triglyceride levels was noticeably higher, equal to 21.2%. In a large Polish study conducted by Brzeziński between 2011 and 2017 as part of the “6-10-14 for Health” program, lipid, carbohydrate, liver enzyme, and TSH parameters were evaluated in children with overweight or obesity. This study revealed that nearly 40% of children with excess body weight were diagnosed with at least one abnormality in the lipid profile, with no statistically significant difference based on the gender of the children. In our study, this percentage was even higher, equal to 52.9%, and confirmed no gender-based significance. In Brzeziński’s study, the most frequently observed disorders were decreased HDL cholesterol levels in about 22% of children and increased LDL cholesterol levels in 15% of the studied population [
12]. In our analysis, the most common lipid disorders were hypertriglyceridemia and elevated LDL cholesterol levels. Another study conducted by Nielsen et al., assessing lipid metabolism among Danish children, showed that lipid disorders occurred in 28% of the studied population and were significantly more common among girls [
29]. A German study by Dathan-Stumpf et al. in 2016 revealed lipid disorders in 24.7% of children [
33]. The frequent occurrence of lipid disorders in the pediatric population should serve as an important warning signal both at the individual and population levels. Importantly, screening tests used to diagnose lipid disorders should be applied from the earliest years. Changing dietary habits and increasing physical activity are crucial to improve and normalize lipid profile parameters. Juárez-López et al. evaluated 11–13-year-old Mexican children for complications related to excess body weight. They diagnosed hypolipoproteinemia in 69%, hypertriglyceridemia in 29%, fasting glucose abnormalities in 4%, and insulin resistance in 51% of the participants. They concluded that regardless of age and gender, a higher degree of insulin resistance is associated with a higher frequency of all components of the metabolic syndrome [
34]. Deeb et al. assessed 216 children from Saudi Arabia with an average age of 10.58 ± 2.97 years. The majority of this group (93%) were children with obesity. They diagnosed dyslipidemia in 55.3%, from which 11.7% had elevated total cholesterol levels, 28.6% had elevated triglyceride levels, 32.7% had elevated LDL cholesterol levels, and 18.0% had low HDL cholesterol levels, emphasizing the significantly increased risk of developing complications associated with excess body weight [
35].
Another important part of this study was the analysis of changes in biochemical parameters after a year of specialized interventions targeted at the children and their families. When comparing the differences in selected biochemical parameters between the 1st and 4th visits in the group of children who completed all specialized visits (Group 4) and underwent laboratory tests (n = 117), a statistically significant increase in glucose concentration by 2.15 ± 8.24 mg/dL, insulin by 2.72 ± 7.09 mg/dL, HOMA-IR by 0.68 ± 1.77, and LDL cholesterol fraction by 3.20 ± 17.0 mg/dL was observed. For other biochemical parameters, an increase in concentration was also noted but without statistical significance. It is worth noting that there was a decrease in the concentration of liver enzymes observed between the 1st and 4th visits and an increase in triglyceride levels, although without statistical significance, which would confirm the impact of dietary changes on triglyceride reduction. Biochemical parameters obtained during the first visit and the fourth visit from children who completed all specialized visits but excluding those who did not undergo laboratory tests after the last visit were compared. Similarly to the previous analysis, a statistically significant increase was observed in glucose concentration, LDL cholesterol fraction, insulin, and HOMA-IR. Additionally, a decrease in AST concentration was found at the borderline of statistical significance. What is more, increased ALT and glucose concentrations were observed in boys (during the first visit), while in the group of girls, there was an increase in carbohydrate metabolism parameters during the fourth visit. This effect could be attributed to the physiologically faster maturation and greater insulin resistance during this period in girls. Consequently, based on the obtained results of the analysis, it was questioned whether insulin concentration norms should be adjusted for gender in addition to age for children. Portuguese researchers observed significantly higher ALT concentrations in boys in their study group. Moreover, there was also a tendency for glucose, insulin, HOMA-IR index, and lipid concentrations (except HDL) to increase in boys, although without statistical significance [
30]. The results align with the analysis conducted during this study. On the contrary, Holst-Schumacher et al., in their research, found higher insulin concentrations and HOMA-IR index accompanied by lower HDL cholesterol concentrations in girls [
36]. Brzeziński emphasized significantly frequent elevated triglyceride in boys with excessive body weight [
12]. In our study, no relationship was found between the gender of the examined children and lipid disorders. Interestingly, a study on cardiovascular risk conducted among young Finns revealed that after 21 years of observation, patients with a complex pattern of dyslipidemia that started in early childhood had significantly increased carotid intima-media thickness (cIMT) compared to the control group with normal lipid levels [
37].
When analyzing individual biochemical parameters, the degree of obesity was taken into consideration. As expected, the higher the body weight, the more complications were found. Higher ALT concentrations were found in obese children compared to overweight, both during the first and fourth visits. In the group of overweight children, ALT levels decreased by 5 U/L between visits, while in the group of obese children, there was an increase of 1.42 U/L. These findings highlight that ALT can be a sensitive marker of liver cell dysfunction in the course of obesity and can increase rapidly with the degree of excessive body weight. However, this conclusion is based on a quite small-sized group.
During the first visit to the center, higher HDL concentrations were observed in the group of children with overweight, and higher HOMA IR, triglyceride, and insulin levels were noted in children with obesity. Higher values of the HOMA-IR index, indicating elevated levels of both glucose and insulin, were observed in obese children during the first visit and were borderline significant during the fourth visit. The observed relationships between HDL cholesterol, triglycerides, and insulin levels within the group of children with obesity were evident only during the first visits. This could result from the improvement in both anthropometric and biochemical parameters in the group of obese children after the completion of the intervention program. However, It is worth noting that these associations are characterized by a low correlation coefficient. Holst-Schumacher also described significantly higher levels of insulin, the HOMA-IR index, and triglycerides in the group of obese children, as well as lower levels of HDL within the same group of patients [
36]. Moreover, research demonstrates that each component of the metabolic syndrome worsens with an increase in body weight. This association is independent of age, gender, and maturation. The prevalence of the metabolic syndrome significantly increases with growing insulin resistance as well [
38].
Taking into account the debatable effectiveness of therapeutic interventions in the treatment of obesity and its complications, there has been a growing interest in preventive strategies. The evaluation of the results presented in this study raises questions about the effectiveness of preventive programs. Based on conducted research, it is evident that initial engagement in the program is high, but motivation tends to decrease over time. It is worth considering the frequency of specialized visits in both preventive programs and outpatient care for the patients with obesity. In this study, patients experienced deterioration of the results mostly between the 3rd and 4th visit, where the gap between visits was the longest, lasting 6 months. This suggests that a 3-month interval between checkpoints may be optimal to maintain motivation and achieve better results. The literature related to intervention programs is extensive, and research results are inconclusive. It is challenging to compare programs due to the diversity of interventions and the involvement of different patient environments. Moreover, limitations in interpretation arise from the lack of data on the long-term effects of the programs and their cost-effectiveness. Waters et al., in their study, confirmed the effectiveness of preventive interventions targeted at children with excess body weight aged 6 to 12 years [
39]. The effectiveness of multidisciplinary intervention programs was confirmed by other authors evaluating a similar age group [
40,
41], however, there are also studies with less optimistic findings regarding the effectiveness of such programs [
42]. It can be concluded that only integrated actions can increase awareness among patients and reverse the growing trend of obesity worldwide. For the greatest success, these actions must be coherent, interdisciplinary, and combine efforts from various environments. A priority area for future research should be preventing obesity from early childhood and shaping health-promoting behaviors at every stage of children’s development. Further research is essential to confirm the effectiveness of implemented interventions.