Current and Emerging Therapies for Chronic Subjective Tinnitus
Abstract
1. Introduction
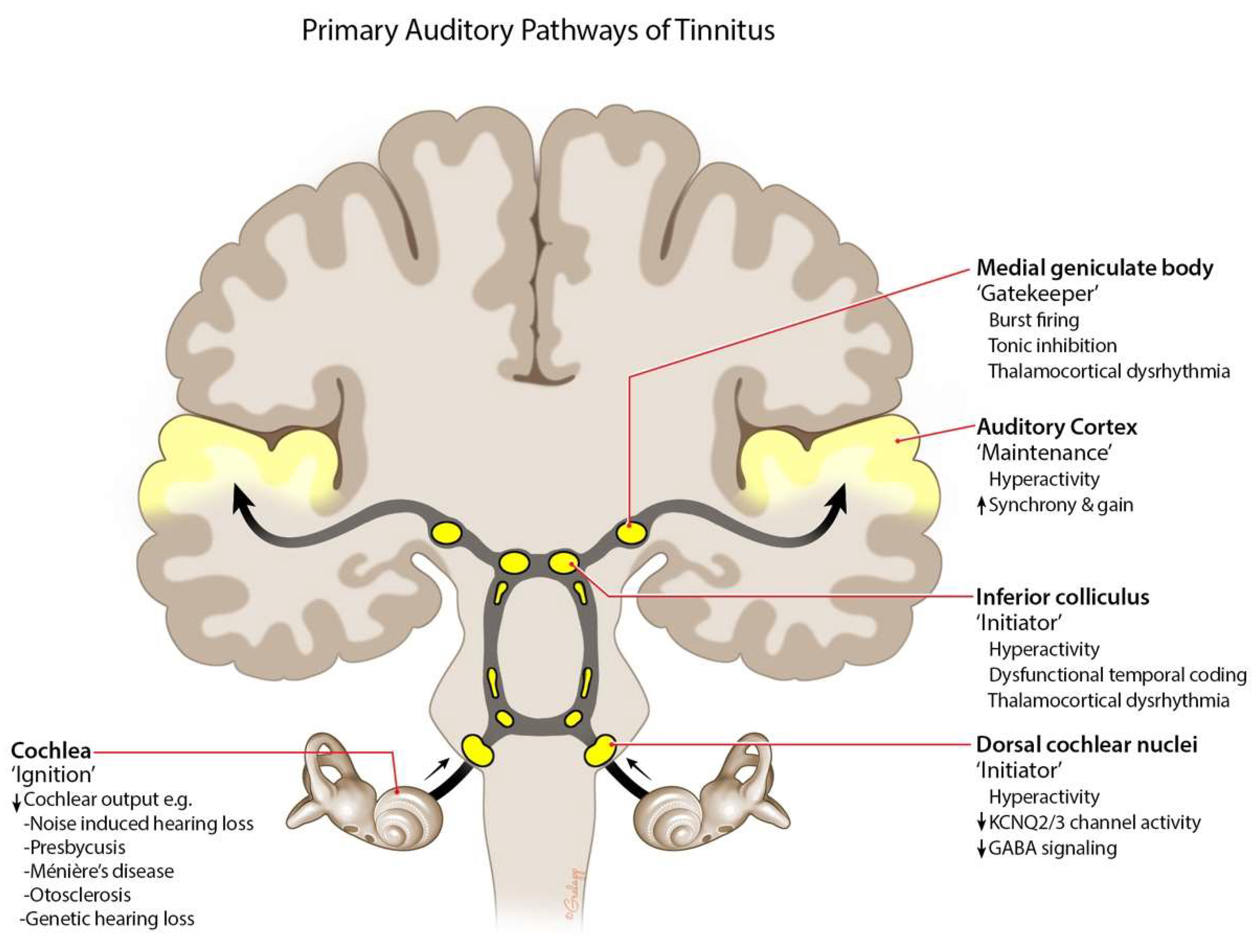
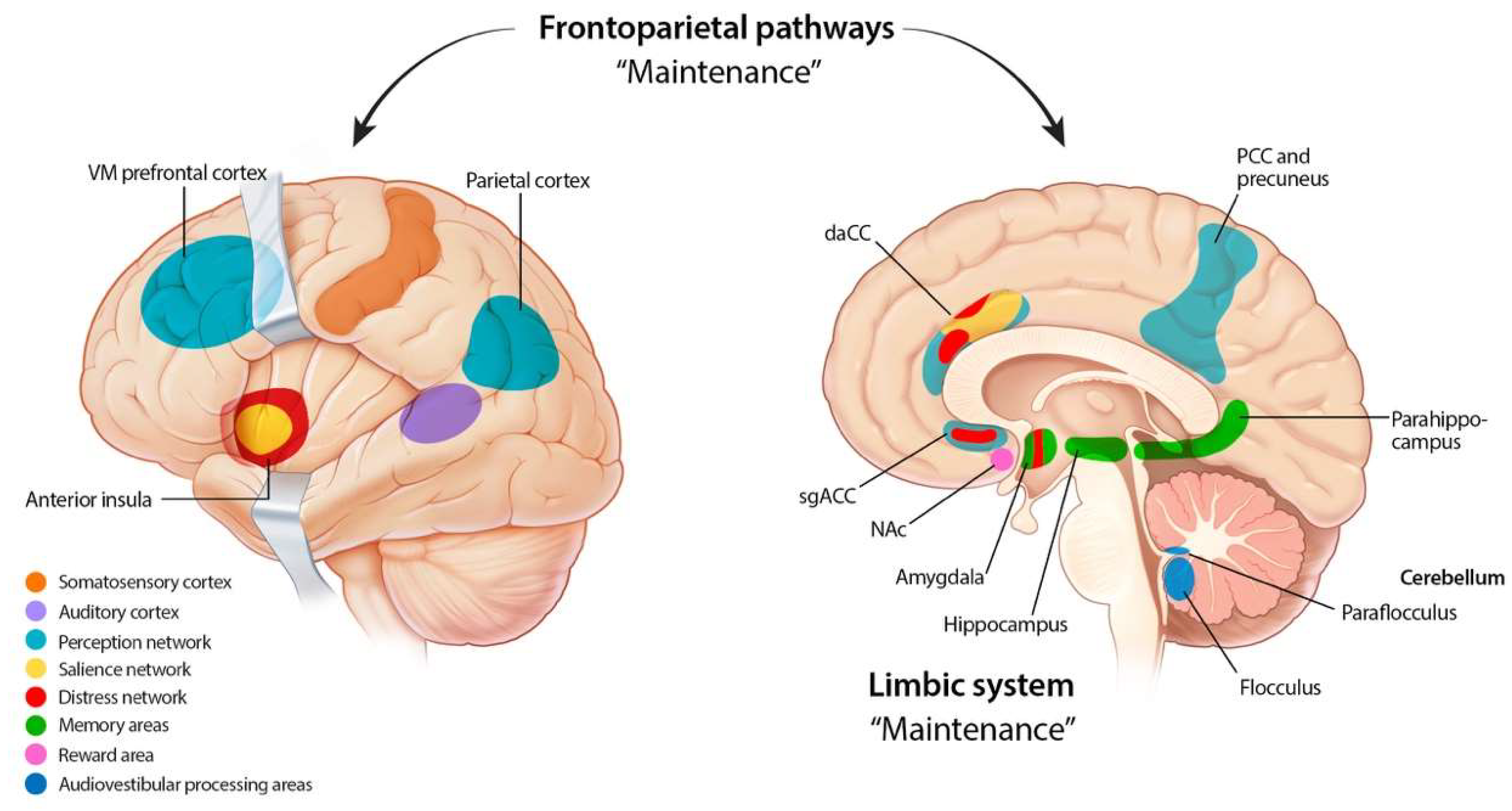
2. Etiology and Pathophysiology
3. Current Options for Tinnitus
3.1. CBT
3.2. Hearing Aids
3.3. Sound Therapy
3.4. Eye-Movement Desensitization Reprocessing
3.5. Cochlear Implantation
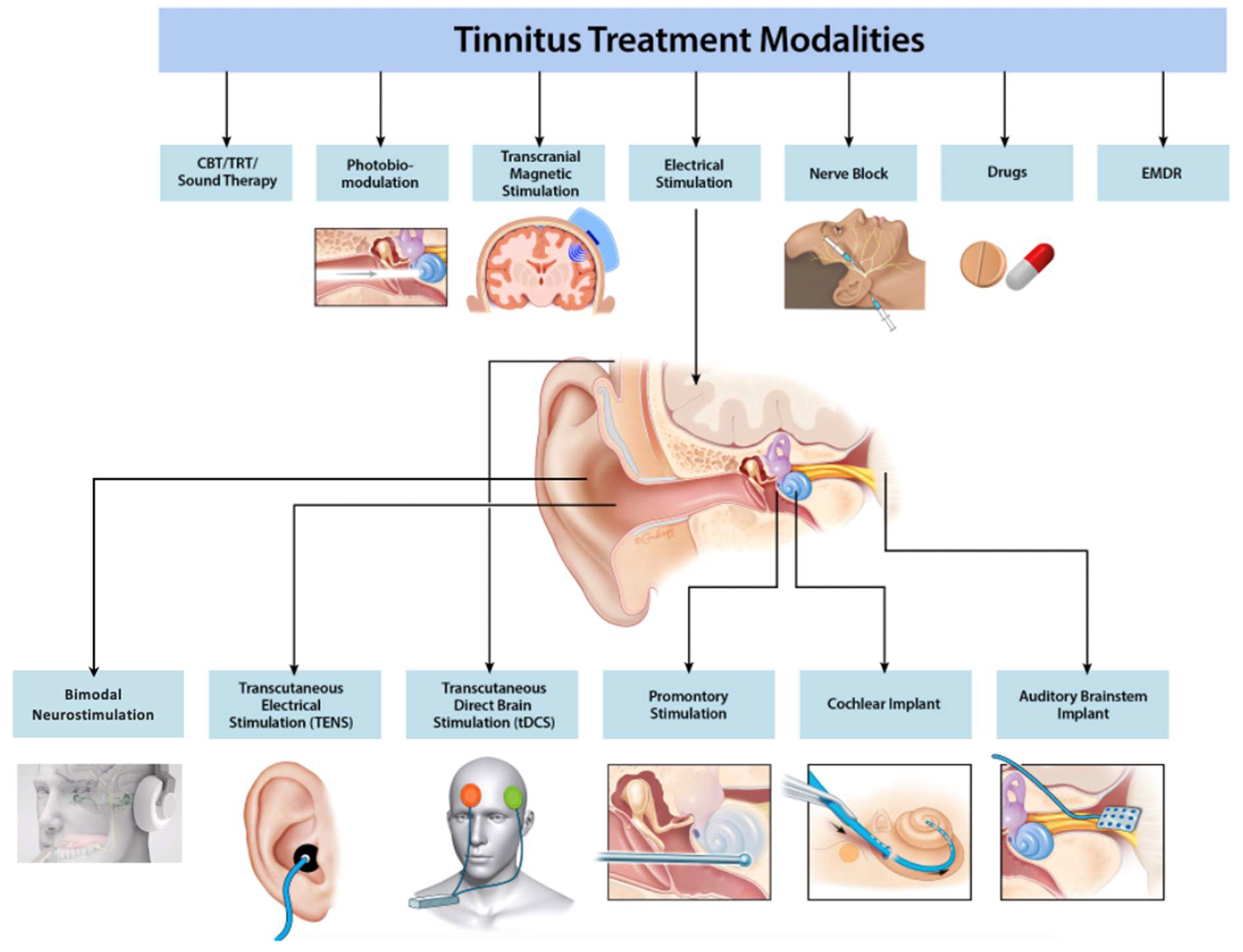
4. Emerging Options for Tinnitus
4.1. Electrical Stimulation
4.1.1. Transcranial and Transcutaneous Stimulation
4.1.2. Promontory and Round Window Stimulation
4.2. Repetitive Transcranial Magnetic Stimulation
4.3. Nerve Block
4.4. Bimodal Neuromodulation
4.5. Pharmaceutical Therapy
5. Other Limited Evidence Treatments
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Dan, B. Titus’s tinnitus. J. Hist. Neurosci. 2005, 14, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Vielsmeier, V.; Santiago Stiel, R.; Kwok, P.; Langguth, B.; Schecklmann, M. From Acute to Chronic Tinnitus: Pilot Data on Predictors and Progression. Front. Neurol. 2020, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Greco, A.; Turchetta, R.; Altissimi, G.; de Vincentiis, M.; Cianfrone, G. Somatosensory tinnitus: Current evidence and future perspectives. J. Int. Med. Res. 2017, 45, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Shargorodsky, J.; Curhan, G.C.; Farwell, W.R. Prevalence and characteristics of tinnitus among US adults. Am. J. Med. 2010, 123, 711–718. [Google Scholar] [CrossRef]
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global Prevalence and Incidence of Tinnitus: A Systematic Review and Meta-Analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef]
- Hallam, R.; McKenna, L.; Shurlock, L. Tinnitus impairs cognitive efficiency. Int. J. Audiol. 2004, 43, 218–226. [Google Scholar] [CrossRef]
- Roberts, L.E.; Husain, F.T.; Eggermont, J.J. Role of attention in the generation and modulation of tinnitus. Neurosci. Biobehav. Rev. 2013, 37, 1754–1773. [Google Scholar] [CrossRef]
- Husain, F.T.; Akrofi, K.; Carpenter-Thompson, J.R.; Schmidt, S.A. Alterations to the attention system in adults with tinnitus are modality specific. Brain Res. 2015, 1620, 81–97. [Google Scholar] [CrossRef]
- Manche, S.K.; Madhavi, J.; Meganadh, K.R.; Jyothy, A. Association of tinnitus and hearing loss in otological disorders: A decade-long epidemiological study in a South Indian population. Braz. J. Otorhinolaryngol. 2016, 82, 643–649. [Google Scholar] [CrossRef]
- Ahmad, N.; Seidman, M. Tinnitus in the older adult: Epidemiology, pathophysiology and treatment options. Drugs Aging 2004, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Shetye, A.; Kennedy, V. Tinnitus in children: An uncommon symptom? Arch. Dis. Child. 2010, 95, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Rosing, S.N.; Schmidt, J.H.; Wedderkopp, N.; Baguley, D.M. Prevalence of tinnitus and hyperacusis in children and adolescents: A systematic review. BMJ Open 2016, 6, e010596. [Google Scholar] [CrossRef] [PubMed]
- Humphriss, R.; Hall, A.J.; Baguley, D.M. Prevalence and characteristics of spontaneous tinnitus in 11-year-old children. Int. J. Audiol. 2016, 55, 142–148. [Google Scholar] [CrossRef]
- De Ridder, D.; Schlee, W.; Vanneste, S.; Londero, A.; Weisz, N.; Kleinjung, T.; Shekhawat, G.S.; Elgoyhen, A.B.; Song, J.J.; Andersson, G.; et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 2021, 260, 1–25. [Google Scholar] [CrossRef]
- Goldstein, E.; Ho, C.X.; Hanna, R.; Elinger, C.; Yaremchuk, K.L.; Seidman, M.D.; Jesse, M.T. Cost of care for subjective tinnitus in relation to patient satisfaction. Otolaryngol. Head Neck Surg. 2015, 152, 518–523. [Google Scholar] [CrossRef]
- Stockdale, D.; McFerran, D.; Brazier, P.; Pritchard, C.; Kay, T.; Dowrick, C.; Hoare, D.J. An economic evaluation of the healthcare cost of tinnitus management in the UK. BMC Health Serv. Res. 2017, 17, 577. [Google Scholar] [CrossRef]
- Treating and Curing Tinnitus Is Part of Our National Commitment to Veterans. Available online: https://www.ata.org/treating-and-curing-tinnitus-is-part-of-our-national-commitment-to-veterans/ (accessed on 20 June 2023).
- Sindhusake, D.; Golding, M.; Wigney, D.; Newall, P.; Jakobsen, K.; Mitchell, P. Factors predicting severity of tinnitus: A population-based assessment. J. Am. Acad. Audiol. 2004, 15, 269–280. [Google Scholar] [CrossRef]
- Cresswell, M.; Casanova, F.; Beaumont, R.N.; Wood, A.R.; Ronan, N.; Hilton, M.P.; Tyrrell, J. Understanding Factors That Cause Tinnitus: A Mendelian Randomization Study in the UK Biobank. Ear Hear. 2022, 43, 70–80. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, H.J.; An, S.Y.; Sim, S.; Park, B.; Kim, S.W.; Lee, J.S.; Hong, S.K.; Choi, H.G. Analysis of the prevalence and associated risk factors of tinnitus in adults. PLoS ONE 2015, 10, e0127578. [Google Scholar] [CrossRef]
- Veile, A.; Zimmermann, H.; Lorenz, E.; Becher, H. Is smoking a risk factor for tinnitus? A systematic review, meta-analysis and estimation of the population attributable risk in Germany. BMJ Open 2018, 8, e016589. [Google Scholar] [CrossRef] [PubMed]
- Dawes, P.; Cruickshanks, K.J.; Marsden, A.; Moore, D.R.; Munro, K.J. Relationship between Diet, Tinnitus, and Hearing Difficulties. Ear Hear. 2020, 41, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Chang, T.Y.; Tyler, R.; Lin, Y.J.; Liang, W.M.; Shau, Y.W.; Lin, W.Y.; Chen, Y.W.; Lin, C.D.; Tsai, M.H. Noise Induced Hearing Loss and Tinnitus-New Research Developments and Remaining Gaps in Disease Assessment, Treatment, and Prevention. Brain Sci. 2020, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Dille, M.F.; Konrad-Martin, D.; Gallun, F.; Helt, W.J.; Gordon, J.S.; Reavis, K.M.; Bratt, G.W.; Fausti, S.A. Tinnitus onset rates from chemotherapeutic agents and ototoxic antibiotics: Results of a large prospective study. J. Am. Acad. Audiol. 2010, 21, 409–417. [Google Scholar] [CrossRef]
- Bhatt, J.M.; Bhattacharyya, N.; Lin, H.W. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope 2017, 127, 466–469. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X.; Yuan, J.; Wu, Y.; Li, Y.; Tong, B.; Qiu, J.; Wu, F.; Liu, Y. Characteristics of tinnitus and factors influencing its severity. Ther. Adv. Chronic Dis. 2022, 13, 20406223221109656. [Google Scholar] [CrossRef]
- Gothelf, D.; Farber, N.; Raveh, E.; Apter, A.; Attias, J. Hyperacusis in Williams syndrome: Characteristics and associated neuroaudiologic abnormalities. Neurology 2006, 66, 390–395. [Google Scholar] [CrossRef]
- Barozzi, S.; Soi, D.; Comiotto, E.; Borghi, A.; Gavioli, C.; Spreafico, E.; Gagliardi, C.; Selicorni, A.; Forti, S.; Ambrosetti, U.; et al. Audiological findings in Williams syndrome: A study of 69 patients. Am. J. Med. Genet. A 2012, 158A, 759–771. [Google Scholar] [CrossRef]
- Henton, A.; Tzounopoulos, T. What’s the buzz? The neuroscience and the treatment of tinnitus. Physiol. Rev. 2021, 101, 1609–1632. [Google Scholar] [CrossRef]
- Jastreboff, P.J.; Gray, W.C.; Gold, S.L. Neurophysiological approach to tinnitus patients. Am. J. Otol. 1996, 17, 236–240. [Google Scholar]
- Liberman, M.C.; Dodds, L.W. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear. Res. 1984, 16, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Vielsmeier, V.; Lehner, A.; Strutz, J.; Steffens, T.; Kreuzer, P.M.; Schecklmann, M.; Landgrebe, M.; Langguth, B.; Kleinjung, T. The Relevance of the High Frequency Audiometry in Tinnitus Patients with Normal Hearing in Conventional Pure-Tone Audiometry. Biomed. Res. Int. 2015, 2015, 302515. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, Y.; Tsuji, J.; Naito, Y.; Fujiki, N.; Yamamoto, N. Characteristics of DPOAE audiogram in tinnitus patients. Hear. Res. 1997, 108, 83–88. [Google Scholar] [CrossRef]
- Chen, G.D.; Tanaka, C.; Henderson, D. Relation between outer hair cell loss and hearing loss in rats exposed to styrene. Hear. Res. 2008, 243, 28–34. [Google Scholar] [CrossRef]
- Sereda, M.; Hall, D.A.; Bosnyak, D.J.; Edmondson-Jones, M.; Roberts, L.E.; Adjamian, P.; Palmer, A.R. Re-examining the relationship between audiometric profile and tinnitus pitch. Int. J. Audiol. 2011, 50, 303–312. [Google Scholar] [CrossRef]
- Wazen, J.J.; Foyt, D.; Sisti, M. Selective cochlear neurectomy for debilitating tinnitus. Ann. Otol. Rhinol. Laryngol. 1997, 106, 568–570. [Google Scholar] [CrossRef]
- Jastreboff, P.J. Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neurosci. Res. 1990, 8, 221–254. [Google Scholar] [CrossRef]
- Knipper, M.; van Dijk, P.; Schulze, H.; Mazurek, B.; Krauss, P.; Scheper, V.; Warnecke, A.; Schlee, W.; Schwabe, K.; Singer, W.; et al. The neural bases of tinnitus: Lessons from deafness and cochlear implants. J. Neurosci. 2020, 40, 7190–7202. [Google Scholar] [CrossRef]
- Shinden, S.; Suzuki, N.; Oishi, N.; Suzuki, D.; Minami, S.; Ogawa, K. Effective sound therapy using a hearing aid and educational counseling in patients with chronic tinnitus. Auris Nasus Larynx 2021, 48, 815–822. [Google Scholar] [CrossRef]
- Brozoski, T.J.; Bauer, C.A. Animal models of tinnitus. Hear. Res. 2016, 338, 88–97. [Google Scholar] [CrossRef]
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus. Trends Neurosci. 2004, 27, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kalappa, B.I.; Brozoski, T.J.; Turner, J.G.; Caspary, D.M. Single unit hyperactivity and bursting in the auditory thalamus of awake rats directly correlates with behavioural evidence of tinnitus. J. Physiol. 2014, 592, 5065–5078. [Google Scholar] [CrossRef] [PubMed]
- Vogler, D.P.; Robertson, D.; Mulders, W.H. Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J. Neurosci. 2011, 31, 6639–6645. [Google Scholar] [CrossRef] [PubMed]
- Melcher, J.R.; Levine, R.A.; Bergevin, C.; Norris, B. The auditory midbrain of people with tinnitus: Abnormal sound-evoked activity revisited. Hear. Res. 2009, 257, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, C.; Martel, D.T.; West, M.; Sutton, M.A.; Shore, S.E. Remodeling of cholinergic input to the hippocampus after noise exposure and tinnitus induction in Guinea pigs. Hippocampus 2019, 29, 669–682. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, C.; Martel, D.T.; West, M.; Sutton, M.A.; Shore, S.E. Noise Exposure Alters Glutamatergic and GABAergic Synaptic Connectivity in the Hippocampus and Its Relevance to Tinnitus. Neural Plast. 2021, 2021, 8833087. [Google Scholar] [CrossRef]
- Li, S.; Kalappa, B.I.; Tzounopoulos, T. Noise-induced plasticity of KCNQ2/3 and HCN channels underlies vulnerability and resilience to tinnitus. Elife 2015, 4, e07242. [Google Scholar] [CrossRef]
- Davies, J.E.; Gander, P.E.; Hall, D.A. Does Chronic Tinnitus Alter the Emotional Response Function of the Amygdala? A Sound-Evoked fMRI Study. Front. Aging Neurosci. 2017, 9, 31. [Google Scholar] [CrossRef]
- Meijers, S.M.; Rademaker, M.; Meijers, R.L.; Stegeman, I.; Smit, A.L. Correlation between Chronic Tinnitus Distress and Symptoms of Depression: A Systematic Review. Front. Neurol. 2022, 13, 870433. [Google Scholar] [CrossRef]
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus: Understanding abnormal and normal auditory perception. Front. Syst. Neurosci. 2012, 6, 53. [Google Scholar] [CrossRef]
- Hullfish, J.; Sedley, W.; Vanneste, S. Prediction and perception: Insights for (and from) tinnitus. Neurosci. Biobehav. Rev. 2019, 102, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carpenter-Thompson, J.R.; Schmidt, S.; McAuley, E.; Husain, F.T. Increased Frontal Response May Underlie Decreased Tinnitus Severity. PLoS ONE 2015, 10, e0144419. [Google Scholar] [CrossRef] [PubMed]
- Brozoski, T.J.; Ciobanu, L.; Bauer, C.A. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI). Hear. Res. 2007, 228, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Brozoski, T.; Brozoski, D.; Wisner, K.; Bauer, C. Chronic tinnitus and unipolar brush cell alterations in the cerebellum and dorsal cochlear nucleus. Hear. Res. 2017, 350, 139–151. [Google Scholar] [CrossRef]
- Bauer, C.A.; Wisner, K.W.; Baizer, J.S.; Brozoski, T.J. Tinnitus, unipolar brush cells, and cerebellar glutamatergic function in an animal model. PLoS ONE 2013, 8, e64726. [Google Scholar] [CrossRef]
- Mennink, L.M.; Van Dijk, J.M.C.; Van Der Laan, B.; Metzemaekers, J.D.M.; Van Laar, P.J.; Van Dijk, P. The relation between flocculus volume and tinnitus after cerebellopontine angle tumor surgery. Hear. Res. 2018, 361, 113–120. [Google Scholar] [CrossRef]
- Chen, J.; Fan, L.; Cai, G.; Hu, B.; Xiong, Y.; Zhang, Z. Altered Amplitude of Low-Frequency Fluctuations and Degree Centrality in Patients with Acute Subjective Tinnitus: A Resting-State Functional Magnetic Resonance Imaging Study. J. Integr. Neurosci. 2022, 21, 116. [Google Scholar] [CrossRef]
- Haider, H.F.; Bojic, T.; Ribeiro, S.F.; Paco, J.; Hall, D.A.; Szczepek, A.J. Pathophysiology of Subjective Tinnitus: Triggers and Maintenance. Front. Neurosci. 2018, 12, 866. [Google Scholar] [CrossRef]
- Bogo, R.; Farah, A.; Karlsson, K.K.; Pedersen, N.L.; Svartengren, M.; Skjönsberg, Å. Prevalence, Incidence Proportion, and Heritability for Tinnitus: A Longitudinal Twin Study. Ear Hear. 2017, 38, 292–300. [Google Scholar] [CrossRef]
- Maas, I.L.; Brüggemann, P.; Requena, T.; Bulla, J.; Edvall, N.K.; Hjelmborg, J.V.B.; Szczepek, A.J.; Canlon, B.; Mazurek, B.; Lopez-Escamez, J.A.; et al. Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genet. Med. 2017, 19, 1007–1012. [Google Scholar] [CrossRef]
- Pawełczyk, M.; Rajkowska, E.; Kotyło, P.; Dudarewicz, A.; Van Camp, G.; Śliwińska-Kowalska, M. Analysis of inner ear potassium recycling genes as potential factors associated with tinnitus. Int. J. Occup. Med. Environ. Health 2012, 25, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Sand, P.G.; Langguth, B.; Kleinjung, T. Deep resequencing of the voltage-gated potassium channel subunit KCNE3 gene in chronic tinnitus. Behav. Brain Funct. 2011, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Sand, P.G.; Langguth, B.; Schecklmann, M.; Kleinjung, T. GDNF and BDNF gene interplay in chronic tinnitus. Int. J. Mol. Epidemiol. Genet. 2012, 3, 245–251. [Google Scholar]
- Clifford, R.E.; Maihofer, A.X.; Stein, M.B.; Ryan, A.F.; Nievergelt, C.M. Novel Risk Loci in Tinnitus and Causal Inference with Neuropsychiatric Disorders among Adults of European Ancestry. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 1015–1025. [Google Scholar] [CrossRef]
- Gilles, A.; Van Camp, G.; Van de Heyning, P.; Fransen, E. A Pilot Genome-Wide Association Study Identifies Potential Metabolic Pathways Involved in Tinnitus. Front. Neurosci. 2017, 11, 71. [Google Scholar] [CrossRef][Green Version]
- Tunkel, D.E.; Bauer, C.A.; Sun, G.H.; Rosenfeld, R.M.; Chandrasekhar, S.S.; Cunningham, E.R., Jr.; Archer, S.M.; Blakley, B.W.; Carter, J.M.; Granieri, E.C.; et al. Clinical practice guideline: Tinnitus. Otolaryngol. Head Neck Surg. 2014, 151, S1–S40. [Google Scholar] [CrossRef]
- Ogawa, K.; Sato, H.; Takahashi, M.; Wada, T.; Naito, Y.; Kawase, T.; Murakami, S.; Hara, A.; Kanzaki, S. Clinical practice guidelines for diagnosis and treatment of chronic tinnitus in Japan. Auris Nasus Larynx 2020, 47, 1–6. [Google Scholar] [CrossRef]
- Cima, R.F.F.; Mazurek, B.; Haider, H.; Kikidis, D.; Lapira, A.; Norena, A.; Hoare, D.J. A multidisciplinary European guideline for tinnitus: Diagnostics, assessment, and treatment. HNO 2019, 67, 10–42. [Google Scholar] [CrossRef]
- Conlon, B.; Hamilton, C.; Meade, E.; Leong, S.L.; Connor, O.C.; Langguth, B.; Vanneste, S.; Hall, D.A.; Hughes, S.; Lim, H.H. Different bimodal neuromodulation settings reduce tinnitus symptoms in a large randomized trial. Sci. Rep. 2022, 12, 10845. [Google Scholar] [CrossRef]
- Conlon, B.; Langguth, B.; Hamilton, C.; Hughes, S.; Meade, E.; Connor, C.O.; Schecklmann, M.; Hall, D.A.; Vanneste, S.; Leong, S.L.; et al. Bimodal neuromodulation combining sound and tongue stimulation reduces tinnitus symptoms in a large randomized clinical study. Sci. Transl. Med. 2020, 12, eabb2830. [Google Scholar] [CrossRef]
- Nakao, M.; Shirotsuki, K.; Sugaya, N. Cognitive-behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. Biopsychosoc. Med. 2021, 15, 16. [Google Scholar] [CrossRef]
- Jun, H.J.; Park, M.K. Cognitive behavioral therapy for tinnitus: Evidence and efficacy. Korean J. Audiol. 2013, 17, 101–104. [Google Scholar] [CrossRef]
- Fuller, T.; Cima, R.; Langguth, B.; Mazurek, B.; Vlaeyen, J.W.; Hoare, D.J. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst. Rev. 2020, 1, CD012614. [Google Scholar] [CrossRef] [PubMed]
- Landry, E.C.; Sandoval, X.C.R.; Simeone, C.N.; Tidball, G.; Lea, J.; Westerberg, B.D. Systematic Review and Network Meta-analysis of Cognitive and/or Behavioral Therapies (CBT) for Tinnitus. Otol. Neurotol. 2020, 41, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Devesa, P.; Perera, R.; Theodoulou, M.; Waddell, A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst. Rev. 2010, 9, CD005233. [Google Scholar] [CrossRef]
- Beukes, E.W.; Andersson, G.; Allen, P.M.; Manchaiah, V.; Baguley, D.M. Effectiveness of Guided Internet-Based Cognitive Behavioral Therapy vs Face-to-Face Clinical Care for Treatment of Tinnitus: A Randomized Clinical Trial. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- McKenna, L.; Marks, E.M.; Hallsworth, C.A.; Schaette, R. Mindfulness-Based Cognitive Therapy as a Treatment for Chronic Tinnitus: A Randomized Controlled Trial. Psychother. Psychosom. 2017, 86, 351–361. [Google Scholar] [CrossRef]
- Hesse, G. Evidence and evidence gaps in tinnitus therapy. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2016, 15, Doc04. [Google Scholar] [CrossRef]
- Hoare, D.J.; Edmondson-Jones, M.; Sereda, M.; Akeroyd, M.A.; Hall, D. Amplification with hearing aids for patients with tinnitus and co-existing hearing loss. Cochrane Database Syst. Rev. 2014, 1, CD010151. [Google Scholar] [CrossRef]
- Vernon, J. Attemps to relieve tinnitus. J. Am. Audiol. Soc. 1977, 2, 124–131. [Google Scholar]
- Hobson, J.; Chisholm, E.; El Refaie, A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst. Rev. 2012, 11, CD006371. [Google Scholar] [CrossRef]
- Henry, J.A.; Stewart, B.J.; Griest, S.; Kaelin, C.; Zaugg, T.L.; Carlson, K. Multisite Randomized Controlled Trial to Compare Two Methods of Tinnitus Intervention to Two Control Conditions. Ear Hear. 2016, 37, e346–e359. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.; Farrell, D.; Barron, I.; Hutchins, J.; Whybrow, D.; Kiernan, M.D. The use of eye-movement desensitization reprocessing (EMDR) therapy in treating post-traumatic stress disorder: A systematic narrative review. Front. Psychol. 2018, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Rikkert, M.; van Rood, Y.; de Roos, C.; Ratter, J.; van den Hout, M. A trauma-focused approach for patients with tinnitus: The effectiveness of eye movement desensitization and reprocessing—A multicentre pilot trial. Eur. J. Psychotraumatol. 2018, 9, 1512248. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.S.; Erskine, S.; Moore, T.; Nunney, I.; Wright, C. Eye movement desensitization and reprocessing as a treatment for tinnitus. Laryngoscope 2019, 129, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Luyten, T.R.; Jacquemin, L.; Van Looveren, N.; Declau, F.; Fransen, E.; Cardon, E.; De Bodt, M.; Topsakal, V.; Van de Heyning, P.; Van Rompaey, V.; et al. Bimodal Therapy for Chronic Subjective Tinnitus: A Randomized Controlled Trial of EMDR and TRT Versus CBT and TRT. Front. Psychol. 2020, 11, 2048. [Google Scholar] [CrossRef]
- Quaranta, N.; Wagstaff, S.; Baguley, D.M. Tinnitus and cochlear implantation. Int. J. Audiol. 2004, 43, 245–251. [Google Scholar] [CrossRef]
- Aschendorff, A.; Pabst, G.; Klenzner, T.; Laszig, R. Tinnitus in Cochlear Implant Users: The Freiburg Experience. Int. Tinnitus J. 1998, 4, 162–164. [Google Scholar]
- Poncet-Wallet, C.; Mamelle, E.; Godey, B.; Truy, E.; Guevara, N.; Ardoint, M.; Gnansia, D.; Hoen, M.; Saai, S.; Mosnier, I.; et al. Prospective Multicentric Follow-Up Study of Cochlear Implantation in Adults with Single-Sided Deafness: Tinnitus and Audiological Outcomes. Otol. Neurotol. 2020, 41, 458–466. [Google Scholar] [CrossRef]
- Ito, J. Tinnitus suppression in cochlear implant patients. Otolaryngol.–Head Neck Surg. 1997, 117, 701–703. [Google Scholar] [CrossRef]
- Miyamoto, R.T.; Bichey, B.G. Cochlear implantation for tinnitus suppression. Otolaryngol. Clin. N. Am. 2003, 36, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, W.; Cantore, I.; Cianfrone, F.; Melillo, P.; Scorpecci, A.; Paludetti, G. Tinnitus modifications after cochlear implantation. Eur. Arch. Otorhinolaryngol. 2007, 264, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Arts, R.A.; George, E.L.; Stokroos, R.J.; Vermeire, K. Review: Cochlear implants as a treatment of tinnitus in single-sided deafness. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.A.; Lee, J.A.; Nguyen, S.A.; McRackan, T.R.; Meyer, T.A.; Lambert, P.R. Cochlear Implantation for Treatment of Tinnitus in Single-sided Deafness: A Systematic Review and Meta-analysis. Otol. Neurotol. 2020, 41, e1004–e1012. [Google Scholar] [CrossRef] [PubMed]
- Punte, A.K.; Vermeire, K.; Hofkens, A.; De Bodt, M.; De Ridder, D.; Van de Heyning, P. Cochlear implantation as a durable tinnitus treatment in single-sided deafness. Cochlear Implants Int. 2011, 12 (Suppl. S1), S26–S29. [Google Scholar] [CrossRef]
- Arts, R.A.; Netz, T.; Janssen, A.M.; George, E.L.; Stokroos, R.J. The occurrence of tinnitus after CI surgery in patients with severe hearing loss: A retrospective study. Int. J. Audiol. 2015, 54, 910–917. [Google Scholar] [CrossRef]
- Richard, C.; Fayad, J.N.; Doherty, J.; Linthicum, F.H., Jr. Round window versus cochleostomy technique in cochlear implantation: Histologic findings. Otol. Neurotol. 2012, 33, 1181–1187. [Google Scholar] [CrossRef]
- Kloostra, F.J.J.; Verbist, J.; Hofman, R.; Free, R.H.; Arnold, R.; van Dijk, P. A Prospective Study of the Effect of Cochlear Implantation on Tinnitus. Audiol. Neurootol. 2018, 23, 356–363. [Google Scholar] [CrossRef]
- Olze, H.; Szczepek, A.J.; Haupt, H.; Forster, U.; Zirke, N.; Grabel, S.; Mazurek, B. Cochlear implantation has a positive influence on quality of life, tinnitus, and psychological comorbidity. Laryngoscope 2011, 121, 2220–2227. [Google Scholar] [CrossRef]
- Olze, H.; Szczepek, A.J.; Haupt, H.; Zirke, N.; Graebel, S.; Mazurek, B. The impact of cochlear implantation on tinnitus, stress and quality of life in postlingually deafened patients. Audiol. Neurootol. 2012, 17, 2–11. [Google Scholar] [CrossRef]
- Miyamoto, R.T.; Wynne, M.K.; McKnight, C.; Bichey, B. Electrical Suppression of Tinnitus via Cochlear Implants. Int. Tinnitus J. 1997, 3, 35–38. [Google Scholar] [PubMed]
- Kuo, M.-F.; Paulus, W.; Nitsche, M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 2014, 85, 948–960. [Google Scholar] [CrossRef]
- Mühlnickel, W.; Elbert, T.; Taub, E.; Flor, H. Reorganization of auditory cortex in tinnitus. Proc. Natl. Acad. Sci. USA 1998, 95, 10340–10343. [Google Scholar] [CrossRef]
- Rauschecker, J.P.; Leaver, A.M.; Mühlau, M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron 2010, 66, 819–826. [Google Scholar] [CrossRef]
- Schlee, W.; Schecklmann, M.; Lehner, A.; Kreuzer, P.M.; Vielsmeier, V.; Poeppl, T.B.; Langguth, B. Reduced variability of auditory alpha activity in chronic tinnitus. Neural Plast. 2014, 2014, 436146. [Google Scholar] [CrossRef] [PubMed]
- Arnold, W.; Bartenstein, P.; Oestreicher, E.; Römer, W.; Schwaiger, M. Focal metabolic activation in the predominant left auditory cortex in patients suffering from tinnitus: A PET study with [18F] deoxyglucose. ORL J. Otorhinolaryngol. Relat. Spec. 1996, 58, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Langguth, B.; Eichhammer, P.; Kreutzer, A.; Maenner, P.; Marienhagen, J.; Kleinjung, T.; Sand, P.; Hajak, G. The impact of auditory cortex activity on characterizing and treating patients with chronic tinnitus—First results from a PET study. Acta Oto-Laryngol. 2006, 126, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.D.S.; Almeida, A.A.; Andrade, S.; Machado, D.; Leitao, M.; Sanchez, T.G.; Rosa, M. Transcranial direct current stimulation improves tinnitus perception and modulates cortical electrical activity in patients with tinnitus: A randomized clinical trial. Neurophysiol. Clin. 2020, 50, 289–300. [Google Scholar] [CrossRef]
- Cavalcanti, K.; Brasil-Neto, J.P.; Allam, N.; Boechat-Barros, R. A Double-blind, Placebo-controlled Study of the Effects of Daily tDCS Sessions Targeting the Dorsolateral Prefrontal Cortex on Tinnitus Handicap Inventory and Visual Analog Scale Scores. Brain Stimul. 2015, 8, 978–980. [Google Scholar] [CrossRef]
- Pal, N.; Maire, R.; Stephan, M.A.; Herrmann, F.R.; Benninger, D.H. Transcranial Direct Current Stimulation for the Treatment of Chronic Tinnitus: A Randomized Controlled Study. Brain Stimul. 2015, 8, 1101–1107. [Google Scholar] [CrossRef]
- Forogh, B.; Mirshaki, Z.; Raissi, G.R.; Shirazi, A.; Mansoori, K.; Ahadi, T. Repeated sessions of transcranial direct current stimulation for treatment of chronic subjective tinnitus: A pilot randomized controlled trial. Neurol. Sci. 2016, 37, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, G.S.; Stinear, C.M.; Searchfield, G.D. Modulation of Perception or Emotion? A Scoping Review of Tinnitus Neuromodulation Using Transcranial Direct Current Stimulation. Neurorehabilit. Neural Repair 2015, 29, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Tutar, B.; Atar, S.; Berkiten, G.; Ustun, O.; Kumral, T.L.; Uyar, Y. The effect of transcutaneous electrical nerve stimulation (TENS) on chronic subjective tinnitus. Am. J. Otolaryngol. 2020, 41, 102326. [Google Scholar] [CrossRef] [PubMed]
- Kapkin, O.; Satar, B.; Yetiser, S. Transcutaneous electrical stimulation of subjective tinnitus. A placebo-controlled, randomized and comparative analysis. ORL J. Otorhinolaryngol. Relat. Spec. 2008, 70, 156–161. [Google Scholar] [CrossRef]
- Steenerson, R.L.; Cronin, G.W. Treatment of tinnitus with electrical stimulation. Otolaryngol.–Head Neck Surg. 1999, 121, 511–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Assouly, K.K.S.; Dullaart, M.J.; Stokroos, R.J.; van Dijk, B.; Stegeman, I.; Smit, A.L. Systematic Review on Intra- and Extracochlear Electrical Stimulation for Tinnitus. Brain Sci. 2021, 11, 1394. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, J.; Wang, B.; Zhang, W.; Xu, M.; Yang, S.; Liu, H. Electrical stimulation to treat tinnitus: A meta-analysis and systemic review of randomized controlled trials. Ther. Adv. Chronic Dis. 2021, 12, 20406223211041069. [Google Scholar] [CrossRef]
- Chen, S.; Du, M.; Wang, Y.; Li, Y.; Tong, B.; Qiu, J.; Wu, F.; Liu, Y. State of the art: Non-invasive electrical stimulation for the treatment of chronic tinnitus. Ther. Adv. Chronic Dis. 2023, 14, 20406223221148061. [Google Scholar] [CrossRef]
- Perez, R.; Shaul, C.; Vardi, M.; Muhanna, N.; Kileny, P.R.; Sichel, J.Y. Multiple electrostimulation treatments to the promontory for tinnitus. Otol. Neurotol. 2015, 36, 366–372. [Google Scholar] [CrossRef]
- Wenzel, G.I.; Sarnes, P.; Warnecke, A.; Stover, T.; Jager, B.; Lesinski-Schiedat, A.; Lenarz, T. Non-penetrating round window electrode stimulation for tinnitus therapy followed by cochlear implantation. Eur. Arch. Otorhinolaryngol. 2015, 272, 3283–3293. [Google Scholar] [CrossRef]
- Zeng, F.G.; Djalilian, H.; Lin, H. Tinnitus treatment with precise and optimal electric stimulation: Opportunities and challenges. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 382–387. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Novel Tinnitus Implant System for the Treatment of Chronic Severe Tinnitus. ClinicalTrials.gov Identifier: NCT03988699. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03988699 (accessed on 9 November 2022).
- Lefaucheur, J.P.; Andre-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, P.M.; Poeppl, T.B.; Vielsmeier, V.; Schecklmann, M.; Langguth, B.; Lehner, A. The more the merrier? Preliminary results regarding treatment duration and stimulation frequency of multisite repetitive transcranial magnetic stimulation in chronic tinnitus. Prog. Brain Res. 2021, 262, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Noh, T.S.; Kyong, J.S.; Chang, M.Y.; Park, M.K.; Lee, J.H.; Oh, S.H.; Kim, J.S.; Chung, C.K.; Suh, M.W. Comparison of Treatment Outcomes Following Either Prefrontal Cortical-only or Dual-site Repetitive Transcranial Magnetic Stimulation in Chronic Tinnitus Patients: A Double-blind Randomized Study. Otol. Neurotol. 2017, 38, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Roland, L.T.; Peelle, J.E.; Kallogjeri, D.; Nicklaus, J.; Piccirillo, J.F. The effect of noninvasive brain stimulation on neural connectivity in Tinnitus: A randomized trial. Laryngoscope 2016, 126, 1201–1206. [Google Scholar] [CrossRef]
- Durmaz, O.; Ates, M.A.; Senol, M.G. Repetitive Transcranial Magnetic Stimulation (rTMS)-Induced Trigeminal Autonomic Cephalalgia. Noro Psikiyatr. Ars. 2015, 52, 309–311. [Google Scholar] [CrossRef][Green Version]
- Khedr, E.M.; Abo-Elfetoh, N.; Rothwell, J.C.; El-Atar, A.; Sayed, E.; Khalifa, H. Contralateral versus ipsilateral rTMS of temporoparietal cortex for the treatment of chronic unilateral tinnitus: Comparative study. Eur. J. Neurol. 2010, 17, 976–983. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, D.Y.; Kim, S.K.; Kim, J.M.; Baek, S.H.; Moon, I.S. Comparison of the outcomes of repetitive transcranial magnetic stimulation to the ipsilateral and contralateral auditory cortex in unilateral tinnitus. Electromagn. Biol. Med. 2014, 33, 211–215. [Google Scholar] [CrossRef]
- Cole, E.J.; Stimpson, K.H.; Bentzley, B.S.; Gulser, M.; Cherian, K.; Tischler, C.; Nejad, R.; Pankow, H.; Choi, E.; Aaron, H.; et al. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression. Am. J. Psychiatry 2020, 177, 716–726. [Google Scholar] [CrossRef]
- Lan, L.; Liu, Y.; Wu, Y.; Xu, Z.G.; Xu, J.J.; Song, J.J.; Salvi, R.; Yin, X.; Chen, Y.C.; Cai, Y. Specific brain network predictors of interventions with different mechanisms for tinnitus patients. EBioMedicine 2022, 76, 103862. [Google Scholar] [CrossRef]
- Sirh, S.J.; Sirh, S.W.; Mun, H.Y.; Sirh, H.M. Integrative Treatment for Tinnitus Combining Repeated Facial and Auriculotemporal Nerve Blocks with Stimulation of Auditory and Non-auditory Nerves. Front. Neurosci. 2022, 16, 758575. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.; Kumar, S. Ultrasound-Guided Occipital Nerve Blocks to Reduce Tinnitus-Associated Otalgia: A Case Series. A A Pract. 2022, 16, e01552. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, J.I.; Sakai, N.; Uemi, N.; Ifukube, T. Effects of greater occipital nerve block on tinnitus and dizziness. Int. Tinnitus J. 1999, 5, 40–46. [Google Scholar] [PubMed]
- Marks, K.L.; Martel, D.T.; Wu, C.; Basura, G.J.; Roberts, L.E.; Schvartz-Leyzac, K.C.; Shore, S.E. Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci. Transl. Med. 2018, 10, eaal3175. [Google Scholar] [CrossRef] [PubMed]
- Koehler, S.D.; Shore, S.E. Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. PLoS ONE 2013, 8, e59828. [Google Scholar] [CrossRef]
- FDA Grants Lenire® Tinnitus Treatment Device De Novo Approval. Available online: https://www.lenire.com/lenire-granted-fda-approval/ (accessed on 20 August 2023).
- McCormack, A.; Edmondson-Jones, M.; Fortnum, H.; Dawes, P.D.; Middleton, H.; Munro, K.J.; Moore, D.R. Investigating the association between tinnitus severity and symptoms of depression and anxiety, while controlling for neuroticism, in a large middle-aged UK population. Int. J. Audiol. 2015, 54, 599–604. [Google Scholar] [CrossRef]
- Baldo, P.; Doree, C.; Molin, P.; McFerran, D.; Cecco, S. Antidepressants for patients with tinnitus. Cochrane Database Syst. Rev. 2012, 9, CD003853. [Google Scholar] [CrossRef]
- Dib, G.C.; Kasse, C.A.; Alves de Andrade, T.; Gurgel Testa, J.R.; Cruz, O.L. Tinnitus treatment with Trazodone. Braz. J. Otorhinolaryngol. 2007, 73, 390–397. [Google Scholar] [CrossRef]
- Robinson, S.K.; Viirre, E.S.; Bailey, K.A.; Gerke, M.A.; Harris, J.P.; Stein, M.B. Randomized placebo-controlled trial of a selective serotonin reuptake inhibitor in the treatment of nondepressed tinnitus subjects. Psychosom. Med. 2005, 67, 981–988. [Google Scholar] [CrossRef]
- Scholz, A. Mechanisms of (local) anaesthetics on voltage-gated sodium and other ion channels. Br. J. Anaesth. 2002, 89, 52–61. [Google Scholar] [CrossRef]
- Kalcioglu, M.T.; Bayindir, T.; Erdem, T.; Ozturan, O. Objective evaluation of the effects of intravenous lidocaine on tinnitus. Hear. Res. 2005, 199, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, J.; Hilders, C.G.; Schoemaker, R.C.; Hulshof, J.H.; Cohen, A.F.; Vermeij, P. Tinnitus suppression by intravenous lidocaine in relation to its plasma concentration. Clin. Pharmacol. Ther. 1993, 54, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Duckert, L.G.; Rees, T.S. Treatment of tinnitus with intravenous lidocaine: A double-blind randomized trial. Otolaryngol. Head Neck Surg. 1983, 91, 550–555. [Google Scholar] [CrossRef]
- Otsuka, K.; Pulec, J.L.; Suzuki, M. Assessment of intravenous lidocaine for the treatment of subjective tinnitus. Ear Nose Throat J. 2003, 82, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Baguley, D.M.; Jones, S.; Wilkins, I.; Axon, P.R.; Moffat, D.A. The inhibitory effect of intravenous lidocaine infusion on tinnitus after translabyrinthine removal of vestibular schwannoma: A double-blind, placebo-controlled, crossover study. Otol. Neurotol. 2005, 26, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Haginomori, S.; Makimoto, K.; Araki, M.; Kawakami, M.; Takahashi, H. Effect of lidocaine injection of EOAE in patients with tinnitus. Acta Otolaryngol. 1995, 115, 488–492. [Google Scholar] [CrossRef]
- Elzayat, S.; El-Sherif, H.; Hegazy, H.; Gabr, T.; El-Tahan, A.R. Tinnitus: Evaluation of Intratympanic Injection of Combined Lidocaine and Corticosteroids. ORL J. Otorhinolaryngol. Relat. Spec. 2016, 78, 159–166. [Google Scholar] [CrossRef]
- Sakata, H.; Kojima, Y.; Koyama, S.; Furuya, N.; Sakata, E. Treatment of cochlear tinnitus with transtympanic infusion of 4% lidocaine into the tympanic cavity. Int. Tinnitus J. 2001, 7, 46–50. [Google Scholar]
- Sieghart, W. Pharmacology of benzodiazepine receptors: An update. J. Psychiatry Neurosci. 1994, 19, 24–29. [Google Scholar] [CrossRef]
- Han, S.S.; Nam, E.C.; Won, J.Y.; Lee, K.U.; Chun, W.; Choi, H.K.; Levine, R.A. Clonazepam quiets tinnitus: A randomised crossover study with Ginkgo biloba. J. Neurol. Neurosurg. Psychiatry 2012, 83, 821–827. [Google Scholar] [CrossRef]
- Hilton, M.P.; Zimmermann, E.F.; Hunt, W.T. Ginkgo biloba for tinnitus. Cochrane Database Syst. Rev. 2013, 3, CD003852. [Google Scholar] [CrossRef] [PubMed]
- Sereda, M.; Xia, J.; Scutt, P.; Hilton, M.P.; El Refaie, A.; Hoare, D.J. Ginkgo biloba for tinnitus. Cochrane Database Syst. Rev. 2022, 11, CD013514. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Brummett, R.; Schleuning, A. Use of alprazolam for relief of tinnitus. A double-blind study. Arch. Otolaryngol. Head Neck Surg. 1993, 119, 842–845. [Google Scholar] [CrossRef]
- Jalali, M.M.; Kousha, A.; Naghavi, S.E.; Soleimani, R.; Banan, R. The effects of alprazolam on tinnitus: A cross-over randomized clinical trial. Med. Sci. Monit. 2009, 15, PI55–PI60. [Google Scholar]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Lesniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Demirkol, N.; Usumez, A.; Demirkol, M.; Sari, F.; Akcaboy, C. Efficacy of Low-Level Laser Therapy in Subjective Tinnitus Patients with Temporomandibular Disorders. Photomed. Laser Surg. 2017, 35, 427–431. [Google Scholar] [CrossRef]
- Dehkordi, M.A.; Einolghozati, S.; Ghasemi, S.M.; Abolbashari, S.; Meshkat, M.; Behzad, H. Effect of low-level laser therapy in the treatment of cochlear tinnitus: A double-blind, placebo-controlled study. Ear Nose Throat J. 2015, 94, 32–36. [Google Scholar]
- Goodman, S.S.; Bentler, R.A.; Dittberner, A.; Mertes, I.B. The effect of low-level laser therapy on hearing. ISRN Otolaryngol. 2013, 2013, 916370. [Google Scholar] [CrossRef]
- Gungor, A.; Dogru, S.; Cincik, H.; Erkul, E.; Poyrazoglu, E. Effectiveness of transmeatal low power laser irradiation for chronic tinnitus. J. Laryngol. Otol. 2008, 122, 447–451. [Google Scholar] [CrossRef]
- Mollasadeghi, A.; Mirmohammadi, S.J.; Mehrparvar, A.H.; Davari, M.H.; Shokouh, P.; Mostaghaci, M.; Baradaranfar, M.H.; Bahaloo, M. Efficacy of low-level laser therapy in the management of tinnitus due to noise-induced hearing loss: A double-blind randomized clinical trial. ScientificWorldJournal 2013, 2013, 596076. [Google Scholar] [CrossRef]
- Teggi, R.; Bellini, C.; Piccioni, L.O.; Palonta, F.; Bussi, M. Transmeatal low-level laser therapy for chronic tinnitus with cochlear dysfunction. Audiol. Neurootol. 2009, 14, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Ueda, H.; Misawa, H.; Suzuki, T.; Tominaga, M.; Ito, A.; Numata, S.; Kasai, S.; Asahi, K.; Vernon, J.A.; et al. Transmeatal low-power laser irradiation for tinnitus. Otol. Neurotol. 2002, 23, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Talluri, S.; Palaparthi, S.M.; Michelogiannakis, D.; Khan, J. Efficacy of photobiomodulation in the management of tinnitus: A systematic review of randomized control trials. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2022, 139, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Balmer, T.S.; Trussell, L.O. Trigeminal Contributions to the Dorsal Cochlear Nucleus in Mouse. Front. Neurosci. 2021, 15, 715954. [Google Scholar] [CrossRef]
- Lee, A.; Abouzari, M.; Akbarpour, M.; Risbud, A.; Lin, H.W.; Djalilian, H.R. A proposed association between subjective nonpulsatile tinnitus and migraine. World J. Otorhinolaryngol. Head Neck Surg. 2023, 9, 107–114. [Google Scholar] [CrossRef]
- Djalilian, H. Treatment of Tinnitus with Migraine Medications. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04404439 (accessed on 20 June 2023).
- Husain, F.T.; Gander, P.E.; Jansen, J.N.; Shen, S. Expectations for Tinnitus Treatment and Outcomes: A Survey Study of Audiologists and Patients. J. Am. Acad. Audiol. 2018, 29, 313–336. [Google Scholar] [CrossRef]
- Simoes, J.P.; Daoud, E.; Shabbir, M.; Amanat, S.; Assouly, K.; Biswas, R.; Casolani, C.; Dode, A.; Enzler, F.; Jacquemin, L.; et al. Multidisciplinary Tinnitus Research: Challenges and Future Directions from the Perspective of Early Stage Researchers. Front. Aging Neurosci. 2021, 13, 647285. [Google Scholar] [CrossRef]
- Duckert, L.G.; Rees, T.S. Placebo effect in tinnitus management. Otolaryngol. Head Neck Surg. 1984, 92, 697–699. [Google Scholar] [CrossRef]
- Meikle, M.B.; Henry, J.A.; Griest, S.E.; Stewart, B.J.; Abrams, H.B.; McArdle, R.; Myers, P.J.; Newman, C.W.; Sandridge, S.; Turk, D.C.; et al. The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef]
- Jacquemin, L.; Mertens, G.; Van de Heyning, P.; Vanderveken, O.M.; Topsakal, V.; De Hertogh, W.; Michiels, S.; Van Rompaey, V.; Gilles, A. Sensitivity to change and convergent validity of the Tinnitus Functional Index (TFI) and the Tinnitus Questionnaire (TQ): Clinical and research perspectives. Hear. Res. 2019, 382, 107796. [Google Scholar] [CrossRef]
- Adamchic, I.; Langguth, B.; Hauptmann, C.; Tass, P.A. Psychometric evaluation of visual analog scale for the assessment of chronic tinnitus. Am. J. Audiol. 2012, 21, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Zeleznik, O.A.; Welling, D.B.; Stankovic, K.; Frueh, L.; Balasubramanian, R.; Curhan, G.C.; Curhan, S.G. Association of Plasma Metabolomic Biomarkers with Persistent Tinnitus: A Population-Based Case-Control Study. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Zeleznik, O.; Welling, B.; Stankovic, K.; Frueh, L.; Balasubramanian, R.; Curhan, G.; Curhan, S. A Population-Based Study of Plasma Metabolomic Profiles of Persistent Tinnitus Identifies Candidate Biomarkers. medRxiv 2022. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in alpha-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef]
- Amanat, S.; Gallego-Martinez, A.; Sollini, J.; Perez-Carpena, P.; Espinosa-Sanchez, J.M.; Aran, I.; Soto-Varela, A.; Batuecas-Caletrio, A.; Canlon, B.; May, P.; et al. Burden of rare variants in synaptic genes in patients with severe tinnitus: An exome based extreme phenotype study. EBioMedicine 2021, 66, 103309. [Google Scholar] [CrossRef]
- Van den Berge, M.J.C.; van Dijk, J.M.C.; Metzemaekers, J.D.M.; Maat, B.; Free, R.H.; van Dijk, P. An auditory brainstem implant for treatment of unilateral tinnitus: Protocol for an interventional pilot study. BMJ Open 2019, 9, e026185. [Google Scholar] [CrossRef]
- Roberts, D.S.; Otto, S.; Chen, B.; Peng, K.A.; Schwartz, M.S.; Brackmann, D.E.; House, J.W. Tinnitus Suppression After Auditory Brainstem Implantation in Patients with Neurofibromatosis Type-2. Otol. Neurotol. 2017, 38, 118–122. [Google Scholar] [CrossRef]
- Tyler, R.; Cacace, A.; Stocking, C.; Tarver, B.; Engineer, N.; Martin, J.; Deshpande, A.; Stecker, N.; Pereira, M.; Kilgard, M.; et al. Vagus Nerve Stimulation Paired with Tones for the Treatment of Tinnitus: A Prospective Randomized Double-blind Controlled Pilot Study in Humans. Sci. Rep. 2017, 7, 11960. [Google Scholar] [CrossRef]
- van Zwieten, G.; Devos, J.V.P.; Kotz, S.A.; Ackermans, L.; Brinkmann, P.; Dauven, L.; George, E.L.J.; Janssen, A.M.L.; Kremer, B.; Leue, C.; et al. A Protocol to Investigate Deep Brain Stimulation for Refractory Tinnitus: From Rat Model to the Set-Up of a Human Pilot Study. Audiol. Res. 2022, 13, 49–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.W.; Kullar, P.; Malhotra, C.; Stankovic, K.M. Current and Emerging Therapies for Chronic Subjective Tinnitus. J. Clin. Med. 2023, 12, 6555. https://doi.org/10.3390/jcm12206555
Park KW, Kullar P, Malhotra C, Stankovic KM. Current and Emerging Therapies for Chronic Subjective Tinnitus. Journal of Clinical Medicine. 2023; 12(20):6555. https://doi.org/10.3390/jcm12206555
Chicago/Turabian StylePark, Ki Wan, Peter Kullar, Charvi Malhotra, and Konstantina M. Stankovic. 2023. "Current and Emerging Therapies for Chronic Subjective Tinnitus" Journal of Clinical Medicine 12, no. 20: 6555. https://doi.org/10.3390/jcm12206555
APA StylePark, K. W., Kullar, P., Malhotra, C., & Stankovic, K. M. (2023). Current and Emerging Therapies for Chronic Subjective Tinnitus. Journal of Clinical Medicine, 12(20), 6555. https://doi.org/10.3390/jcm12206555








