Is Burning Mouth Syndrome Associated with Extraoral Dryness? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Registration
2.2. Search Strategy
2.3. Literature Search
2.3.1. Inclusion and Exclusion Criteria
2.3.2. Search Terms in the Search Databases
2.4. Management Tool for the Systematic Review
2.5. Screening of Articles
2.6. Synthesizing the Evidence
2.7. Quality Assessment
2.8. Prerequisite Checks for a Meta-Analysis: Data Size and Data Heterogeneity
2.9. Data Synthesis
3. Results
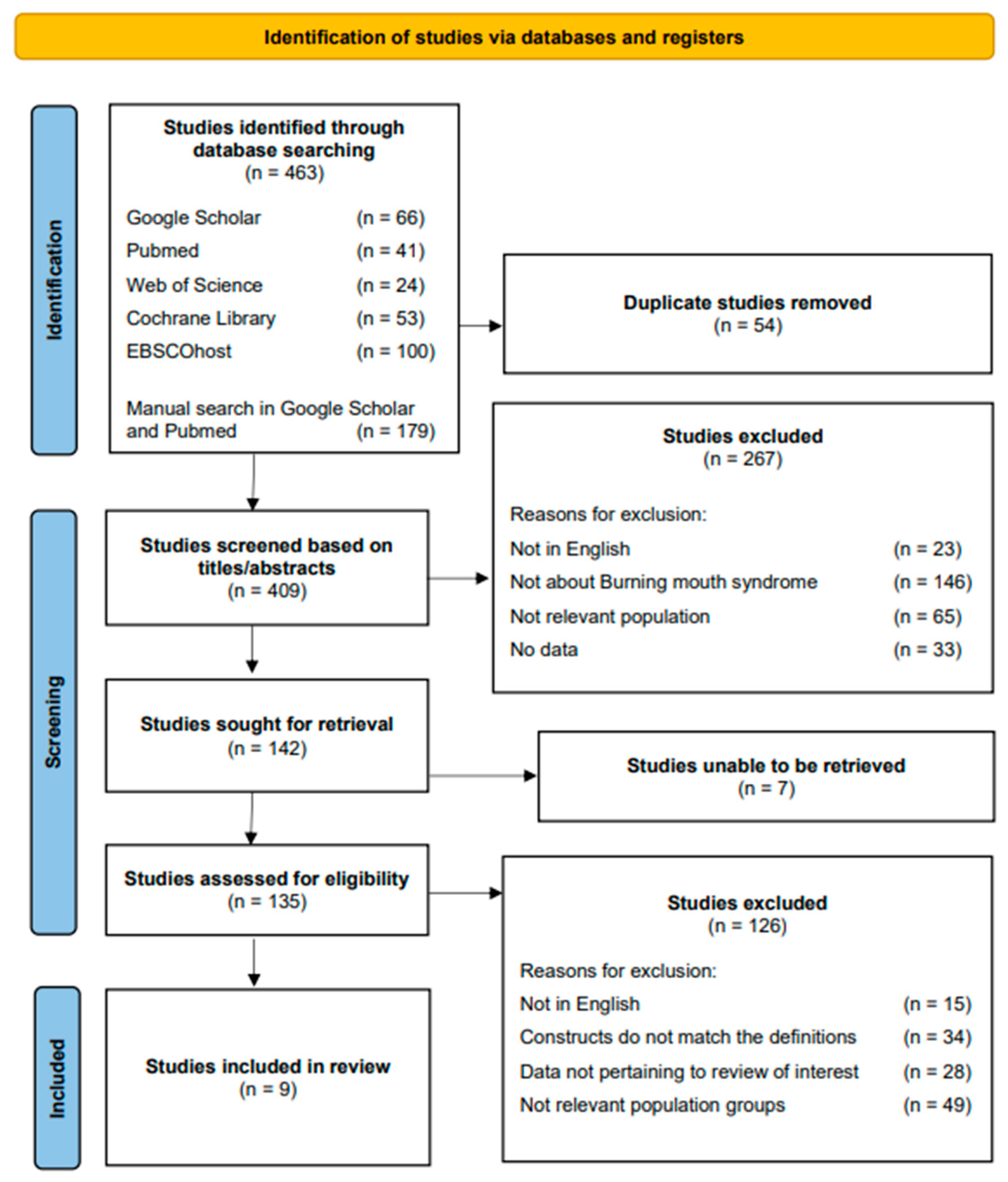
3.1. Literature Search Results on PRISMA
3.2. Description of the Included Studies
3.3. Quality Assessment of the Included Studies
3.4. Summary Tables
3.5. Extraoral Dryness
4. Discussion
4.1. Demographic Characteristics
4.2. Extraoral Dryness
4.3. Other Findings
5. Strength and Limitations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2023. [Google Scholar] [CrossRef]
- Spanemberg, J.C.; Cardoso, J.A.; Slob, E.M.G.B.; López-López, J. Quality of life related to oral health and its impact in adults. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, M.D.; Pollio, A.; Fortuna, G.; Leuci, S.; Ruoppo, E.; Adamo, D.; Zarrelli, C. Unexplained somatic comorbidities in patients with burning mouth syndrome: A controlled clinical study. J. Orofac. Pain 2011, 25, 131–140. [Google Scholar]
- Herbert, C. Oral health and mental health in healthy adults, a topic of primary prevention and health care, empirical results from two online studies. Curr. Psychol. 2023, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Alsabbagh, R.; Ouanounou, A. Burning Mouth Syndrome: Etiology, clinical presentations, and treatment alternatives. Dent. Rev. 2022, 2, 100036. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS) the International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef]
- Klasser, G.D.; Epstein, J.B. Oral burning and burning mouth syndrome. J. Am. Dent. Assoc. 2012, 143, 1317–1319. [Google Scholar] [CrossRef]
- Coculescu, E.C.; Radu, A.; Coculescu, B.I. Burning mouth syndrome: A review on diagnosis and treatment. J. Med. Life 2014, 7, 512–515. [Google Scholar]
- McMillan, R.; Forssell, H.; Buchanan, J.A.; Glenny, A.M.; Weldon, J.C.; Zakrzewska, J.M. Interventions for treating burning mouth syndrome. Cochrane Database Syst. Rev. 2016, 11, Cd002779. [Google Scholar] [CrossRef] [PubMed]
- Ariyawardana, A.; Chmieliauskaite, M.; Farag, A.M.; Albuquerque, R.; Forssell, H.; Nasri-Heir, C.; Klasser, G.D.; Sardella, A.; Mignogna, M.D.; Ingram, M.; et al. World Workshop on Oral Medicine VII: Burning mouth syndrome: A systematic review of disease definitions and diagnostic criteria utilized in randomized clinical trials. Oral Dis. 2019, 25, 141–156. [Google Scholar] [CrossRef]
- Tan, H.L.; Renton, T. Burning mouth syndrome: An update. Cephalalgia Rep. 2020, 3, 2515816320970143. [Google Scholar] [CrossRef]
- Jääskeläinen, S.K.; Woda, A. Burning mouth syndrome. Cephalalgia 2017, 37, 627–647. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, W.; Yan, J.; Noma, N.; Young, A.; Yan, Z. Worldwide prevalence estimates of burning mouth syndrome: A systematic review and meta-analysis. Oral Dis. 2022, 28, 1431–1440. [Google Scholar] [CrossRef]
- Kohorst, J.J.; Bruce, A.J.; Torgerson, R.R.; Schenck, L.A.; Davis, M.D.P. The prevalence of burning mouth syndrome: A population-based study. Br. J. Dermatol. 2015, 172, 1654–1656. [Google Scholar] [CrossRef] [PubMed]
- Momin, S. Burning Mouth Syndrome-A Frustrating Problem. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 580. [Google Scholar] [CrossRef]
- Souza, F.T.A.; Santos, T.P.M.; Bernardes, V.F.; Teixeira, A.L.; Kümmer, A.M.; Silva, T.A.; Abreu, M.H.N.G. The impact of burning mouth syndrome on health-related quality of life. Health Qual. Life Outcomes 2011, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Lehman, B.J.; David, D.M.; Gruber, J.A. Rethinking the biopsychosocial model of health: Understanding health as a dynamic system. Soc. Personal. Psychol. Compass 2017, 11, e12328. [Google Scholar] [CrossRef]
- Adamo, D.; Spagnuolo, G. Burning Mouth Syndrome: An Overview and Future Perspectives. Int. J. Environ. Res. Public Health 2022, 20, 682. [Google Scholar] [CrossRef]
- Mitsikostas, D.D.; Ljubisavljevic, S.; Deligianni, C.I. Refractory burning mouth syndrome: Clinical and paraclinical evaluation, comorbidities, treatment and outcome. J. Headache Pain 2017, 18, 40. [Google Scholar] [CrossRef][Green Version]
- Malta, C.E.N.; Costa, F.W.G.; Dias, C.C.; Carlos, A.; Sousa, F.B.; Silva, P.G.B.; Teófilo, C.R. Association of anxiety, depression, and stress with burning mouth syndrome: A case-control study. Gen. Dent. 2021, 69, 46–52. [Google Scholar]
- Galli, F.; Lodi, G.; Sardella, A.; Vegni, E. Role of psychological factors in burning mouth syndrome: A systematic review and meta-analysis. Cephalalgia 2017, 37, 265–277. [Google Scholar] [CrossRef]
- Al Quran, F.A. Psychological profile in burning mouth syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 339–344. [Google Scholar] [CrossRef]
- Pereira, J.V.; Normando, A.G.C.; Rodrigues-Fernandes, C.I.; Rivera, C.; Santos-Silva, A.R.; Lopes, M.A. The impact on quality of life in patients with burning mouth syndrome: A systematic review and meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Grushka, M. Clinical features of burning mouth syndrome. Oral Surg. Oral Med. Oral Pathol. 1987, 63, 30–36. [Google Scholar] [CrossRef]
- Acharya, S.; Carlén, A.; Wenneberg, B.; Jontell, M.; Hägglin, C. Clinical characterization of women with burning mouth syndrome in a case-control study. Acta Odontol. Scand. 2018, 76, 279–286. [Google Scholar] [CrossRef]
- Acharya, S.; Jin, C.; Bylund, J.; Shen, Q.; Kamali-Moghaddam, M.; Jontell, M.; Carlén, A.; Karlsson, N.G. Reduced sialyl-Lewis(x) on salivary MUC7 from patients with burning mouth syndrome. Mol. Omics 2019, 15, 331–339. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, F.; McBride, K.A.; George, E.S.; Steiner, G.Z. Conducting a Systematic Review: A Practical Guide. In Handbook of Research Methods in Health Social Sciences; Liamputtong, P., Ed.; Springer: Singapore, 2018; pp. 1–22. [Google Scholar] [CrossRef]
- Muka, T.; Glisic, M.; Milic, J.; Verhoog, S.; Bohlius, J.; Bramer, W.; Chowdhury, R.; Franco, O.H. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur. J. Epidemiol. 2020, 35, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Sarkis-Onofre, R.; Catalá-López, F.; Aromataris, E.; Lockwood, C. How to properly use the PRISMA Statement. Syst. Rev. 2021, 10, 117. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Moher, D.; Shekelle, P. Why prospective registration of systematic reviews makes sense. Syst. Rev. 2012, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, R. PICO: Model for clinical questions. Evid. Based Med. Pract. 2018, 3, 2. [Google Scholar]
- Riva, J.J.; Malik, K.M.; Burnie, S.J.; Endicott, A.R.; Busse, J.W. What is your research question? An introduction to the PICOT format for clinicians. J. Can. Chiropr. Assoc. 2012, 56, 167–171. [Google Scholar]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: Oxford, UK, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- Cavaleri, R.; Bhole, S.; Arora, A. Critical Appraisal of Quantitative Research. In Handbook of Research Methods in Health Social Sciences; Liamputtong, P., Ed.; Springer: Singapore, 2019; pp. 1027–1049. [Google Scholar] [CrossRef]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Brennan, S.E. Synthesizing and Presenting Findings Using Other Methods. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: Oxford, UK, 2019; pp. 321–347. [Google Scholar] [CrossRef]
- Denison, H.J.; Dodds, R.M.; Ntani, G.; Cooper, R.; Cooper, C.; Sayer, A.A.; Baird, J. How to get started with a systematic review in epidemiology: An introductory guide for early career researchers. Arch. Public Health 2013, 71, 21. [Google Scholar] [CrossRef]
- Lamey, P.J.; Freeman, R.; Eddie, S.A.; Pankhurst, C.; Rees, T. Vulnerability and presenting symptoms in burning mouth syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2005, 99, 48–54. [Google Scholar] [CrossRef]
- Monteserín-Matesanz, M.; Domínguez-Gordillo, A.A.; Esparza-Gómez, G.C.; Jiménez-Ortega, L.; Cerero-Lapiedra, R. Central sensitization in burning mouth syndrome: A practical approach using questionnaires. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 292–300. [Google Scholar] [CrossRef]
- Werfalli, S.; Drangsholt, M.; Johnsen, J.M.; Jeffrey, S.K.; Dakhil, S.; Presland, R.B.; LeResche, L. Saliva flow rates and clinical characteristics of patients with burning mouth syndrome: A case-control study. Int. J. Oral Maxillofac. Surg. 2021, 50, 1187–1194. [Google Scholar] [CrossRef]
- de Pedro, M.; López-Pintor, R.M.; Casañas, E.; Hernández, G. General health status of a sample of patients with burning mouth syndrome: A case-control study. Oral Dis. 2020, 26, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, D.R.; Birman, E.G.; Migliari, D.A.; da Silveira, F.R. Burning mouth syndrome: Clinical profile of Brazilian patients and oral carriage of Candida species. Braz. Dent. J. 2007, 18, 341–345. [Google Scholar] [CrossRef]
- Freilich, J.E.; Kuten-Shorrer, M.; Treister, N.S.; Woo, S.B.; Villa, A. Burning mouth syndrome: A diagnostic challenge. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 120–124. [Google Scholar] [CrossRef]
- Weatherspoon, D.; Chattopadhyay, A. International Classification of Diseases Codes and their Use in Dentistry. J. Dent. Oral Craniofacial Epidemiol. 2013, 1, 20–26. [Google Scholar]
- The National Heart, Lung, and Blood Institute Institute (NHLBI). Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 12 September 2023).
- Lijmer, J.G.; Mol, B.W.; Heisterkamp, S.; Bonsel, G.J.; Prins, M.H.; van der Meulen, J.H.; Bossuyt, P.M. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999, 282, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J. Systematic reviews in health care: Systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001, 323, 157–162. [Google Scholar] [CrossRef]
- White-Chu, E.F.; Reddy, M. Dry skin in the elderly: Complexities of a common problem. Clin. Dermatol. 2011, 29, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Kamp, E.; Musbahi, E.; DeGiovanni, C. Menopause, skin and common dermatoses. Part 4: Oral disorders. Clin. Exp. Dermatol. 2022, 47, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Naftolin, F. Menopause and the Skin: Old Favorites and New Innovations in Cosmeceuticals for Estrogen-Deficient Skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef]
- Rahrovan, S.; Fanian, F.; Mehryan, P.; Humbert, P.; Firooz, A. Male versus female skin: What dermatologists and cosmeticians should know. Int. J. Womens Dermatol. 2018, 4, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Phuong, C.; Maibach, H.I. Gender Differences in Skin. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1729–1755. [Google Scholar] [CrossRef]
- Engebretsen, K.A.; Johansen, J.D.; Kezic, S.; Linneberg, A.; Thyssen, J.P. The effect of environmental humidity and temperature on skin barrier function and dermatitis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 223–249. [Google Scholar] [CrossRef]
- Firooz, A.; Sadr, B.; Babakoohi, S.; Sarraf-Yazdy, M.; Fanian, F.; Kazerouni-Timsar, A.; Nassiri-Kashani, M.; Naghizadeh, M.M.; Dowlati, Y. Variation of biophysical parameters of the skin with age, gender, and body region. Sci. World J. 2012, 2012, 386936. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.R.; Mo, J.; Goodman, R.S. The dermatological manifestations of extreme weather events: A comprehensive review of skin disease and vulnerability. J. Clim. Chang. Health 2022, 8, 100162. [Google Scholar] [CrossRef]
- Sainani, K.L. Understanding Odds Ratios. PM&R 2011, 3, 263–267. [Google Scholar] [CrossRef]
- Szumilas, M. Explaining odds ratios. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 227–229. [Google Scholar]
- Bland, J.M.; Altman, D.G. The odds ratio. BMJ 2000, 320, 1468. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Fourie, J.; Bouckaert, M.; Khammissa, R.A.G.; Ballyram, R.; Lemmer, J. Burning Mouth Syndrome: Aetiopathogenesis and Principles of Management. Pain Res. Manag. 2017, 2017, 1926269. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Kim, Y.S.; Ko, I.; Kim, D.-K. Association Between Burning Mouth Syndrome and the Development of Depression, Anxiety, Dementia, and Parkinson Disease. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Kim, Y.; Yoo, T.; Han, P.; Inman, J.C. Burning mouth syndrome: A systematic review of treatments. Oral Dis. 2018, 24, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Cabras, M.; Gambino, A.; Broccoletti, R.; De Paola, S.; Sciascia, S.; Arduino, P.G. Effectiveness of Nonpharmacologic Treatments of Burning Mouth Syndrome: A Systematic Review. J. Oral Facial Pain Headache 2021, 35, 175–198. [Google Scholar] [CrossRef]
- Thakkar, J.P.; Lane, C.J. Hyposalivation and Xerostomia and Burning Mouth Syndrome: Medical Management. Oral Maxillofac. Surg. Clin. 2022, 34, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Lavareze, L.; Scarini, J.F.; Egal, E.S.A.; Emerick, C.; Crescencio, L.R.; Altemani, A.; Mariano, F.V. Xerostomia in Burning Mouth Syndrome: A Dry Sea or Different Water? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, e235. [Google Scholar] [CrossRef]
- Nagler, R.M.; Hershkovich, O. Age-related changes in unstimulated salivary function and composition and its relations to medications and oral sensorial complaints. Aging Clin. Exp. Res. 2005, 17, 358–366. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hong, I.K.; Na, S.Y.; Eun, Y.G. Evaluation of salivary function in patients with burning mouth syndrome. Oral Dis. 2015, 21, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, C.; Ito, K.; Takamatsu, K.; Ogawa, M.; Kajii, Y.; Nohno, K.; Sugano, A.; Funayama, S.; Katakura, A.; Nomura, T.; et al. Factors associated with xerostomia in perimenopausal women. J. Obstet. Gynaecol. Res. 2021, 47, 3661–3668. [Google Scholar] [CrossRef]
- Ciesielska, A.; Kusiak, A.; Ossowska, A.; Grzybowska, M.E. Changes in the Oral Cavity in Menopausal Women—A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 253. [Google Scholar] [CrossRef]
- binti Tengku, L.D.; Noor, A. Burning mouth syndrome caused by xerostomia secondary to amlodipine. Dent. J. 2020, 53, 187–190. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Furuta, K.; Ueno, M.; Egawa, M.; Yoshino, A.; Kondo, S.; Nariai, Y.; Ishibashi, H.; Kinoshita, Y.; Sekine, J. Oral symptoms including dental erosion in gastroesophageal reflux disease are associated with decreased salivary flow volume and swallowing function. J. Gastroenterol. 2012, 47, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Wang, G.; Song, X.; Qian, Y.; Liao, Z.; Sui, L.; Ai, L.; Xia, Y. Understanding the Connection between Gut Homeostasis and Psychological Stress. J. Nutr. 2023, 153, 924–939. [Google Scholar] [CrossRef]
- Teruel, A.; Patel, S. Burning mouth syndrome: A review of etiology, diagnosis, and management. Gen. Dent. 2019, 67, 24–29. [Google Scholar] [PubMed]
- Hsieh, J.W.; Daskalou, D.; Macario, S.; Voruz, F.; Landis, B.N. How to Manage Taste Disorders. Curr. Otorhinolaryngol. Rep. 2022, 10, 385–392. [Google Scholar] [CrossRef]
- Dutt, P.; Chaudhary, S.; Kumar, P. Oral health and menopause: A comprehensive review on current knowledge and associated dental management. Ann. Med. Health Sci. Res. 2013, 3, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Adamo, D.; Sardella, A.; Varoni, E.; Lajolo, C.; Biasotto, M.; Ottaviani, G.; Vescovi, P.; Simonazzi, T.; Pentenero, M.; Ardore, M.; et al. The association between burning mouth syndrome and sleep disturbance: A case-control multicentre study. Oral Dis. 2018, 24, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, F.; Farahmand, F.; Hosseinpour, H.; Shahriarirad, R.; Sabet Eghlidi, A. The Association between Emotional Stress, Sleep Disturbance, Depression, and Burning Mouth Syndrome. Biomed. Res. Int. 2021, 2021, 5555316. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Steinberg, J.; Patel, P. Insomnia in the Elderly: A Review. J. Clin. Sleep Med. 2018, 14, 1017–1024. [Google Scholar] [CrossRef]
- Suzuki, K.; Miyamoto, M.; Hirata, K. Sleep disorders in the elderly: Diagnosis and management. J. Gen. Fam. Med. 2017, 18, 61–71. [Google Scholar] [CrossRef]
- Morin, C.M.; Jarrin, D.C. Epidemiology of Insomnia: Prevalence, Course, Risk Factors, and Public Health Burden. Sleep Med. Clin. 2022, 17, 173–191. [Google Scholar] [CrossRef]
- Arakane, M.; Castillo, C.; Rosero, M.F.; Peñafiel, R.; Pérez-López, F.R.; Chedraui, P. Factors relating to insomnia during the menopausal transition as evaluated by the Insomnia Severity Index. Maturitas 2011, 69, 157–161. [Google Scholar] [CrossRef]
- Jankovskis, V.; Selga, G. Candidiasis and Other Bacterial Infections among Patients Diagnosed with Burning Mouth Syndrome. Medicina 2022, 58, 1029. [Google Scholar] [CrossRef] [PubMed]
- Dugan, C.; Parlatescu, I.; Dobre, M.; Pîrvu, R.E.; Milanesi, E. Insights on brain functions in burning mouth syndrome. Front. Syst. Neurosci. 2022, 16, 975126. [Google Scholar] [CrossRef]
- Gough, D.; Davies, P.; Jamtvedt, G.; Langlois, E.; Littell, J.; Lotfi, T.; Masset, E.; Merlin, T.; Pullin, A.S.; Ritskes-Hoitinga, M.; et al. Evidence Synthesis International (ESI): Position Statement. Syst. Rev. 2020, 9, 155. [Google Scholar] [CrossRef]
- Murad, M.H.; Noor, A.; Mouaz, A.; Fares, A. New evidence pyramid. Evid. Based Med. 2016, 21, 125. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Barak, G.; Truong, G.; Parker, M.W. Hierarchy of Evidence within the Medical Literature. Hosp. Pediatr. 2022, 12, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.K. Evidence-Based Medicine and Levels of Evidence. Am. Orthopt. J. 2010, 60, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Katikireddi, S.V.; Sowden, A.; Thomson, H. Lack of transparency in reporting narrative synthesis of quantitative data: A methodological assessment of systematic reviews. J. Clin. Epidemiol. 2019, 105, 1–9. [Google Scholar] [CrossRef]
| Population | Intervention | Comparison | Outcome |
|---|---|---|---|
| Burning mouth syndrome patients (aged women) | Not applicable | Extraoral dryness and what type/to which degree Comorbidities Diagnostic criteria | Exploring the etiology of burning mouth syndrome by investigating to which degree extraoral dryness is found |
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | ● BMS patients ● Aged women | ● Men, children, and teens ● Animal studies and in vitro studies |
| Study design | ● Observational studies ● Experimental studies | ● Abstracts ● Non-full text journals ● Opinion papers ● Letters |
| Data/outcome | ● Extraoral dryness in BMS ● Comorbidities in BMS ● Empirical quantitative data | ● Qualitative data |
| Databases Searched | Search Terms |
|---|---|
| Google Scholar | 1. “Burning mouth syndrome”|“Burning mouth disorder” AND prevalence “Dry skin”|“Xerosis”|“Skin disease” body|“body region” 2. “Burning mouth syndrome” OR “Burning mouth disorder” AND prevalence “Dry skin” OR “Xerosis” OR “Skin disease” AND “body” OR “body region” 3. “Burning mouth syndrome” OR “Burning mouth disorder” AND prevalence “Dry skin” OR “Xerosis” OR “Dermatitis” OR “Skin disease” AND “body” OR “body region” 4. “Burning mouth syndrome patients” OR “BMS patients” AND prevalence “Dry skin” OR “Xerosis” OR “Dermatitis” OR “Skin disease” AND “body” OR “body region” 5. “Burning mouth syndrome patients” AND “dryness” AND “association” |
| PubMed | 1. (“Burning mouth syndrome patients” OR “BMS patients”) AND (Dry skin OR Xerosis OR Dermatitis OR Skin disease) 2. (“Burning mouth syndrome patient*” OR “BMS patient*”) AND (Association) 3. (“Burning mouth syndrome patient*” OR “BMS patient*”) AND (“comorbidit*”) |
| Web of Science | 1. (“Burning mouth syndrome patient*” OR “BMS patient*”) (Topic) and (Dry skin OR Xerosis OR Dermatitis OR Skin disease*) (Topic) 2. (“Burning mouth syndrome patient*” OR “BMS patient*”) (Topic) and (“Comorbidit*”) (Topic) |
| COCHRANE Library | 1. (“Burning mouth syndrome patient*”) OR (“BMS patient*”) AND (Comorbidit*) |
| EBSCOhost | 1. (((“burning mouth syndrome”) OR (“BMS”) AND patient*)) AND (comorbidit*) 2. (((“burning mouth syndrome”) OR (“BMS”) AND patient*)) AND (Dry skin OR Xerosis OR Dermatitis OR Skin disease*) |
| Study ID | Diagnostic Criteria for BMS | Diagnostic Criteria for the Control Groups |
|---|---|---|
| Study 1 [25] | “Complaint of persistent oral burning for >3 months in an otherwise clinically normal oral mucosa” | Sex- and age-matched participants |
| Study 2 [42] | “Burning sensation in an otherwise clinically normal oral mucosa” | Sex-, age-, marital-, and employment status-matched participants with no prior diagnosis of BMS |
| Study 3 [43] | ICHD-3 clinical definition: 13.11 | Participants who did not meet the ICHD-3 clinical definition: 13.11 diagnostic criteria |
| Study 4 [44] | ICD-10-CM diagnosis code K14.6 | Participants with no prior diagnosis of BMS or other oral complications |
| Study 5 [4] | “Participants of >18 years old with chronic oral pain daily for >6 months, without clinically evident causative lesions in an otherwise clinically normal oral mucosa” | >18-year-old participants without evident oral lesions of unexplained oral symptoms |
| Study 6 [26] | ICHD-3 clinical definition: 13.11 | Age-matched women who did not meet the ICHD-3 clinical definition: 13.11 diagnostic criteria |
| Study 7 [45] | ICHD-3 clinical definition: 13.11 | Sex- and age-matched participants who did not meet the ICHD-3 clinical definition: 13.11 diagnostic criteria |
| Study 8 [46] | “Burning sensation for >6 months, without clinically evident causative change” | Sex- and age-matched participants without oral burning or lesions |
| Study 9 [46] | ICHD-3 clinical definition: 13.11 | Not applicable |
| Study ID | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study 1 [25] | Y | Y | N | Y | Y | Y | N | Y | N | N | N | N | Fair (6) |
| Study 2 [42] | Y | Y | N | Y | Y | N | Y | Y | Y | Y | N | Y | Fair (9) |
| Study 3 [43] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | Fair (9) |
| Study 4 [44] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | Fair (9) |
| Study 5 [4] | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | N | Fair (9) |
| Study 6 [26] | Y | Y | N | Y | Y | Y | Y | Y | N | N | Y | N | Fair (8) |
| Study 7 [45] | Y | Y | N | Y | Y | Y | N | N | Y | Y | N | N | Fair (7) |
| Study 8 [46] | Y | Y | N | Y | Y | Y | N | Y | Y | Y | N | N | Fair (8) |
| Study ID | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Study 9 [47] | Y | Y | Y | N | N | Y | Y | N | Y | Fair (6) |
| Study ID | Study Title | Year Published | Place of Origin | Study Design | Aims |
|---|---|---|---|---|---|
| Study 1 [25] | Clinical features of burning mouth syndrome | 1987 | Canada | Case–control | Comparison of clinical features between BMS patients and age- and sex-matched control subjects |
| Study 2 [42] | Vulnerability and presenting symptoms in burning mouth syndrome | 2005 | UK | Case–control | Comparison of clinical features and health quality between BMS patients and age- and sex-matched control subjects, as well as to examine the role of vulnerability factors and differentiate them from presenting symptomology in patients with BMS |
| Study 3 [43] | Central sensitization in burning mouth syndrome: a practical approach using questionnaires | 2022 | Spain | Case–control | Comparison of central sensitization presence between BMS patients and age- and sex-matched control subjects, as well as measuring the extent of pain, the presence of associated symptoms, and other chronic pain conditions |
| Study 4 [44] | Saliva flow rates and clinical characteristics of patients with burning mouth syndrome: a case–control study | 2021 | USA | Case–control | Comparison of unstimulated and stimulated saliva flow rates, mucosal hydration, and xerostomia between BMS patients and age- and sex-matched control subjects |
| Study 5 [4] | Unexplained somatic comorbidities in patients with burning mouth syndrome: a controlled clinical study | 2011 | Italy | Case–control | Comparison of unexplained extraoral symptoms prevalence in BMS patients, oral lichen planus patients, and age- and sex-matched control subjects |
| Study 6 [26] | Clinical characterization of women with burning mouth syndrome in a case–control study | 2018 | Sweden | Case–control | Comparison of underlying factors, clinical characteristics, and patients’ self-reported oral and general health factors associated between BMS patients and age- and sex-matched control subjects |
| Study 7 [45] | General health status of a sample of patients with burning mouth syndrome: a case–control study | 2020 | Spain | Case–control | Comparison of general health between BMS patients and age- and sex-matched control subjects |
| Study 8 [46] | Burning mouth syndrome: clinical profile of Brazilian patients and oral carriage of Candida species | 2007 | Brazil | Case-control | Comparison of clinical profile between BMS patients and age- and sex-matched control subjects |
| Study 9 [47] | Burning mouth syndrome: a diagnostic challenge | 2020 | USA | Case series | To characterize the diagnostic process for BMS patients and identify pitfalls encountered in the workup and management of BMS |
| Study ID | Participants | Sex | Age | Diagnostic Criteria (BMS) | Diagnostic Criteria (Control) |
|---|---|---|---|---|---|
| Study 1 [25] | BMS n = 54 Control n = 27 | BMS and Control 84/102 women (82%) | BMS and Control Weighted mean of 57.88 | Complaint of persistent oral burning for >3 months in an otherwise clinically normal oral mucosa | Sex- and age-matched participants |
| Study 2 [42] | BMS n = 84 Control n = 73 | BMS and Control 138/160 women (88%) | BMS and Control Mean of 65 | Burning sensation in an otherwise clinically normal oral mucosa | Sex-, age-, marital-, and employment status-matched participants without prior experience of burning mouth syndrome or cognitive impairment |
| Study 3 [43] | BMS n = 40 Control n = 42 | BMS 37/40 women (92.5%) Control 39/42 women (92.9%) | BMS Weighted mean of 61.96 | ICHD-3 clinical definition: 13.11: Intraoral burning or dysesthesia sensation recurring daily for >2 h for >3 months without clinically evident causative lesions | Participants who did not meet the ICHD-3 clinical definition: 13.11 diagnostic criteria |
| Study 4 [44] | BMS n = 22 Control n = 28 | BMS All women (100%) Control All women (100%) | BMS Weighted mean of 58.64 | ICD-10-CM diagnosis code K14.6: Intraoral burning or dysesthesia sensation daily for >2 h for >3 months in an otherwise clinically normal oral mucosa | Participants with no prior diagnosis of BMS and who answered specific questions regarding oral disturbances in the past 3 months |
| Study 5 [4] | BMS n = 124 Control n = 102 | “Mostly” women * | BMS Weighted mean of 57.23 | Participants of >18 years old with chronic oral pain daily for >6 months without clinically evident causative lesions in an otherwise clinically normal oral mucosa | Participants of >18 years old without evident oral lesions or unexplained oral symptoms, no history of psychiatric disorder, and first-time consultation in such a study |
| Study 6 [26] | BMS n = 56 Control n = 56 | BMS All women (100%) Control All women (100%) | BMS Weighted mean of 67.75 | ICHD-3 clinical definition: 13.11: Intraoral burning or dysesthesia sensation recurring daily for >2 h for >3 months without clinically evident causative lesions | Age-matched women who did not meet the ICHD-3 clinical definition: 13.11 diagnostic criteria, and other oral mucosal changes, infections, and illnesses |
| Study 7 [45] | BMS n = 20 Control n = 40 | BMS 16/20 women (80%) Control 32/40 women (80%) | BMS Weighted mean of 63.72 | ICHD-3 clinical definition: 13.11: Intraoral burning or dysesthesia sensation recurring daily for >2 h for >3 months without clinically evident causative lesions | Sex- and age-matched participants, who did not meet the ICHD-3 clinical definition: 13.11 diagnostic criteria |
| Study 8 [46] | BMS n = 31 | BMS 28/31 women (90.3%) | BMS Mean of 61.3 | Burning sensation for >6 months, without clinically evident causative change | Sex- and age-matched participants without burning complaints and without oral lesions |
| Study 9 [47] | BMS n =102 | BMS 88/102 women (86.3%) | BMS Median of 60 | ICHD-3 clinical definition: 13.11: Intraoral burning or dysesthesia sensation recurring daily for >2 h for >3 months without clinically evident causative lesions | No comparator |
| Study ID | Extraoral Dryness Outcomes for BMS | Extraoral Dryness Outcomes for Control | BMS Types | Reported Associated Symptoms | Conclusions |
|---|---|---|---|---|---|
| Study 1 [25] | Dry eyes 18/54 (33.3%) | Dry eyes 4/27 (14.8%) | Not reported | Dysgeusia Insomnia Xerostomia | The study found little evidence to support earlier theories that dentures, oral habits, and nutritional deficiencies are significant causes of burning mouth syndrome (BMS), although some differences were found between the BMS and control subjects. However, the study suggested that changes in salivary composition might be linked to the subjective xerostomia prevalent in BMS subjects. Compared to the control subjects, the BMS subjects reported a higher prevalence of symptoms such as dry mouth, thirst, taste and sleep disturbances, headaches, nonspecific health problems, pain complaints, and severe menopausal symptoms but had no differences in other oral or dental features or the prevalence of candidiasis infection. |
| Study 2 [42] | Skin problems 23/84 (27.3%) | Skin problems 7/73 (9.6%) | Not reported | Carcinophobia Chronic fatigue Circadian rhythm disorders Dizziness Gastrointestinal problems Insomnia Rheumatoid arthritis Skin alterations Somatic complaints ** | The study reports patients with BMS had poorer overall health, more illnesses and gastrointestinal problems, chronic fatigue, disturbed sleep patterns, and more anxiety and depression compared to control subjects. The study proposes that perceptions of ill health may be related to presenting symptoms and life experiences and that vulnerability factors may be associated with experiencing emotional distress as a bodily illness. The study recommends considering vulnerability factors as potential markers for those who experience emotional distress as bodily illness in BMS patients. |
| Study 3 [43] | Dry eyes 19/40 (47.5%) | Dry eyes 11/42 (26.2%) | Not reported | Cognitive impairment Common mental illnesses Dysgeusia Dizziness Insomnia Myasthenia Xerostomia | The study results suggest the presence of CS in BMS patients, and the study proposes that evaluating the degree of CS is useful for complementing clinical diagnosis and aiding therapeutic decision-making. The study found that patients with BMS had significantly higher scores for nonrestorative sleep, sleep disorders, fatigue, and anxiety than the controls and that these factors should be considered in the management of patients with BMS. Symptoms related to BMS and those associated with CS both obtained high scores in patients with BMS, suggesting that the list of comorbidities closely associated with BMS is a useful tool for identifying this disorder. |
| Study 4 [44] | Dry eyes 12/22 (54.5%) Genital dryness 11/22 (50%) | Dry eyes 11/28 (39.3%) Genital dryness 5/28 (17.8%) | Not reported | Gastrointestinal problems Genital dryness | The study of burning mouth syndrome (BMS) in older women found that BMS cases had lower USWS flow rates and higher prevalence of xerostomia, vaginal dryness, and GI disease compared to controls. The study found a significant difference in the mean USWS flow rate in cases compared to controls, suggesting that the sensation of oral dryness is correlated with a reduction in salivation. Medication usage is possibly a contributing factor in BMS patients using more than one medication. Gastrointestinal disease was more commonly reported among BMS cases than the controls. Vaginal dryness was significantly more common among BMS cases. The study had several strengths and limitations, including a well-characterized sample of cases and controls. |
| Study 5 [4] | Genital dryness 15/124 (12.1%) | Genital dryness 0/130 (0%) | Not reported | Gastrointestinal problems Globus sensation Myofascial pain Ocular burning Otorhinolaryngological problems Palpitations Tension-type headaches Tinnitus | The study aimed to assess the prevalence of unexplained extraoral symptoms in patients with burning mouth syndrome (BMS) compared to patients with oral lichen planus (OLP) and healthy individuals. The study collected data from 124 BMS patients, 112 OLP patients, and 102 healthy patients. The results showed that 96.1% of BMS patients reported unexplained extraoral symptoms compared to 9.3% in OLP patients and 15.7% in healthy individuals. BMS patients reported painful symptomatology in different bodily regions more frequently than OLP patients and healthy individuals. The study suggests that BMS may be classified as a complex somatoform disorder rather than a neuropathic pain entity and that various medical disciplines should be involved in the diagnostic process. |
| Study 6 [26] | Skin problems 22/56 (39.3%) | Skin problems 8/56 (14.3%) | Not reported | Chronic pain Xerostomia | The study compared patients with BMS and age-matched controls. Skin diseases were strongly associated with BMS, as were self-reported symptoms of xerostomia. The groups did not differ with respect to background factors. The BMS group reported poorer general and oral health and poorer life satisfaction than the controls. Hormonal status may play a role in the pathogenesis, but studies on hormone replacement therapy show contradictory results. Patients with BMS reported more allergies and medications than controls. Bruxofacets were more common in patients with BMS but did not contribute to the regression model. |
| Study 7 [45] | Xerostomia Inventory * Skin problems 1.85 ± 1.31 Dry eyes 3.70 ± 0.92 Dry lips 4.05 ± 0.83 Dry nose cavity 2.70 ± 1.30 | Xerostomia Inventory * Skin problems 1.85 ± 1.05 Dry eyes 1.65 ± 0.89 Dry lips 2.23 ± 1.05 Dry nose cavity 1.98 ± 0.85 | Not reported | Asthma Chronic pain Fibromyalgia Osteoarthritis Sleepiness Xerostomia | The study reports that burning mouth syndrome (BMS) patients suffer from more comorbidities and consume more medications than controls. Mental, behavioral, or neurodevelopmental disorders were found more frequently in BMS patients, who also used four times more drugs, especially for the nervous and cardiovascular systems, as well as the alimentary tract and metabolism. BMS patients had lower iron and higher folic acid levels than the controls, and their general health status, oral health impact, sleepiness, psychological status, and xerostomia levels were significantly worse. BMS is usually associated with mental and behavioral disorders like depressive and anxiety disorders, making the positive correlation in the use of psycholeptics and psychoanaleptics logical. |
| Study 8 [46] | Dry lips 17/31 (54.8%) | No relevant comparator | Type 1 (48.4%) Type 2 (35.5%) Type 3 (16.1%) | Carcinophobia Xerostomia | The study reports that the majority of BMS patients were postmenopausal women with chronic medication use, particularly antihypertensives and antidepressants, and secondary complaints such as dry mouth and altered taste. Anemia and prosthetic adjustments did not significantly affect BMS symptoms. The study found positive cultures for Candida species in 45.16% of patients, but this did not confirm the presence of C. albicans as an associated factor in BMS etiology. The study highlights the importance of interdisciplinary investigation and patient education to address psychological factors and lifestyle changes associated with BMS. |
| Study 9 [47] | Dry eyes 35/102 (34.3%) | No comparator | Not reported | Candidiasis *** Chronic pain, Common mental illnesses Dysgeusia Hyposalivation Xerostomia | The study reports that burning mouth syndrome (BMS) poses a diagnostic challenge and causes significant delays in diagnosis, often with patients seeing multiple providers before presenting to an oral medicine specialist. Misdiagnosis is common, with candidiasis being the most frequent provisional diagnosis. Patients often present with extraoral comorbidities, including anxiety and depression. BMS is underrecognized and underappreciated by both medical and dental professionals. A clinician with more familiarity with BMS can quickly diagnose and begin the proper management regimen without delay. |
| Study ID | Extraoral Dryness Outcomes for BMS | Extraoral Dryness Outcomes for Controls | Odds Ratio (95% Confidence Interval) |
|---|---|---|---|
| Study 1 [25] | Dry eyes 18/54 (33.3%) | Dry eyes 4/27 (14.8%) | Dry eyes 2.88 (0.86, 9.58) |
| Study 2 [42] | Skin problems 23/84 (27.4%) | Skin problems 7/73 (9.6%) | Skin problems 3.56 (1.43, 8.88) |
| Study 3 [43] | Dry eyes 19/40 (47.5%) | Dry eyes 11/42 (26.2%) | Dry eyes 2.55 (1.01, 6.44) |
| Study 4 [44] | Dry eyes 12/22 (54.5%) Genital dryness 11/22 (50%) | Dry eyes 11/28 (39.3%) Genital dryness 5/28 (17.8%) | Dry eyes 1.85 (0.60, 5.75) Genital dryness 4.60 (1.28, 16.51) |
| Study 5 [4] | Genital dryness 15/124 (12.1%) | Genital dryness 0/130 (0%) | NA |
| Study 6 [26] | Skin problems 22/56 (39.3%) | Skin problems 8/56 (14.3%) | Skin problems 3.88 (1.55, 9.75) |
| Study 7 [45] | Xerostomia Inventory * Skin problems 1.85 ± 1.31 Dry eyes 3.70 ± 0.92 Dry lips 4.05 ± 0.83 Dry nose cavity 2.70 ± 1.30 | Xerostomia Inventory * Skin problems 1.85 ± 1.05 Dry eyes 1.65 ± 0.89 Dry lips 2.23 ± 1.05 Dry nose cavity 1.98 ± 0.85 | NA |
| Study 8 [46] | Dry lips 17/31 (54.8%) | No data ** | NA |
| Study 9 [47] | Dry eyes 35/102 (34.3%) | No comparator | NA |
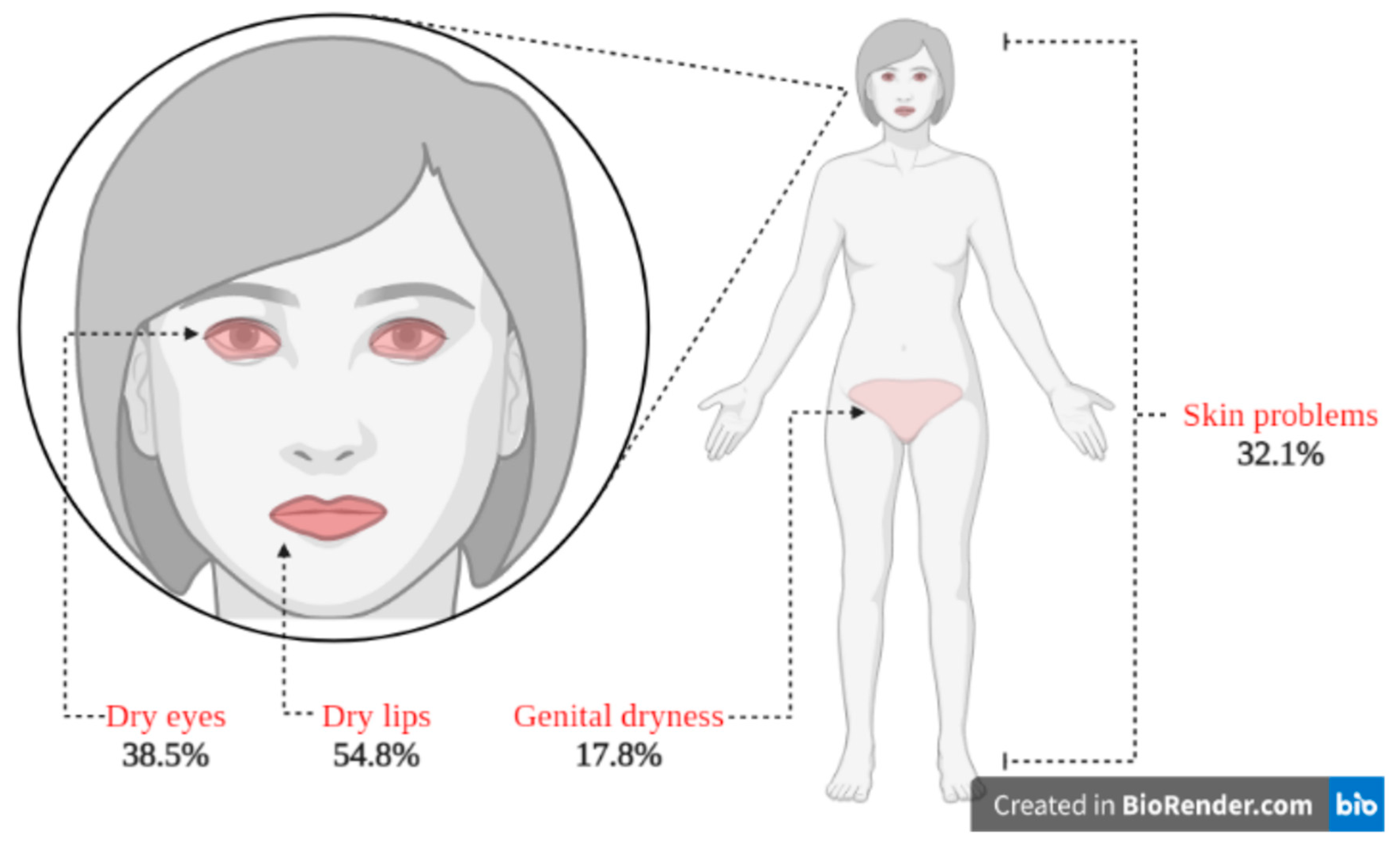
| Summed Reported Extraoral Dryness Values | In BMS Patients | In the Control Group | Odds Ratio (95% Confidence Interval) |
|---|---|---|---|
| Dry eyes | = 38.5% | = 26.8% | 1.7 (1.01, 2.88) |
| Genital dryness | = 17.8% | = 3.2% | 6.6 (2.47, 17.78) |
| Skin problems | = 32.1% | = 11.6% | 3.6 (1.89, 6.86) |
| Dry lips | = 54.8% | No comparator | Not applicable |
| Overall dryness | = 32.1% | = 12.0% | 2.7 (1.88, 3.84) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, K.D.T.; DosSantos, M.F.; Gazerani, P. Is Burning Mouth Syndrome Associated with Extraoral Dryness? A Systematic Review. J. Clin. Med. 2023, 12, 6525. https://doi.org/10.3390/jcm12206525
Le KDT, DosSantos MF, Gazerani P. Is Burning Mouth Syndrome Associated with Extraoral Dryness? A Systematic Review. Journal of Clinical Medicine. 2023; 12(20):6525. https://doi.org/10.3390/jcm12206525
Chicago/Turabian StyleLe, Kim Devon Terga, Marcos Fabio DosSantos, and Parisa Gazerani. 2023. "Is Burning Mouth Syndrome Associated with Extraoral Dryness? A Systematic Review" Journal of Clinical Medicine 12, no. 20: 6525. https://doi.org/10.3390/jcm12206525
APA StyleLe, K. D. T., DosSantos, M. F., & Gazerani, P. (2023). Is Burning Mouth Syndrome Associated with Extraoral Dryness? A Systematic Review. Journal of Clinical Medicine, 12(20), 6525. https://doi.org/10.3390/jcm12206525







